Long-Term Adverse Effects and Survival in Patients with Myasthenia Gravis Treated with Azathioprine: A Retrospective Cohort
Abstract
1. Introduction
2. Methods
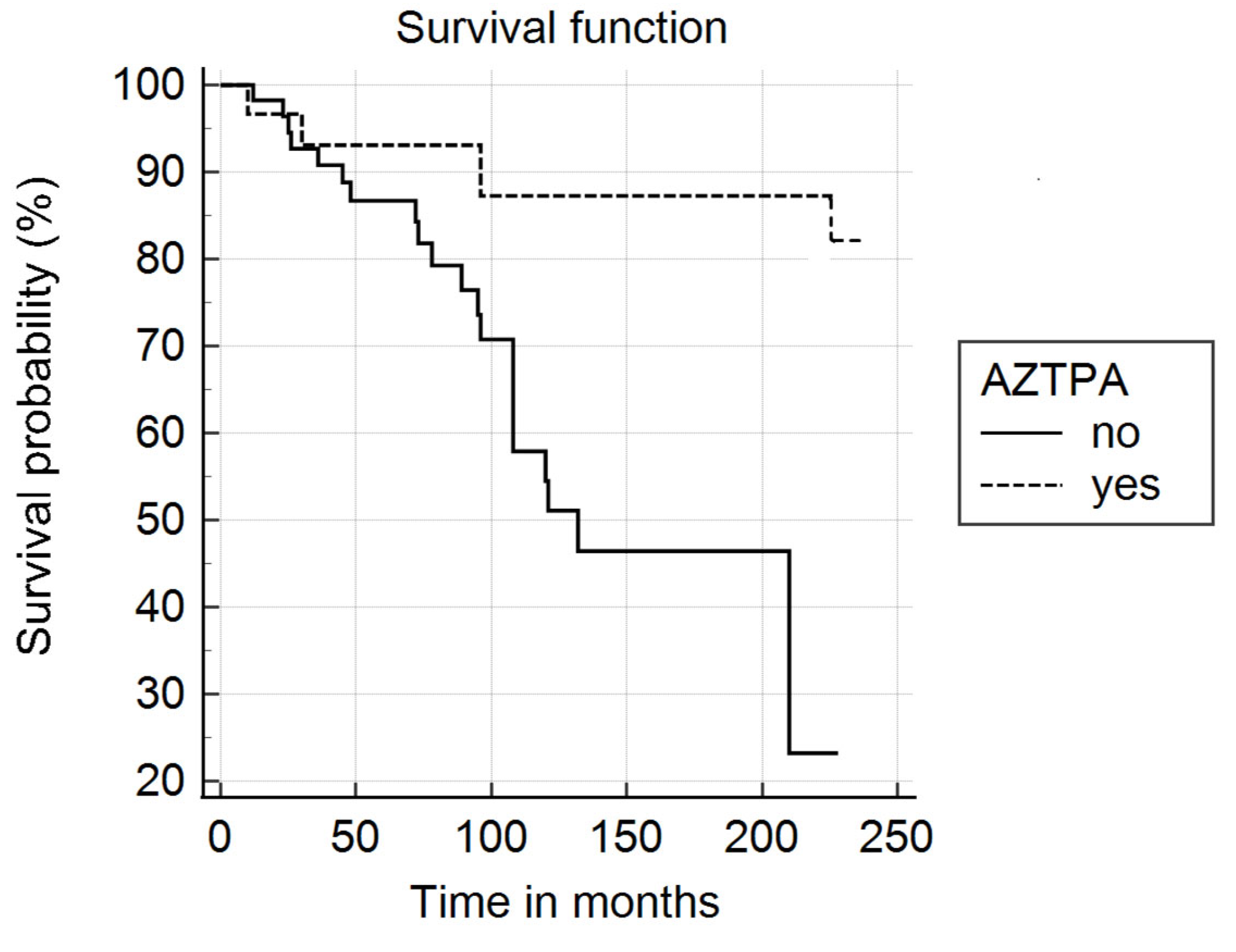
3. Results
Vaccination Status Against COVID-19 and Seasonal Flu Data
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, J.F. Myasthenia gravis: The role of complement at the neuromuscular junction. Ann. N. Y. Acad. Sci. 2018, 141, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Manu, P.; Rogozea, L.M.; Roman-Filip, C. Pharmacological management of myasthenia gravis: A century of expert opinions in Cecil textbook of Medicine. Am. J. Ther. 2021, 28, e631–e637. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Evoli, A. Immunosuppressive therapies in Myasthenia gravis. Autoimmunity 2010, 43, 428–435. [Google Scholar] [CrossRef]
- Prado, M.B.; Adiao, K.J.B. Methotrexate in generalized myasthenia gravis: A systematic review. Acta Neurol. Belg. 2023, 123, 1679–1691. [Google Scholar] [CrossRef]
- Bi, Z.; Cao, Y.; Liu, C.; Gui, M.; Lin, J.; Zhang, Q.; Li, Y.; Ji, S.; Bu, B. Remission and relapses of myasthenia gravis on long-term tacrolimus: A retrospective cross-sectional study of a Chinese cohort. Ther. Adv. Chronic Dis. 2022, 13, 20406223221122538. [Google Scholar] [CrossRef]
- Mantegazza, R.; Antocci, C.; Peluchetti, D.; Sghirlanzoni, A.; Cornelio, F. Azathioprine as a single drug or in combination with steroids in the treatment of myasthenia gravis. J. Neurol. 1988, 235, 449–453. [Google Scholar] [CrossRef]
- Palace, J.; Newsom-Davies, J.; Lecky, B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia gravis study group. Neurology 1998, 50, 1778–1783. [Google Scholar] [CrossRef]
- Myasthenia Gravis Clinical Study Group. A randomized clinical trial comparing prednisone and azathioprine in myasthenia gravis. Results of the second interim analysis. J. Neurol. Neurosurg. Psychiatry 1992, 56, 1157–1163. [Google Scholar] [CrossRef]
- Bromberg, M.B.; Wald, J.J.; Forshew, D.A.; Feldman, E.L.; Albers, J.W. Randomized trial of azathioprine or prednisone for initial immunosuppressive treatment of myasthenia gravis. J. Neurol. Sci. 1997, 150, 59–62. [Google Scholar] [CrossRef]
- Rozsa, C.; Mikor, A.; Kasa, K.; Illes, Z.; Komoly, S. Long-term effects of immunosuppressive treatment on myasthenia gravis. Eur. J. Neurol. 2009, 16, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Rózsa, C.; Mikor, A.; Kasa, K.; Illes, Z.; Komoly, S. Mysthenia gravis treatment in the elderly presents with a significant iatrogenic risk: A multicentric retrospective study. J. Neurol. 2023, 270, 5819–5826. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Xu, L.; Jiang, B.; Jin, T.; Shi, T.; Xu, B. Cancer occurrence following azathioprine treatment in myasthenia gravis patients. A systematic review and meta-analysis. J. Clin. Neurosci. 2021, 88, 70–74. [Google Scholar] [CrossRef]
- Pedersen, E.G.; Pottegård, A.; Hallas, J.; Friis, S.; Hansen, K.; Jensen, P.E.H.; Gaist, D. Risk of non-melanoma skin cancer in myasthenia gravis treated with azathioprine. Eur. J. Neurol. 2014, 21, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Salort-Campana, E.; Laforet, P.; de Pouvourville, G.; Crochard, A.; Chollet, G.; Nevoret, C.; Emery, C.; Bouée, S.; Tard, C. Epidemiology of myasthenia gravis in France. A retrospective claim database study (STAMINA). Rev. Neurol. 2024, 180, 202–210. [Google Scholar] [CrossRef]
- Wolfe, G.I.; Herbelin, L.; Nations, S.P.; Foster, B.; Bryan, W.W.; Barohn, R.J. Myasthenia gravis activities of daily living profile. Neurology 1999, 52, 1487–1489. [Google Scholar] [CrossRef]
- Crisafulli, S.; Boccanegra, B.; Carollo, M.; Bottani, E.; Mantuano, P.; Trifirò, G.; De Luca, A. Myasthenia gravis treatment: From old drugs to innovative therapies with a glimpse into the future. CNS Drugs 2024, 38, 15–32. [Google Scholar] [CrossRef]
- Westerberg, E.; Punga, A.R. Mortality rates and causes of death in Swedish myasthenia gravis patients. Neuromuscul. Disord. 2020, 10, 815–824. [Google Scholar] [CrossRef]
- Basta, I.; PekmezoviĆ, T.; Peric, S.; NikoliĆ, A.; RakoČeviĆ-StojanoviĆ, V.; SteviĆ, Z.; LavrniĆ, D. Survival and mortality of adult-onset myasthenia gravis in the population of Belgrade, Serbia. Muscle Nerve 2018, 58, 708–712. [Google Scholar] [CrossRef]
- Hansen, J.S.; Danielsen, D.H.; Somnier, F.E.; Frøslev, T.; Jakobsen, J.; Johnsen, S.P.; Andersen, H. Mortality in myasthenia gravis. A nation-wide population-based follow-up study in Denmark. Muscle Nerve 2016, 53, 73–77. [Google Scholar] [CrossRef]
- Salahat, A.A.; Abdul Jabbar, A.B.; Sharma, R.; Ting Chen, Y.; Bernitsas, E. Demographic and geographic trends in myasthenia gravis-ralated mortality in the United States, 1999–2022. Neurology 2025, 104, e213505. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Michels, M.; Heininger, K.; Besinger, U.; Toyka, K.V. Azathioprine toxicity during long-term of immunosupression of generalized myasthenia gravis. Neurology 1988, 38, 258. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Goyal, V.; Srivastava, A.K.; Shukla, G.; Behri, M. Remission and relapse of myasthenia gravis on long-term azathioprine: An ambispective study. Muscle Nerve 2016, 54, 405–412. [Google Scholar] [CrossRef] [PubMed]
- McGurgan, I.J.; McGuigan, C. Non-melanoma skin cancer risk awareness in azathioprine-treated myasthenia gravis patients. Brain Behav. 2015, 5, e00396. [Google Scholar] [CrossRef]
- Kassardjian, C.D.; Widdifield, J.; Paterson, J.M.; Kopp, A.; Nagamuthu, C.; Barnett, C.; Tu, K.; Breiner, A. Serious infections in patients with myasthenia gravis: Population-based cohort study. Eur. J. Neurol. 2020, 27, 702–708. [Google Scholar] [CrossRef]
- Yaman, A.; Aydin, F.K. Therapeutic and prognostic features in myasthenia gravis patients followed in a tertiary neuromuscular diseases center in Turkey. Front. Neurol. 2023, 14, 1176636. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, B.; Yang, H.; Wang, L.; Liu, W.; Duan, R.-S.; Zhang, M.; Zeng, P.; Du, C.; Yang, L.; et al. Immunotherapy choice and maintenance for generalized myasthenagravis in China. CNS Neurosci. Ther. 2020, 26, 1241–1254. [Google Scholar] [CrossRef]
- Fonseca, V.; Havard, C.W. Long termtreatment of myasthenia gravis with azathioprine. Postgrad. Med. J. 1990, 66, 102–105. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Thomas, L.; Thibault, D.; Blevins, J.; Desai, R.; Krueger, A.; Bibeau, K.; Liu, B.; Guptill, J.T.; et al. Comparative effectiveness of azathioprine and mycophenolate-mofetil for myasthenia gravis (PROMISE-MG): A prospective cohort study. Lancet Neurol. 2024, 23, 267–276. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Andersen, H.; Andersen, L.K.; Boldingh, M.; Laakso, S.; Leopoldsdottir, M.O.; Madsen, S.; Piehl, F.; Popperud, T.H.; Punga, A.R.; et al. Generalized myasthenia gravis with acetylcholine receptor antibodies. A guidance for treatment. Eur. J. Neurol. 2024, 31, e16229. [Google Scholar] [CrossRef]
- Melzer, N.; Ruck, T.; Fuhr, P.; Gold, R.; Hohlfeld, R.; Marx, A.; Melms, A.; Tackenberg, B.; Schalke, B.; Schneider-Gold, C.; et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: A supplement to the guidelines of the German neurological society. J. Neurol. 2016, 273, 1473–1494. [Google Scholar] [CrossRef]
| Number of patients: 90 |
| Mean age at baseline: 74.2 y; SD = 13.4 y; range: 32–95 y |
| Median age: 72 years |
| Sex: 48 women, 42 men |
| Clinical form: Ocular in 20 patients, generalized in 70 |
| Thymoma found in three patients; two operated on |
| Thymectomy without thymoma found in one case |
| Patients on azathioprine: 31 |
| Mean follow-up for the cohort: 103.87 months (8.6 years); SD: 51; range: 18–240 months |
| Median follow-up: 96 months |
| Mean follow-up for patients on AZTP: 105.4 months; SD: 48.7; range: 18–240 months |
| Median follow-up: 94 months |
| Grade I. Any ocular muscle weakness; may have weakness of eye closure. All other muscle strength is normal: 20 patients |
| Grade IIa. Predominantly affecting limb, axial muscles, or both. May also have lesser involvement of oropharyngeal muscles: 22 patients |
| Grade IIb. Predominantly affecting oropharyngeal or respiratory muscles, or both. May also have lesser or equal involvement of limb or axial muscles, or both: 19 patients |
| Grade IIIa. Predominantly affecting limb or axial muscles, or both. May also have lesser involvement of oropharyngeal muscles: 8 patients |
| Grade IIIb. Predominantly affecting oropharyngeal or respiratory muscles, or both. May also have lesser or equal involvement of limb or axial muscles, or both: 6 patients |
| Grade IVa. Predominantly affecting limb or axial muscles, or both. May also have lesser involvement of oropharyngeal muscles: 6 patients |
| Grade IVb. Predominantly affecting oropharyngeal or respiratory muscles, or both. May also have lesser or equal involvement of limb or axial muscles, or both: 8 patients |
| Grade V. Defined as intubation, with or without mechanical ventilation, except when employed during routine postoperative management. The use of a feeding tube without intubation places the patient in class IVb: 1 patient |
| Predictor | Hazard Ratio | 95% CI | Significance |
|---|---|---|---|
| Azathioprine | 0.3897 | 0.10–11.43 | 0.156 |
| Age | 1.126 | 1.06–1.191 | <0.0001 |
| Clinical form | 1.259 | 1.07–1.56 | 0.033 |
| Sex | 0.54 | 0.23–1.26 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrego, P.J. Long-Term Adverse Effects and Survival in Patients with Myasthenia Gravis Treated with Azathioprine: A Retrospective Cohort. J. Clin. Med. 2025, 14, 3945. https://doi.org/10.3390/jcm14113945
Modrego PJ. Long-Term Adverse Effects and Survival in Patients with Myasthenia Gravis Treated with Azathioprine: A Retrospective Cohort. Journal of Clinical Medicine. 2025; 14(11):3945. https://doi.org/10.3390/jcm14113945
Chicago/Turabian StyleModrego, Pedro J. 2025. "Long-Term Adverse Effects and Survival in Patients with Myasthenia Gravis Treated with Azathioprine: A Retrospective Cohort" Journal of Clinical Medicine 14, no. 11: 3945. https://doi.org/10.3390/jcm14113945
APA StyleModrego, P. J. (2025). Long-Term Adverse Effects and Survival in Patients with Myasthenia Gravis Treated with Azathioprine: A Retrospective Cohort. Journal of Clinical Medicine, 14(11), 3945. https://doi.org/10.3390/jcm14113945





