Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Search Strategy
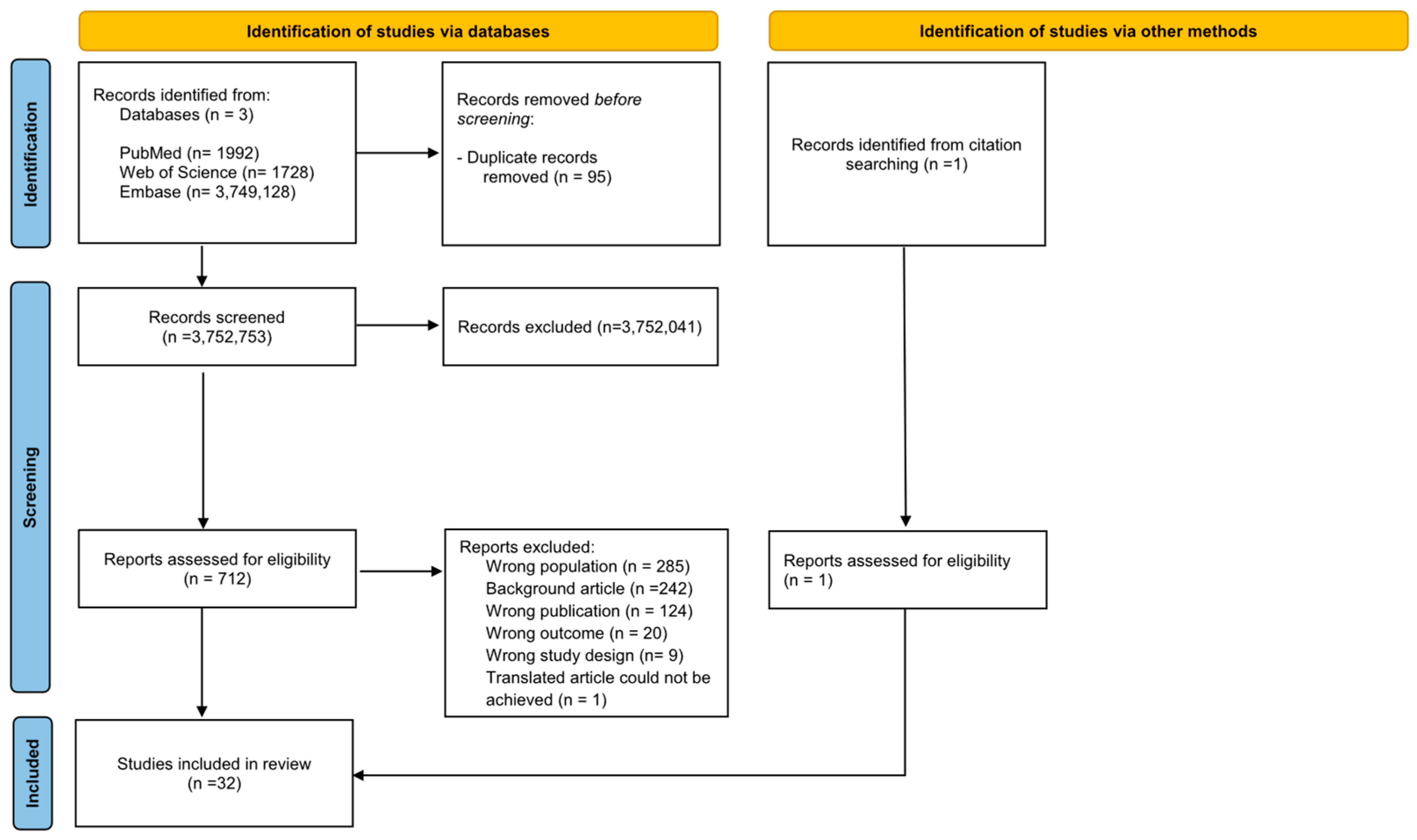
2.2. Selection Procedures
2.3. Data Extraction and Evaluation
2.4. Strategy for Data Synthesis
2.5. Quality Assessment
3. Results
3.1. Sample Characteristics
3.2. Domains Investigated and Outcome Measures
3.3. Safety
- -
- MPH: Most frequent AEs were decreased appetite, sleep disturbances, irritability, and gastrointestinal symptoms. 28 out of 388 patients (7.2%) discontinued.
- -
- ATX: Similar profile to MPH, with 45 dropouts out of 306 patients (14.7%).
- -
- GFC: Common AEs included irritability and sleep problems (notably insomnia and mid-sleep awakenings). 2 out of 87 patients (2.3%) withdrew.
- -
- Clonidine: Drowsiness and irritability were reported; no dropouts.
3.4. Non-Pharmacological Intervention
3.5. Pharmacological Intervention
3.5.1. Methylphenidate
3.5.2. Atomoxetine
3.5.3. Guanfacina
3.5.4. Other Pharmacological Interventions
3.6. Response Moderators
3.7. Quality Assessment
4. Discussion
5. Limitations
6. Future Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| MPH | Methylphenidate |
| ATX | Atomoxetine |
| GFC | Guanfacina |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| RCTs | Randomized Controlled Studies |
| ICD | International Classification of Diseases |
| IQ | Intellectual Quotient |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| NOS | Newcastle-Ottawa Scale |
| AHRQ | Agency for Healthcare Research and Quality |
| ABC | Aberrant Behavior Checklist |
| CPRS | Conners Parent Rating Scales–Revised |
| SNAPIV | Swanson, Noland, and Pelham Scale IV |
| CGI | Clinical Global Improvement |
| AE | Adverse Events |
| VR-CBT | Virtual reality-based cognitive-behavioral therapy |
| LSP | Therapist-led program |
| PT | Parent Training |
| RIS | Risperidone |
| ARI | Aripiprazole |
| CONSORT | Consolidated Standards of Reporting Trials |
| ED | Emotion Dysregulation |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders., 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Bougeard, C.; Picarel-Blanchot, F.; Schmid, R.; Campbell, R.; Buitelaar, J. Prevalence of Autism Spectrum Disorder and Co-Morbidities in Children and Adolescents: A Systematic Literature Review. Focus (Am. Psychiatr. Publ.) 2024, 22, 212–228. [Google Scholar] [CrossRef] [PubMed]
- Kenny, L.; Hattersley, C.; Molins, B.; Buckley, C.; Povey, C.; Pellicano, E. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 2016, 20, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.-C.; Lombardo, M.V.; Ruigrok, A.N.; Chakrabarti, B.; Auyeung, B.; Szatmari, P.; Happé, F.; Baron-Cohen, S.; MRC AIMS Consortium. Quantifying and exploring camouflaging in men and women with autism. Autism 2017, 21, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Baron-Cohen, S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Fucà, E.; Guerrera, S.; Valeri, G.; Casula, L.; Novello, R.L.; Menghini, D.; Vicari, S. Psychiatric comorbidities in children and adolescents with high-functioning autism spectrum disorder: A study on prevalence, distribution and clinical features in an Italian sample. J. Clin. Med. 2023, 12, 677. [Google Scholar] [CrossRef]
- Fuld, S. Autism spectrum disorder: The impact of stressful and traumatic life events and implications for clinical practice. Clin. Soc. Work. J. 2018, 46, 210–219. [Google Scholar] [CrossRef]
- Visser, J.C.; Rommelse, N.; Greven, C.; Buitelaar, J.K. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of the current evidence and future directions. Child. Adolesc. Psychiatry Ment. Health 2016, 10, 17. [Google Scholar]
- Leitner, Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children—What do we know? Front. Hum. Neurosci. 2014, 8, 268. [Google Scholar] [CrossRef]
- Antshel, K.M.; Zhang-James, Y.; Wagner, K.E.; Ledesma, A.; Faraone, S.V. An update on the comorbidity of ADHD and ASD: A focus on clinical management. Expert Rev. Neurother. 2016, 16, 279–293. [Google Scholar] [CrossRef]
- Sokolova, E.; Oerlemans, A.M.; Rommelse, N.N.; Groot, P.; Hartman, C.A.; Glennon, J.C.; Claassen, T.; Heskes, T.; Buitelaar, J.K. A causal and mediation analysis of the comorbidity between attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). J. Autism Dev. Disord. 2017, 47, 1595–1604. [Google Scholar] [CrossRef]
- Stergiakouli, E.; Thapar, A.; Davey Smith, G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: Evidence against confounding. JAMA Pediatr. 2016, 170, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Mahendiran, T.; Brian, J.; Dupuis, A.; Muhe, N.; Wong, P.Y.; Iaboni, A.; Anagnostou, E. Meta-analysis of sex differences in social and communication function in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. Front. Psychiatry 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.F.; Posner, J. Moving towards causality in attention-deficit hyperactivity disorder: Overview of neural and genetic mechanisms. Lancet Psychiatry 2016, 3, 555–567. [Google Scholar] [CrossRef]
- Simon, V.; Czobor, P.; Bálint, S.; Mészáros, Á.; Bitter, I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br. J. Psychiatry 2009, 194, 204–211. [Google Scholar] [CrossRef]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E.J. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Schwörer, M.C.; Reinelt, T.; Petermann, F.; Petermann, U. Influence of executive functions on the self-reported health-related quality of life of children with ADHD. Qual. Life Res. 2020, 29, 1183–1192. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef]
- Murray, M.L.; Hsia, Y.; Glaser, K.; Simonoff, E.; Murphy, D.G.; Asherson, P.J.; Eklund, H.; Wong, I.C. Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology 2014, 231, 1011–1021. [Google Scholar] [CrossRef]
- Aman, M.G.; Langworthy, K.S. Pharmacotherapy for hyperactivity in children with autism and other pervasive developmental disorders. J. Autism Dev. Disord. 2000, 30, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Persico, A.M.; Postorino, V.; Simmaco, M.; Fatta, L.M.; Vanacore, N.; Armando, M.; uratolo, P.; Vicari, S.; Mazzone, L.; Laviola, G.; et al. Toward a pharmacogenetic algorithm for the personalization of drug treatments in autism spectrum disorder and attention-deficit hyperactivity disorder. Pharmacol. Res. 2021, 165, 105442. [Google Scholar]
- Sikora, D.M.; Vora, P.; Coury, D.L.; Rosenberg, D. Attention-deficit/hyperactivity disorder symptoms, adaptive functioning, and quality of life in children with autism spectrum disorder. Pediatrics 2012, 130 (Suppl. 2), S91–S97. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.M.; Oerlemans, A.M.; Van Steijn, D.J.; Lappenschaar, M.G.; De Sonneville, L.M.; Buitelaar, J.K.; Rommelse, N.N. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 1160–1172.e3. [Google Scholar] [CrossRef]
- Storebø, O.J.; Elmose Andersen, M.; Skoog, M.; Joost Hansen, S.; Simonsen, E.; Pedersen, N.; Tendal, B.; Callesen, H.E.; Faltinsen, E.; Gluud, C.; et al. Social skills training for attention deficit hyperactivity disorder (ADHD) in children aged 5 to 18 years. Cochrane Database Syst. Rev. 2019, 2019, CD008223. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097-269. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Publishing: Washington, DC, USA, 1994. [Google Scholar]
- Slee, V.N. The International Classification of Diseases: Ninth revision (ICD-9). Ann. Intern. Med. 1978, 88, 424–426. [Google Scholar] [CrossRef]
- World Health Organization. CD-10: International Statistical Classification of Diseases and Related Health Problems. 10th Revision; Disanto, S., Translator; Italian Collaborating Centre of WHO for the Family of International Classifications: Milan, Italy, 2001. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality (AHRQ). Guidance for Assessing the Quality and Risk of Bias of Randomized Controlled Trials; U.S. Department of Health and Human Services: Rockville, MD, USA, 2012.
- Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 2005, 62, 1266–1274. [Google Scholar] [CrossRef]
- Posey, D.J.; Wiegand, R.E.; Wilkerson, J.; Maynard, M.; Stigler, K.A.; McDougle, C.J. Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J. Child. Adolesc. Psychopharmacol. 2006, 16, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Scahill, L.; Bearss, K.; Sarhangian, R.; McDougle, C.J.; Arnold, L.E.; Aman, M.G.; McCracken, J.T.; Tierney, E.; Gillespie, S.; Postorino, V.; et al. Using a patient-centered outcome measure to test methylphenidate versus placebo in children with autism spectrum disorder. J. Child. Adolesc. Psychopharmacol. 2017, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Santosh, P.J.; Baird, G.; Pityaratstian, N.; Tavare, E.; Gringras, P. Impact of comorbid autism spectrum disorders on stimulant response in children with attention deficit hyperactivity disorder: A retrospective and prospective effectiveness study. Child. Care Health Dev. 2006, 32, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Taylor, E.; Baird, G.; Bernard, S.; Chadwick, O.; Liang, H.; Whitwell, S.; Riemer, K.; Sharma, K.; Sharma, S.P.; et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J. Child. Psychol. Psychiatry 2013, 54, 527–535. [Google Scholar] [CrossRef]
- Pearson, D.A.; Santos, C.W.; Aman, M.G.; Arnold, L.E.; Casat, C.D.; Mansour, R.; Lane, D.M.; Loveland, K.A.; Bukstein, O.G.; Jerger, S.W.; et al. Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J. Child. Adolesc. Psychopharmacol. 2013, 23, 337–351. [Google Scholar] [CrossRef]
- Pearson, D.A.; Santos, C.W.; Aman, M.G.; Arnold, L.E.; Lane, D.M.; Loveland, K.A.; Mansour, R.; Ward, A.R.; Casat, C.D.; Jerger, S.; et al. Effects of extended-release methylphenidate treatment on cognitive task performance in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Child. Adolesc. Psychopharmacol. 2020, 30, 414–426. [Google Scholar] [CrossRef]
- Golubchik, P.; Rapaport, M.; Weizman, A. The effect of methylphenidate on anxiety and depression symptoms in patients with Asperger syndrome and comorbid attention deficit/hyperactivity disorder. Int. Clin. Psychopharmacol. 2017, 32, 289–293. [Google Scholar] [CrossRef]
- Kim, S.J.; Shonka, S.; French, W.P.; Strickland, J.; Miller, L.; Stein, M.A. Dose-response effects of long-acting liquid methylphenidate in children with attention deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD): A pilot study. J. Autism Dev. Disord. 2017, 47, 2307–2313. [Google Scholar] [CrossRef]
- Peled, J.; Cassuto, H.; Berger, I. Processing speed as a marker to stimulant effect in clinical sample of children with high functioning autism spectrum disorder. Nord. J. Psychiatry 2020, 74, 163–167. [Google Scholar] [CrossRef]
- Ventura, P.; de Giambattista, C.; Trerotoli, P.; Cavone, M.; Di Gioia, A.; Margari, L. Methylphenidate use for emotional dysregulation in children and adolescents with ADHD and ASD: A naturalistic study. J. Clin. Med. 2022, 11, 2922. [Google Scholar] [CrossRef]
- Arnold, L.E.; Aman, M.G.; Cook, A.M.; Witwer, A.N.; Hall, K.L.; Thompson, S.; Ramadan, Y. Atomoxetine for hyperactivity in autism spectrum disorders: Placebo-controlled crossover pilot trial. J. Am. Acad. Child. Adolesc. Psychiatry 2006, 45, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Troost, P.W.; Steenhuis, M.-P.; Tuynman-Qua, H.G.; Kalverdijk, L.J.; Buitelaar, J.K.; Minderaa, R.B.; Hoekstra, P.J. Atomoxetine for attention-deficit/hyperactivity disorder symptoms in children with pervasive developmental disorders: A pilot study. J. Child. Adolesc. Psychopharmacol. 2006, 16, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Harfterkamp, M.; van de Loo-Neus, G.; Minderaa, R.B.; van der Gaag, R.-J.; Escobar, R.; Schacht, A.; Pamulapati, S.; Buitelaar, J.K.; Hoekstra, P.J. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Harfterkamp, M.; Buitelaar, J.K.; Minderaa, R.B.; van de Loo-Neus, G.; van der Gaag, R.J.; Hoekstra, P.J. Atomoxetine in autism spectrum disorder: No effects on social functioning; some beneficial effects on stereotyped behaviors, inappropriate speech, and fear of change. J. Child. Adolesc. Psychopharmacol. 2014, 24, 481–485. [Google Scholar] [CrossRef]
- Harfterkamp, M.; Buitelaar, J.K.; Minderaa, R.B.; van de Loo-Neus, G.; van der Gaag, R.J.; Hoekstra, P.J. Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: An open-label extension study. J. Child. Adolesc. Psychopharmacol. 2013, 23, 194–199. [Google Scholar] [CrossRef]
- Fernández-Jaén, A.; Fernández-Mayoralas, D.M.; Calleja-Pérez, B.; Muñoz-Jareño, N.; Campos Díaz, M.R.; López-Arribas, S. Efficacy of atomoxetine for the treatment of ADHD symptoms in patients with pervasive developmental disorders: A prospective, open-label study. J. Atten. Disord. 2013, 17, 497–505. [Google Scholar] [CrossRef]
- Handen, B.L.; Aman, M.G.; Arnold, L.E.; Hyman, S.L.; Tumuluru, R.V.; Lecavalier, L.; Corbett-Dick, P.; Pan, X.; Hollway, J.A.; Buchan-Page, K.A.; et al. Atomoxetine, parent training, and their combination in children with autism spectrum disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 905–915. [Google Scholar] [CrossRef]
- Tumuluru, R.V.; Corbett-Dick, P.; Aman, M.G.; Smith, T.; Arnold, L.E.; Pan, X.; Buchan-Page, K.A.; Brown, N.V.; Ryan, M.M.; Hyman, S.L.; et al. Adverse events of atomoxetine in a double-blind placebo-controlled study in children with autism. J. Child. Adolesc. Psychopharmacol. 2017, 27, 708–714. [Google Scholar] [CrossRef]
- Smith, T.; Aman, M.G.; Arnold, L.E.; Silverman, L.B.; Lecavalier, L.; Hollway, J.; Tumuluru, R.; Hyman, S.L.; Buchan-Page, K.A.; Hellings, J.; et al. Atomoxetine and parent training for children with autism and attention-deficit/hyperactivity disorder: A 24-week extension study. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 868–876.e2. [Google Scholar] [CrossRef]
- Kilincaslan, A.; Mutluer, T.D.; Pasabeyoglu, B.; Tutkunkardas, M.D.; Mukaddes, N.M. Effects of atomoxetine in individuals with attention-deficit/hyperactivity disorder and low-functioning autism spectrum disorder. J. Child. Adolesc. Psychopharmacol. 2016, 26, 798–806. [Google Scholar] [CrossRef]
- Scahill, L.; Pachler, M. Treatment of hyperactivity in children with pervasive developmental disorders. J. Child. Adolesc. Psychiatr. Nurs. 2007, 20, 59–62. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.T.; Aman, M.G.; McDougle, C.J.; Tierney, E.; Shiraga, S.; Whelan, F.; Arnold, L.E.; Posey, D.; Ritz, L.; Vitiello, B.; et al. Possible influence of variant of the P-glycoprotein gene (MDR1/ABCB1) on clinical response to guanfacina in children with pervasive developmental disorders and hyperactivity. J. Child. Adolesc. Psychopharmacol. 2010, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Scahill, L.; McCracken, J.T.; King, B.H.; Rockhill, C.; Shah, B.; Politte, L.; Sanders, R.; Minjarez, M.; Cowen, J.; Mullett, J.; et al. Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 2015, 172, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Politte, L.C.; Scahill, L.; Figueroa, J.; McCracken, J.T.; King, B.; McDougle, C.J. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: An analysis of secondary outcome measures. Neuropsychopharmacology 2018, 43, 1772–1778. [Google Scholar] [CrossRef]
- Jaselskis, C.A.; Cook, E.H., Jr.; Fletcher, K.E.; Leventhal, B.L. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J. Clin. Psychopharmacol. 1992, 12, 322–327. [Google Scholar] [CrossRef]
- Lamberti, M.; Siracusano, R.; Italiano, D.; Alosi, N.; Cucinotta, F.; Di Rosa, G.; Germanò, E.; Spina, E.; Gagliano, A. Head-to-head comparison of aripiprazole and risperidone in the treatment of ADHD symptoms in children with autistic spectrum disorder and ADHD: A pilot, open-label, randomized controlled study. Paediatr. Drugs 2016, 18, 319–329. [Google Scholar] [CrossRef]
- Aman, M.G.; Singh, N.N.; Stewart, A.W.; Field, C.J. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am. J. Ment. Defic. 1985, 89, 485–491. [Google Scholar]
- Conners, C.K. Conners’ Rating Scales–Revised: Technical Manual; Multi-Health Systems: North Tonawanda, NY, USA, 1997. [Google Scholar]
- Swanson, J.M.; Nolan, W.; Pelham, W.E. The SNAP-IV Teacher and Parent Rating Scale. Available online: https://www.caddra.ca (accessed on 17 April 2025).
- Guy, W. Clinical Global Impressions. In ECDEU Assessment Manual for Psychopharmacology; Guy, W., Ed.; U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration: Rockville, MD, USA, 1976; pp. 217–222. [Google Scholar]
- Chan, J.K.-Y.; Cheung, T.C.-K.; Chan, C.-W.; Fang, F.; Lai, K.Y.-C.; Sun, X.; O’reilly, H.; Golan, O.; Allison, C.; Baron-Cohen, S.; et al. Enhancing emotion recognition in young autistic children with or without attention-deficit/hyperactivity disorder in Hong Kong using a Chinese App version of The Transporters. Autism 2024, 28, 945–958. [Google Scholar] [CrossRef]
- Chu, L.; Shen, L.; Ma, C.; Chen, J.; Tian, Y.; Zhang, C.; Gong, Z.; Li, M.; Wang, C.; Pan, L.; et al. Effects of a nonwearable digital therapeutic intervention on preschoolers with autism spectrum disorder in China: Open-label randomized controlled trial. J. Med. Internet Res. 2023, 25, e45836. [Google Scholar] [CrossRef]
- Patel, K.; Curtis, L.T. A comprehensive approach to treating autism and attention-deficit hyperactivity disorder: A prepilot study. J. Altern. Complement. Med. 2007, 13, 1091–1097. [Google Scholar] [CrossRef]
- Yerys, B.E.; Bertollo, J.R.; Kenworthy, L.; Dawson, G.; Marco, E.J.; Schultz, R.T.; Sikich, L. Brief report: Pilot study of a novel interactive digital treatment to improve cognitive control in children with autism spectrum disorder and co-occurring ADHD symptoms. J. Autism Dev. Disord. 2019, 49, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Lau-Zhu, A.; Fritz, A.; McLoughlin, G. Overlaps and distinctions between attention deficit/hyperactivity disorder and autism spectrum disorder in young adulthood: Systematic review and guiding framework for EEG-imaging research. Neurosci. Biobehav. Rev. 2019, 96, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Karalunas, S.L.; Hawkey, E.; Gustafsson, H.; Miller, M.; Langhorst, M.; Cordova, M.; Fair, D.; Nigg, J.T. Overlapping and distinct cognitive impairments in attention-deficit/hyperactivity and autism spectrum disorder without intellectual disability. J. Abnorm. Child. Psychol. 2018, 46, 1705–1716. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Murray, M.J.; Morrow, J.D.; Cothren, S.; Purichia, H.; Yurich, K.K.L.; Bouder, J.N. Use of Gilliam Asperger’s disorder scale in differentiating high and low functioning autism and ADHD. Psychol. Rep. 2011, 108, 3–13. [Google Scholar] [CrossRef]
- Sturm, H.; Fernell, E.; Gillberg, C. Autism spectrum disorders in children with normal intellectual levels: Associated impairments and subgroups. Dev. Med. Child. Neurol. 2004, 46, 444–447. [Google Scholar] [CrossRef]
- de Boo, G.M.; Prins, P.J. Social incompetence in children with ADHD: Possible moderators and mediators in social-skills training. Clin. Psychol. Rev. 2007, 27, 78–97. [Google Scholar] [CrossRef]
- Craig, F.; Lamanna, A.L.; Margari, F.; Matera, E.; Simone, M.; Margari, L. Overlap between autism spectrum disorders and attention deficit hyperactivity disorder: Searching for distinctive/common clinical features. Autism Res. 2015, 8, 328–337. [Google Scholar] [CrossRef]
- Posey, D.J.; Aman, M.G.; McCracken, J.T.; Scahill, L.; Tierney, E.; Arnold, L.E.; Vitiello, B.; Chuang, S.Z.; Davies, M.; Ramadan, Y.; et al. Positive effects of methylphenidate on inattention and hyperactivity in pervasive developmental disorders: An analysis of secondary measures. Biol. Psychiatry 2007, 61, 538–544. [Google Scholar] [CrossRef]
- Man, K.K.C.; Lau, W.C.Y.; Coghill, D.; Besag, F.M.C.; Cross, J.H.; Ip, P.; Wong, I.C.K. Association between methylphenidate treatment and risk of seizure: A population-based, self-controlled case-series study. Lancet Child. Adolesc. Health 2020, 4, 435–443. [Google Scholar] [CrossRef]
- Sturman, N.; Deckx, L.; van Driel, M.L. Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst. Rev. 2017, 11, CD011144. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, S.L.; Ghuman, J.K.; Ghuman, H.S.; Karpov, I.; Schuster, J.M. Efficacy of atomoxetine in the treatment of attention-deficit hyperactivity disorder in patients with common comorbidities in children, adolescents and adults: A review. Ther. Adv. Psychopharmacol. 2016, 6, 317–334. [Google Scholar] [CrossRef]
- Rezaei, G.; Hosseini, S.A.; Sari, A.A.; Olyaeemanesh, A.; Lotfi, M.H.; Yassini, M.; Bidaki, R.; Nouri, B. Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran. 2016, 30, 325. [Google Scholar] [PubMed]
- Garnock-Jones, K.P.; Keating, G.M. Atomoxetine: A review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr. Drugs 2009, 11, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, T.; Winterstein, A.G.; Olfson, M.; Huang, C.; Saidi, A.; Crystal, S. Pre-existing cardiovascular conditions and pharmacological treatment of adult ADHD. Pharmacoepidemiol. Drug Saf. 2010, 19, 457–464. [Google Scholar] [CrossRef]
- Reichart, C.G.; Nolen, W.A. Earlier onset of bipolar disorder in children by antidepressants or stimulants? An hypothesis. J. Affect. Disord. 2004, 78, 81–84. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). NICE Guidelines for the Treatment of Adult ADHD. 2008. Available online: https://www.nice.org.uk/guidance/cg72 (accessed on 15 February 2016).
- D’Alò, G.L.; De Crescenzo, F.; Amato, L.; Cruciani, F.; Davoli, M.; Fulceri, F.; Minozzi, S.; Mitrova, Z.; Morgano, G.P.; Nardocci, F.; et al. Acceptability, equity, and feasibility of using antipsychotics in children and adolescents with autism spectrum disorder: A systematic review. BMC Psychiatry 2020, 20, 561. [Google Scholar] [CrossRef]
- Safavi, P.; Hasanpour-Dehkordi, A.; AmirAhmadi, M. Comparison of risperidone and aripiprazole in the treatment of preschool children with disruptive behavior disorder and attention deficit-hyperactivity disorder: A randomized clinical trial. J. Adv. Pharm. Technol. Res. 2016, 7, 43–47. [Google Scholar] [CrossRef]
- Lv, Y.B.; Cheng, W.; Wang, M.H.; Wang, X.M.; Hu, Y.L.; Lv, L.Q. Multimodal integrated intervention for children with attention-deficit/hyperactivity disorder. World J. Clin. Cases 2023, 11, 4267–4276. [Google Scholar] [CrossRef]
- Steeger, C.M.; Gondoli, D.M.; Gibson, B.S.; Morrissey, R.A. Combined cognitive and parent training interventions for adolescents with ADHD and their mothers: A randomized controlled trial. Child. Neuropsychol. 2016, 22, 394–419. [Google Scholar] [CrossRef]
- Scarselli, V.; Martucci, M.; Novelli, M.; Galosi, S.; Romani, M.; Sogos, C. Diagnostic and therapeutic challenges of comorbid ASD, ADHD and psychosis: A case report. Behav. Sci. 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Lebeña, A.; Faresjö, Å.; Faresjö, T.; Ludvigsson, J. Clinical implications of ADHD, ASD, and their co-occurrence in early adulthood—The prospective ABIS-study. BMC Psychiatry 2023, 23, 851. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hours, C.; Recasens, C.; Baleyte, J.M. ASD and ADHD comorbidity: What are we talking about? Front. Psychiatry 2022, 13, 837424. [Google Scholar] [CrossRef]
- Cucinotta, F.; Ricciardello, A.; Turriziani, L.; Mancini, A.; Keller, R.; Sacco, R.; Persico, A.M. Efficacy and Safety of Q10 Ubiquinol With Vitamins B and E in Neurodevelopmental Disorders: A Retrospective Chart Review. Front. Psychiatry 2022, 13, 829516. [Google Scholar] [CrossRef]
| Reference | Study Design | Sample Size | Ethnicity % | Sex Ratio M:F | Age Range in Years (Mean ± SD) | IQ, Mean (SD) or % of Individuals with Intellectualdisability |
|---|---|---|---|---|---|---|
| RUPP., 2005 [35]; Posey et al., 2006 [36]; Scahill et al., 2017 [37] | randomized crossover trial | 72 | 73.3% White/Non-Hispanic, 15% Black or African American, 6.7% Asian, 5% Hispanic | 9:1 | 5–14 (7.4 ± 2.1) | IQ 40% ≥ 70 50% < 70 10% missing data |
| RUPP., 2005 [35] | OPEN-LABEL extention study | 35 | // | // | 5–14 (7.4 ± 2.1) | // |
| Santosh et al., 2006 [38] | Retrospective | ADHD: 113 | N.R. | 4:1 | NR (13.1 ± 3.18) | 5.65% |
| ASD + ADHD: 61 | NR (12.4 ± 3.01) | 7.32% | ||||
| Prospective, quasiexperimental, within-subjects | ADHD: 25 | NR | 4.5:1 | NR (11.6 ± 2.5) | 95.2 (16.1) | |
| ASD + ADHD: 27 | 9:1 | NR (10.6 ± 2.7) | 84.3 (25.1) | |||
| Simonoff et al., 2013 [39] | randomized controlled double-blind trial | 61 | N.R. | 1:0.5 | 7–15 (11.5 ± 2.3) | 53 (10.5) |
| 61 | 2.8:1 | 7–15 (10.9 ± 2.4) | 54 (9.6) | |||
| Pearson et al., 2013; 2020 [40,41] | Single-blind, within-subject, dose crossover, placebo-controlled design | 24 | 75% Caucasian, 16.6% African-American, 4.17% Asian, 4.17% multiple races | 3.8:1 | 7.1–12.7 (8.8 ± 1.6) | 85.0 (16.8) |
| Golubchik et al., 2017 [42] | open-label design | 12 | N.R. | 4.5:1 | 8–18 (11.3 ± 2.0) | ≥IQ 70 |
| Kim et al., 2017 [43] | Pilot randomized study | Low dose group: 9 | 55.56% Caucasian, 18.52% multiple races, 14.81% NR, 7.41% other, 3.70% Asian | 9:1 | 5–14(9.33 ± 2.92) | NR |
| medium dose group: 18 | 9:1 | 5–15 (9.11 ± 3.12) | NR | |||
| Peled et al., 2019 [44] | Within subject | 40 | N.R. | 9:1 | 6–18 (9.0 ± N.R.) | IQ in the normal range |
| Ventura et al., 2022 [45] | Naturalistic study | ASD + ADHD: 29 | Caucasian 100% | 4.5:1 | 6–18 (13.3 ± NR) | 96.3 (NR) |
| ADHD: 41 | 1:0 | 6–18 (13.4 ± NR) | 97.2 (NR) | |||
| Arnold et al., 2006 [46] | double-blind, placebo-controlled, crossover pilot study | 16† | 81.3% White,12.5% African, 6.2% Asian | 3:1 | 5–15 (9.26 ± 2.93) | mental age ≥ 18 months |
| Troost et al., 2006 [47] | Pilot, within subject study | 12 | N.R. | 4.5:1 | 6–14 (10.2 ± 2.8) | ≥IQ 70 |
| Posey et al., 2006 [36] | prospective, open label study | 16 | 68.75% white 12.5% African american 12.5% Hispanic/Latino; 6.25% african american/white | 4.5:1 | 6–14 (7.7 ± 2.2) | 93.9 ± 18.0 |
| Harfeterkamp et al. 2012; 2014 [48,49] | double-blind RCT | 48 | 100% White, 0% African | 9:1 | 6–16 (9.9 ± 2.7) | 91.0 (16.4) |
| 49 | 98% White, 2% African | 9:2 | 6–17 (10 ± 2.9) | 94.6 (17.7) | ||
| Harfeterkamp et al. 2013 [50] | open-label extension study | 42 | N.R. | 9:1 | 6–16 (10.0 ± 2.8) | 93.1 (17.3) |
| 46 | 4.5:1 | 6–17 (10.0 ± 3.0) | ||||
| Fernandez-Jaen et al., 2013 [51] | prospective, open label study | 24† | N.R. | 3:2 | 5–17 (8.8 ± 3.38) | 80% |
| Handen et al. 2015 [52]; Tumuluru et al., 2017 [53] | double-blind RCT | 32 | 87.5 Caucasian, 3.1% African | 9:0 | 5–14.11 (8.0 ± 1.9) | 83.3 (21.6) |
| 32 | 84.4% Caucasian, 6.3% African | 4.5:1 | 5–14.11 (8.6 ± 2.3) | 78.7 (25.9) | ||
| 32 | 81.3% Caucasian, 6.3% African | 4.5:1 | 5–14.11 (7.7 ± 1.5) | 77.9 (25.7) | ||
| 32 | 75.0% Caucasian, 15.6% African | 9:1 | 5–14.11 (8.2 ± 2.4) | 86.7 (23.7) | ||
| Smith et al., 2016 [54] | open-label extension study | 28 | 85.7% Caucasian, 3.6% African American, 0% Other, 10.7% Multiracial | 5:1 | 5–14.11 (8.0 ± 1.8) | 85.4 ± 22.9 |
| 15 | 60.0% Caucasian, 20.0% African American, 6.7% Other, 13.3% Multiracial | 2:1 | 5–14.11 (8.1 ± 2.0) | 74.3 ± 26.6 | ||
| 19 | 79.0% Caucasian, 5.3% African American, 5.3% Other, 10.5% Multiracial | 18:1 | 5–14.11 (7.7 ± 1.3) | 82.9 ± 24.1 | ||
| 22 | 81.8% Caucasian, 13.6% African American, 0% Other, 4.6% Multiracial | 3:1 | 5–14.11 (8.4 ± 2.5) | 89.4 ± 22.9 | ||
| Kilincaslan et al., 2016 [55] | naturalistic retrospective study | 37 | N.R. | 3:1 | 6–17 (10.16 ± 3.60) | <70; mild 27% moderate 46% severe 27% |
| Scahill et al., 2007 [56]; McCracken et al., 2010 [57] | Prospective Open-label Trial | 25 | 72% caucasian, 24% black, 4% Hispanic | 11.5.1 | 9.03 (±3.14) | 31.56% |
| Scalhill et al., 2015 [58]; Politte et al., 2018 [59] | randomized, placebo-controlled trial | 30 | 56.67% white, 23.3% black, 13.33% asian, 3.33% pacific islander, 3.33% mixer | 9:1 | 5–14 (8.5 ± 2.25) | 36.7% |
| 32 | 68.75% white, 12.50% black, 3.13% asian, 3.13% pacific islander, 9.38% mixer | 4.5:1 | 33.0% | |||
| Jaselskis et al., 1992 [60] | double blind placebo controlled crossover study | 8 | N.R. | 8 | 5–13.4 (8.1 ± 2.8) | 30–75 (59 ± 16) |
| Lamberti et al., 2016 [61] | Pilot, Open-Label, Randomized Controlled Study | 22 | NR | 4.5:1 | 6–13 (7.8 ± 2.3) | ≥55 |
| 22 | NR | 3:1 | 6–13 (8.4 ± 2.9) |
| Reference | Study Design | Sample Size | Investigated Domains | Outcome Measures | Pharmacological Treatment | Mean or Range Dose | Treatment Duration | Efficacy |
|---|---|---|---|---|---|---|---|---|
| RUPP., 2005 [35]; Posey et al., 2006 [36]; Scahill et al., 2017 [37] | Randomized crossover trial | 72 | Hyperactivity, irritability, social withdrawal, stereotyped behaviors, inappropriate speech, distractibility, impulsivity, challenging behaviours | ABC (parent and teacher raters), CGI (RUPP 2005 [35], Scahill 2017 [37]); SNAP-IV, CYBOCS-PDD, (Posey 2006 [36]); PTPs (Scahill 2017 [37]); | Placebo | // | 1 week for each dose | // |
| MPH IR | ~0.125 mg/kg ×2/day + half dose at 4 P.M. | Hyperactivity (both parents and teachers), inattention | ||||||
| ~0.25 mg/kg ×2/day + half dose at 4 P.M. | Hyperactivity (both parents and teachers); ADHD-related problems targeted by parents | |||||||
| ~0.5 mg/kg ×2/day + half dose at 4 P.M. | Hyperactivity (both parents and teachers); ADHD-related problems targeted by parents. The greatest improvement was recorded at the highest dose | |||||||
| RUPP., 2005 [35] | OPEN LABEL extension study | 35 | hyperactivity, irritability, social withdrawal, stereotyped behaviors, inappropriate speech | ABC (parent and teacher raters) | MPH IR | adjusted on clinical judgment | 8 weeks | Hyperactivity (both parents and teachers) |
| Santosh et al., 2006 [38] | Retrospective | ADHD: 113 | therapeutic effects on degree of illness | CGI and TEI | MPH | MPH 30 mg/day in 3 divided doses | 8 weeks | The clinical response was not statistically different between the groups |
| ASD + ADHD: 61 | MPH 30 mg/day in 3 divided doses | |||||||
| Prospective, quasiexperimental, within-subjects | ADHD: 25 | hyperactivity, impulsivity, inattention, oppositionality, aggressivity, intermittent explosive rage, antisocial behav., tics, social communication, language, clumsiness, repetitive behav, circumscribed interests, cognitive rigidity, self-injury, stereotypies, hypersensitivity, lack of remorse, obsessions, low mood, depressive ideation, warries, panic, fears, labile mood, somatic symp., eating probl, sleep probl., bed wetting, hallucination, seizures | PONS-C, CGI | MPH | mean dose 31.7 mg/day | 8 weeks | hyperactivity, impulsivity, inattention, oppositionality, aggression and intermittent explosive rage | |
| ASD + ADHD: 27 | mean dose 33.8 mg/day | hyperactivity, impulsivity, inattention, oppositionality, aggression and intermittent explosive rage | ||||||
| Simonoff, et al., 2012 [39] | randomized controlled double-blind trial | 61 | inattention, hyperactivity, impulsivity, irritability, social withdrawal, stereotyped behaviors, inappropriate speech | CPRS-R, CTRS-R, ABC, CGI-I | Placebo | // | 16 weeks (at least 1 week of dose titration for each dose; after the optimal dose for each participant was prescribed for the remaining of 16 weeks) | // |
| 61 | MPH IR | 0.5 mg/kg ×3/day | hyperactivity and Conners ADHD index (both parents and teachers); 40% improved at CGI | |||||
| 1.0 mg/kg ×3/day | ||||||||
| 1.5 mg/kg ×3/day | ||||||||
| Pearson et al., 2013; 2020 [40,41] | Single-blind, within-subject, dose crossover, placebo-controlled design | 24 | inattention, hyperactivity, impulsivity, emotional lability, irritability, oppositional, social withdrawal, social skills, social communication, stereotypy, and inappropriate speech (Pearson D.A. et al., 2013 [40]). Sustained attention, selective attention, impulsivity/inhibition (Pearson D.A. et al., 2020 [41]) | CPRS-R, CTRS-R, SNAP-IV, ACTeRS, ABC, SCQ, VAS, CGI (Pearson D.A. et al., 2013 [40]); CPT, SCT, PSIT, GDS, MFFT, SST (Pearson D.A. et al., 2020 [41]) | Placebo | // | 1 week for each dose | // |
| MPH (ER morning + IR afternoon doses) | LA 0.21 mg/kg + IR 0.14 mg/kg | teachers detected significant improvements in hyperactivity and impulsivity (Pearson D.A. et al., 2013 [40]); gain in sustained attention, selective attention both auditive and visive, impulsivity/inhibition | ||||||
| 0.35 mg/kg + 0.24 mg/kg | Both parents and teachers: hyperactivity, impulsivity, social skills (Pearson D.A. et al., 2013 [40]); gain in sustained attention, selective attention both auditive and visive, impulsivity/inhibition | |||||||
| 0.48 mg/kg + 0.27 mg/kg | Both parents and teachers: oppositional behavior, hyperactivity, impulsivity, attention, irritability, inappropriate speech, and social skills. These effects were greater than the medium dose. Clinicians reported the most dramatic improvement. (Pearson D.A. et al., 2013 [40]); greater improvement in sustained attention at the highest dose, selective attention both auditive and visive, impulsivity/inhibition. | |||||||
| Golubchik et al., 2017 [42] | open-label design | 12 | inattention, hyperactivity, impulsivity, anxiety and depression symptoms | ADHD-RS, SCARED, CDI | MPH | 10–54 mg/day | 12 weeks | attention-deficit/hyperactivity symptoms, school-related anxiety |
| Kim et al., 2017 [43] | Pilot randomized study | Low dose group: 9 | inattention, hyperactivity, impulsivity, irritability, social withdrawal, inappropriate speech, stereotyped behaviors. | ADHD-RS-INV, CGI-I, ABC | MPH ER | 0.29 mg/kg | 6 weeks | attention, hyperactivity, impulsivity and clinical global improvement for low and medium doses, with greater improvement at the highest dose. Additionally, irritability, lethargy, inappropriate speech, and stereotyped behaviors only for medium dose. |
| medium dose group: 18 | 0.5 mg/kg | |||||||
| Peled et al., 2019 [44] | Within subject | 40 | inattention, hyperactivity, processing speed, impulsivity | MOXO-CPT | MPH IR | 10 mg i.r. | single dose | Processing speed |
| Ventura et al., 2022 [45] | Naturalistic study | ASD + ADHD: 29 | Emotional disregulation | CBCL | MPH ER | 0.8 mg/kg | 12 weeks | significant reduction in emotion dysregulation |
| ADHD: 41 | 0.78 mg/kg | 12 weeks | significant reduction in emotion dysregulation | |||||
| Arnold et al., 2006 [46] | double-blind, placebo-controlled, crossover pilot study | 16† | inattention, hyperactivity, impulsivity, irritability, social withdrawal, stereotyped behaviors, inappropriate speech; Self-injury, Compulsions, Rituals, Restrictive | ABC hyperactivity subscale, RBSR, CGI-S, CGI-I, CPT, DMST, ACT | Placebo | // | 6 weeks | // |
| ATX | 1.4 mg/kg/day in split dose (maximum daily dose) | hyperactivity, impulsivity, social withdrawall | ||||||
| Troost et al., 2006 [47] | Pilot, within subject study | 12 | inattention, hyperactivity, impulsivity, oppositional, irritability, social withdrawal, stereotypy, inappropriate speech | ADHD-RS IV, CPRS-R, ABC; CGI-ADHD-S | ATX | 0.5–1.8 mg/kg/day (divided into two doses based on patient-preference) | 10 weeks | Hyperactivity, attention |
| Posey et al., 2006 [36] | prospective, open label study | 16 | inattention, hyperactivity, impulsivity, irritability, social withdrawal, stereotype, inappropriate speech, social responsiveness. | SNAP-IV reported both by parents and teachers, ABC, CGI-I, SRS | ATX | 1.2–1.4 mg/kg/day | 8 weeks | adhd total score reported both by parents and teachers; irritability, social withdrawal, stereotype, inappropriate speech reported by parent |
| Harfeterkamp et al. 2012; 2014 [48,49] | double-blind RCT | 48 | inattention, hyperactivity, impulsivity (Harfeterkamp M., et al. 2012 [48]); irritability, social withdrawal, stereotypic behavior, inappropriate speech, subtle social, communicative, and repetitive behaviors (Harfeterkamp M., et al. 2014 [49]); | ADHD RS-IV, CTRS-R:S, CGI-ADHD-S (Harfeterkamp M., et al. 2012 [48]); ABC, CSBQ (Harfeterkamp M., et al. 2014 [49]) | ATX | 1.2 mg/kg/day | 8 weeks | Hyperactivity, inattention, and impulsivity, reported both by parents and teachers (Harfeterkamp M., et al. 2012 and 2014 [48,49]); inappropriate speech, and stereotypic behavior, fear of changes (Harfeterkamp M., et al. 2014 [49]) |
| 49 | Placebo | // | // | |||||
| Harfeterkamp et al. 2013 [50] | open-label exstension study | 42 | inattention, hyperactivity, impulsivity | ADHD-RS, CGI | ATX | 0.8–1.2 mg/kg/day, based on tolerability | 20 weeks | Hyperactivity, inattention, impulsivity |
| 46 | ||||||||
| Fernandez-Jaen et al., 2013 [51] | prospective, open label study | 24† | inattention, hyperactivity, impulsivity, conduct problems | ADHD RS-IV, CPRS-R, CTRS-R:S, CGI-I | ATX | 39.79 mg/day (±12.46) | 16 weeks | Hyperactivity, inattention, impulsivity, reported both by parents and teachers |
| Handen et al. 2015 [52]; Tumuluru, et al., 2017 [53] | double-blind RCT | 32 | inattention, hyperactivity, impulsivity; non-compliance (parents and teachers raters); irritability, social withdrawal, stereotypy, inappropriate speech (Handen B. L., et al. 2015 [52]) | SNAP IV and ABC (parent and teacher rater), CGI-ADHD, HSQ, SSQ (Handen B. L., et al. 2015 [52]) | ATX + PT | 1.35 mg/kg/split twice daily | 10 weeks (6 w tritation + 4 w manteinment) | Hyperactivity, inattention, impulsivity, reported by parents; inappropriate speech, home non-compliance |
| 32 | ATX | 1.38 mg/kg/split twice daily | Hyperactivity, inattention, impulsivity, reported by parents; home non-compliance | |||||
| 32 | Placebo + PT | // | Hyperactivity, inattention, impulsivity, irritability, reported by parents; inappropriate speech (by parents and teachers), school non-compliance. | |||||
| 32 | Placebo | // | None | |||||
| Smith et al., 2016 [54] | open-label extension study | 28 | inattention, hyperactivity, impulsivity; non-compliance (parents), irritability, social withdrawal, stereotypy, inappropriate speech | SNAP IV and ABC (parent rater), CGI-ADHD, HSQ | ATX | 1.16 mg/kg | 24 weeks | Hyperactivity, inattention, impulsivity, home non-compliance |
| 15 | Placebo | // | // | |||||
| 19 | ATX + PT | 1.16 mg/kg | Hyperactivity, inattention, impulsivity, home non-compliance | |||||
| 22 | Placebo + PT | // | no significant difference | |||||
| Kilincaslan et al., 2016 [55] | naturalistic retrospective study | 37 | inattention, hyperactivity, impulsivity, irritability, social withdrawal, stereotyped behaviors, inappropriate speech; | ADHD-RS, ABC, CGI, CARS | ATX | 1.20 ± 0.11 mg/kg/day | 12 weeks | Hyperactivity, inattention, impulsivity, social withdrawal |
| Scahill et al. 2007 [56]; McCracken et al. 2010 [57] | Prospective Open-label Trial | 25 | inattention, hyperactivity, irritability, social withdrawal, stereotypy, inappropriate speech | ABC, SNAPIV (both parents and teachers), CGI-I (Scahill, L et al. 2006) [56] | Guanfacine | 3–5 mg/day | 8-weeks | Hyperactivity, Inattention (parents and teachers raters), Irritability, Social withdrawal, Stereotypy (parents raters) |
| Scalhill, et al. 2015 [58]; Politte et al. 2018 [59] | randomized, placebo-controlled trial | 30 | inattention, hyperactivity, irritability, social withdrawal, stereotypy, inappropriate speech, working memory, motor planning (Scailhill, L. et al. 2015 [58]); oppositional behavior, non-compliance, anxiety, repetitive behavior, and sleep disturbance (Politte, L. et al. 2018 [59]) | ABC, ADHD-RS, CGI-I, RPDR, NEPSY II (Scailhill, L. et al. 2015 [58]), HSQM, CASI, CYBOCS-ASD, CSHQ (Politte, L. et al. 2018 [59]) | ER Guanfacine | 3–4 mg/day | 8 weeks | Hyperactivity, inattention, stereotypy, inappropriate speech (Scailhill, L. et al. 2015 [58]); hyperactivity, oppositional and repetitive behavior (Politte, L. et al. 2018 [59]) |
| 32 | Placebo | // | // | |||||
| Jaselskis et al., 1992 [60] | double blind placebo controlled crossover study | 8 | ADHD Index, inattention, hyperactivity, impulsivity, irritability, social withdrawal, stereotypy, inappropriate speech, oppositivity, non-compliance | CPRS-R, CTRS-R, ACTeRS, ABC (parent and teachers raters), HSS, CGI-I | Placebo | // | 6 weeks | // |
| Clonidine | 0.15–0.20 mg/day | ADHD Index (parents raters), hyperactivity, irritability, stereotypy, inappropriate speech, oppositional behaviours (teachers raters) | ||||||
| Lamberti et al., 2016 [61] | Pilot, Open-Label, Randomized Controlled Study | 22 | general functioning, inattention, hyperactivity, impulsivity, oppositional, ADHD Index, severity of illness | C-GAS, CGI-S, ADHD R-S, CPRS-R:S | Risperidone | 3 mg/day | 12 weeks | ADHD-RS index, hyperactivity, illness severity |
| 24 weeks | ADHD-RS index, hyperactivity, illness severity | |||||||
| 22 | Aripiprazole | 15 mg/day | 12 weeks | ADHD-RS index, hyperactivity, inattention, illness severity, increased global functioning | ||||
| 24 weeks | ADHD-RS index, hyperactivity, inattention, illness severity, increased global functioning |
| Reference | Pharmacological Agent | Mean or Range Dose | Safety Measures | Side Effects/Adverse Event (Mostly Reported) | Withdrawal Due to AEs |
|---|---|---|---|---|---|
| RUPP., 2005 [35]; Posey et al., 2006 [36]; Scahill et al., 2017 [56] | Placebo | // | survey on side effects filled by parents; physical examination (blood pressure, pulse, body weight, height, temperature) | bradycardia, diarrhea | n. 6 subjects showed intolerable adverse effects with >1 dosage level and dropped out of the test-dose phase. N. 7 exited during the cross-over phases. The highest dose was considered missing for 16 subjects who showed intolerable adverse effects. |
| MPH IR | ~0.125 mg/kg ×2/day + half dose at 4 P.M. | Sleep difficulties, irritability, emotional outbursts | |||
| ~0.25 mg/kg ×2/day + half dose at 4 P.M. | decreased appetite, sleep difficulties, irritability, and emotional outbursts. | ||||
| ~0.5 mg/kg ×2/day + half dose at 4 P.M. | decreased appetite, sleep difficulties, stomach or abdominal discomfort, irritability, and emotional outbursts. | ||||
| RUPP., 2005 [35] | MPH IR | adjusted on clinical judgment | survey on side effects filled by parents; physical examination (blood pressure, pulse, body weight, height, temperature) | not specified | n. 3 for adverse effects, lack of efficacy, or declined participation (not specified) |
| Santosh et al., 2006 [38] | MPH | 30 mg/day in 3 divided doses | // | low mood, sleep difficulties, depressive ideas | // |
| 30 mg/day in 3 divided doses | low mood, sleep difficulties, appetite problems, dysphoria, obsessional behavior | ||||
| MPH | mean dose 31.7 mg/day | NR | nausea, giddiness, headache, sleep difficulties | // | |
| mean dose 33.8 mg/day | sleep difficulties | ||||
| Simonoff et al., 2012 [39] | Placebo | // | Parental interview: sleep difficulties, loss of appetite, sadness, crying spells, anxiety, repetitive behavior, social withdrawal, daydreams, irritable, stomach ache, headache, drowsy, excitability, anger, nightmares, tics, tremors of hands. Medical evaluation: weight, pulse, blood pressure | no significant differences in AE between the EG and CG. The most reported AEs: Sleep difficulties, decreased appetite, weight change | n. 5 participants due to irritability, poor appetite and aggression, nausea and diarrhea, blurred vision, urticaria, and irritability combined with social withdrawal. n. 5 withdrew consent after being randomly assigned to their groups. |
| MPH i.r. | 0.5 mg/kg ×3/day | ||||
| 1.0 mg/kg ×3/day | |||||
| 1.5 mg/kg ×3/day | |||||
| Pearson et al., 2013; 2020 [40,41] | Placebo | // | Physician desk reference survey on side effects filled by parents | // | // |
| MPH (ER morning + IR afternoon doses) | LA 0.21 mg/kg + IR 0.14 mg/kg | // | n. 5 patients discontinued Afternoon IR-MPH for the presence of: irritability (n. 5/5), decreased sleep (n. 2/5), increased stereotyped behaviors (n.2/5) | ||
| 0.35 mg/kg + 0.24 mg/kg | // | ||||
| 0.48 mg/kg + 0.27 mg/kg | Decreased appetite, sleep difficulties (Pearson D.A. et al., 2013 [40]); | ||||
| Golubchik et al., 2017 [42] | MPH | 10–54 mg/day | spontaneous self-reports of adverse effects | minor side effects (not specified) | // |
| Kim et al., 2017 [43] | MPH ER | 0.29 mg/kg | HALP Sleep Questionnaire, RISK-K, C-SSRS | Rebound at the end of the day, aggression, and irritability were significantly more reported for medium dose. Other reported sleep difficulties and decreased appetite. | n. 1 withdrawal at week 4 due to time commitment issues |
| 0.5 mg/kg | |||||
| Peled et al., 2019 [44] | MPH IR | 10 mg i.r. | // | N.R. | // |
| Ventura P. et al., 2022 [45] | MPH ER | 0.8 mg/kg | // | Decreased appetite, abdominal discomfort, headache, palpitation, irritability, anxiety, insomnia, hyperfocusing | n. 2 ASD patients interrupted treatment for restlessness, increased stereotyped behaviours. |
| 0.78 mg/kg | |||||
| Arnold et al., 2006 [46] | Placebo | // | physical examination, weekly vital signs, weight, EKG, spontaneously reported Aes. Clinician-rated 16-item side effects scale (based on side effects abstracted from the package insert) | no significant differences in AE between the EG and CG. Most frequently reported Heart rate, Upset stomach, Nausea/vomiting, Tiredness/fatigue, Racing heart | // |
| ATX | 1.4 mg/kg/day in split dose (maximum daily dose) | ||||
| Troost et al., 2006 [47] | ATX | 0.5–1.8 mg/kg/day (divided into two doses based on patient-preference) | weekly open-ended questioning, vital signs, physical assessment, routine laboratory tests, EKG. | Decreased appetite, irritability, sleep problems, mean heart rate increased | n. 3 gastrointestinal compliance, n. 2 anxiety and increased aggressivity |
| Posey et al., 2006 [36] | ATX | 1.2–1.4 mg/kg/day | open-ended question | sedation, irritability, decreased appetite, decrease/increase thirst | n. 1 swallow pills, n.2 for irritability |
| Harfeterkamp et al., 2012; 2014 [48,49] | ATX | 1.2 mg/kg/day | open-ended questioning | nausea, decreased appetite, sleep difficulties | N. 1 adverse event (fatigue), n. 2 protocol violation, n. 1 no efficacy, n. 1 parents decision |
| Placebo | // | // | n. 2 protocol violation, n.1 physician decision | ||
| Harfeterkamp et al., 2013 [50] | ATX | 0.8–1.2 mg/kg/day, based on tolerability | open-ended questioning | abdominal pain, decreased appetite, fatigue, headache, nausea | n. 11 adverse events, n. 4 lack of efficacy |
| Fernandez-Jaen et al., 2013 [51] | ATX | 39.79 mg/day (±12.46) | // | sleep difficulties, gastrointestinal symptoms, irritability | n. 3 for adverse events and no efficacy, N. 2 for no efficacy. |
| Handen et al. 2015 [52]; Tumuluru et al., 2017 [53] | ATX + PT | 1.35 mg/kg/split twice daily | Side effects checklist (filled by parents), Side effects review (parents and subjects), blood pressure, pulse, height, and weight, EKG, blood work (Tumuluru, E. V., et al., 2017 [53]) | The only significant difference between ATX and placebo was the higher rate of decreased appetite in ATX. Most frequently reported: mood swing, restlessness, upset stomach, decreased appetite, constipation, sleep problems | n. 5 patients discontinued due to behavioral difficulties or irritability |
| ATX | 1.38 mg/kg/split twice daily | ||||
| Placebo + PT | // | n. 10 on placebo withdrew due to increased behavioral difficulties and physical side effects (e.g., GI issues and insomnia). | |||
| Placebo | // | ||||
| Smith et al., 2016 [54] | ATX | 1.16 mg/kg | this 24 week extension study reported maintenance of responder status in pregresso ATX responders; improvement in adhd symptoms reported in no-placebo responders; no any improvement from acute trial were retrived | decreased appetite, nausea, vomiting, constipation, headache, mood swing, sleep difficulties, fatigue | n. 4 behavioral deterioration, n. 8 side-effects, n. 2 parent request |
| Placebo | // | // | |||
| ATX + PT | 1.16 mg/kg | decreased appetite, nausea, vomiting, constipation, headache, mood swing, sleep difficulties, fatigue | |||
| Placebo + PT | // | // | |||
| Kilincaslan et al. 2016 [55] | ATX | 1.20 ± 0.11 mg/kg/day | BSERS | uninterested in others, drowsiness, dizziness, increased blood pressure | n.5 adverse events (increased motor activity, talkativeness, irritability, temper outbursts, increased blood pressure) |
| Scahill et al., 2006 [56]; McCracken et al., 2010 [57] | Guanfacine | 3–5 mg/day | vital signs, height and weight, systematic review of adverse events (Scahill, L et al. 2006 [56]) | Sedation, Irritability, sleep difficulties | NR |
| Scalhill et al., 2015 [58]; Politte, et al. 2018 [59] | ER Guanfacine | 3–4 mg/day | Blood pressure, pulse, height, weight, 34-item question on AE | Drowsiness, fatigue, decreased appetite, dry mouth, emotional/tearful, irritability, anxiety, sleep difficulties | N.2 subjects due to AEs, N.2 due to lack efficacy |
| Placebo | // | headache, excessive talking, increased energy | N. 4 subjects due to lack of efficacy | ||
| Jaselskis et al., 1992 [60] | Placebo | // | // | // | // |
| Clonidine | 0.15–0.20 mg/day | blood pressure, symptom checklist | hypotension, irritability, drowsiness, decreased activity, sleep difficulties | ||
| Lamberti et al., 2016 [61] | Risperidone | 3 mg/day | ECG, blood pressure, pulse, body weight, height, body mass index, abdominal circumference, laboratory test (fasting blood glucose, insulin and lipid levels, prolactin, other general blood tests). AIMS | n.11 increased appetite, n.8 weight gain, n.4 drowsiness; n.3 higher plasma prolactin level | n.1 lack of compliance, n.1 decline to return, n.1 restlessness with akatisia |
| Aripiprazole | 15 mg/day | n.5 increased appetite, n.4 weight gain, n.4 drowsiness | n.1 lack of compliance, n. 2 decline to return, n. 1 restlessness with sleep difficulties |
| Reference | Study Design | Sample Size | Ethnicity % | Sex Ratio M:F | Age Range in Years (Mean ± SD) | Mean IQ (SD) | Investigated Domains | Outcome Measures | Treatment | N. Sessions (Duration; Frequency) | Therapeutic Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chan et al., 2023 [66] | RCT | 95 (26 ASD; 24 ASD + ADHD; 22 ASD control; 23 non-autistic) | 100% Chinese | ASD 12:1 ASD + ADHD 24:0 ASD: 4.5:1 Non autism 10.5:1 | 4–6 (5.11 ± 0.87) | 100–105 (11.71) | Emotion recognition | EVT; ERT1/2/3 | The Transporters—App-based emotion recognition training: daily viewing of animated episodes (15 emotions) using vehicle characters with human faces in social story contexts | 28 days (15–20 min/day; daily for 4 weeks) | Significant improvement in emotion recognition in both ASD and ASD + ADHD groups vs. control; Learning was generalizable to novel situations. |
| Chu et al., 2023 [67] | RCT | 78 (53 ASD+ ADHD) | 100% Chinese | 3.6:1 | 3–6 (5.02 ± 0.52) | IQ > 70 | Autism symptoms, inattention, hyperactivity, impulsivity, response inhibition | ABC, CARS, ADHD-RS-IV, Go/No-Go task (accuracy and reaction time) | VR-CBT: Virtual reality-based cognitive-behavioral therapy with interactive games projected on the floor to train attention, inhibition and social skills; LSP: Structured therapist-led program that adapts learning strategies to individual profiles. | 40 sessions (2×/week; 20 min VR-CBT + 1 h LSP per session for 20 weeks) | In the ASD + ADHD subgroup, significant reduction in hyperactivity-impulsivity severity and in total scores of ADHD-RS-IV. No significant change in inattention symptoms. |
| Patel et al., 2007 [68] | open-label observational | 10 | NR | 9:1 | 4–10 (5.9) | NR | motor, social, behavioral, and educational skills, symptoms of autism and ADHD, Urinary metals, and metabolic analysis | motor, behavioral, and educational skills evaluated through an ad hoc questionnaire rated by parents, teachers, and the physician; blood laboratory tests, urinary heavy metals, intradermal testing, organic acid analysis | Multidimensional treatment protocol: (I) environmental control and avoidance of triggers; (II) an organic diet; (III) gastrointestinal support; (IV) antigen injection therapy; (V) nutritional supplements; (VI) chelation therapy; (VI) glutathione and methylcobalamin integration; (VII) usual therapies. | 3–6 months | Parents reported improvement in social interaction, concentration, writing, language, and behaviors. Urinary lead burden decreased significantly |
| Yerys et al., 2018 [69] | RCT | 11 | 9.1% Black 9.1% Multir.72.7% White 9.1% NR | 11:0 | 9–13 (11.25 ± 1.12) | 98.36 (13.11) | sustained attention, inattention, hyperactivity, impulsivity, Behavior Regulation, Emotion Regulation, Cognitive Regulation, psychomotor speed and accuracy, retaining and updating visuospatial information, social skills, problem behaviors | TOVA, CANTAB, ADHD Rating Scale-IV, BRIEF-2, SSIS. | Project Evo—multi-tasking gameplay: a perceptual discrimination attention/memory task and a continuous visuomotor driving-type task on a tablet. | 20 sessions (25 min; five times a week for 4 weeks) | Significant improvement in ADHD-RS-index, Behavior Regulation, Emotion Regulation, Cognitive Regulation, social skills, and Problem Behaviors. |
| 8 | 12.5% Multir.87.5% White | 6:2 | 9–13 (11.26 ± 1.88) | 111.12 (16.99) | educational-based intervention: a task that requires children to generate words from an array of letters on a tablet | // |
| Publication | Exposed Representation | Ascertainment of Exposure | Selection of the Non-Exposed | Outcome Was Not Present at Start of Study | Comparability of Cohorts | Assessment of Outcome | Sufficient Follow-Up Time | Adequacy of Follow-Up of Cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|
| Posey et al., 2006 [36] (A) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 3 |
| Santosh et al., 2006 [38] (M) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 5 |
| Scahill et al., 2006 [56] (G) | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 3 |
| Troost et al., 2006 [47] (A) | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 4 |
| Patel et al., 2007 [68] (NP) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 4 |
| McCracken et al., 2010 [57] (G) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Fernández-Jaén et al., 2013 [51] (A) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Kilincaslan et al., 2016 [55] (A) | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 4 |
| Lamberti et al.,2016 [61] (R) | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 |
| Golubchik et al.,2017 [42] (M) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 3 |
| Yerys et al., 2018 [69] (NP) | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 3 |
| Peled et al., 2019 [44] (M) | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 4 |
| Ventura et al., 2022 [45] (M) | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 3 |
| Publication | Random Sequence Generation | Allocation Concealment | Selective Reporting | Other Bias | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | AHRQ Standard |
|---|---|---|---|---|---|---|---|---|
| Jaselskis et al.,1992 [60] (C) | Low | Low | Unclear | Unclear | Low | Low | Unclear | Poor |
| Arnold et al., 2006 [46] (A) | High | High | Unclear | Unclear | Low | Unclear | Low | Poor |
| Handen et al., 2006 [52] (A) | Low | Low | Unclear | Unclear | Low | Low | Unclear | Fair |
| Posey et al., 2007 [36] (M) | High | High | Unclear | Unclear | Low | Low | Low | Poor |
| Harfterkamp et al., 2012 [48] (A) | Low | Low | Low | Unclear | Low | Low | Unclear | Fair |
| Simonoff et al.,2012 [39] (M) | Low | Low | Low | Low | Low | Low | Low | Good |
| Harfterkamp et al., 2013 [50] (A) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | Poor |
| Pearson et al., 2013 [40] (M) | Unclear | Low | Unclear | Low | Low | Low | Low | Fair |
| Harfterkamp et al., 2014 [49] (A) | Low | Low | Low | Unclear | Low | Unclear | Low | Fair |
| Scahill et al., 2015 [58] (G) | Low | Low | Low | Low | Low | Low | Low | Good |
| Kim et al., 2017 [43] (M) | Unclear | Low | Unclear | Unclear | Low | Unclear | Low | Poor |
| Scahill et al., 2017 [37] (M) | Unclear | Unclear | Unclear | Unclear | Low | Low | Low | Poor |
| Smith et al., 2017 [54] (A) | Unclear | Unclear | Unclear | Unclear | Low | Unclear | Low | Poor |
| Tumuluru et al., 2017 [53] (A) | High | High | Unclear | Unclear | Low | Unclear | Unclear | Poor |
| Politte et al., 2018 [59] (G) | Low | Unclear | Low | Unclear | Low | Unclear | Unclear | Poor |
| Pearson et al., 2020 [41] (M) | Unclear | Low | Unclear | Unclear | Low | Low | Low | Poor |
| Chu et al.,2023 [67] (NP) | Low | Low | Unclear | Unclear | High | High | Low | Poor |
| Chan et al.2024 [66] (NP) | Unclear | Low | Unclear | Unclear | High | High | Low | Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Domenico, C.; Alito, A.; Leonardi, G.; Pironti, E.; Di Cara, M.; Piccolo, A.; Settimo, C.; Quartarone, A.; Gagliano, A.; Cucinotta, F. Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions. J. Clin. Med. 2025, 14, 4000. https://doi.org/10.3390/jcm14114000
De Domenico C, Alito A, Leonardi G, Pironti E, Di Cara M, Piccolo A, Settimo C, Quartarone A, Gagliano A, Cucinotta F. Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions. Journal of Clinical Medicine. 2025; 14(11):4000. https://doi.org/10.3390/jcm14114000
Chicago/Turabian StyleDe Domenico, Carmela, Angelo Alito, Giulia Leonardi, Erica Pironti, Marcella Di Cara, Adriana Piccolo, Carmela Settimo, Angelo Quartarone, Antonella Gagliano, and Francesca Cucinotta. 2025. "Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions" Journal of Clinical Medicine 14, no. 11: 4000. https://doi.org/10.3390/jcm14114000
APA StyleDe Domenico, C., Alito, A., Leonardi, G., Pironti, E., Di Cara, M., Piccolo, A., Settimo, C., Quartarone, A., Gagliano, A., & Cucinotta, F. (2025). Children and Adolescents with Co-Occurring Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder: A Systematic Review of Multimodal Interventions. Journal of Clinical Medicine, 14(11), 4000. https://doi.org/10.3390/jcm14114000






