Recruitment Challenges in Spinal Cord Stimulation Trial for Motor Recovery in Patients with Chronic Complete Spinal Cord Injury
Abstract
1. Introduction
2. Methods
2.1. Addressing the Limited Patient Population and Strict Eligibility Criteria
2.2. Mitigating Patient Skepticism and Fear of Invasive Procedures
2.3. Reducing Potential Medical Complications
2.4. Overcoming Geographical and Logistical Barrier
2.5. Addressing Emotional and Psychological Barrier
2.6. Study Dedicated Clinical Research Coordinator (CRC)
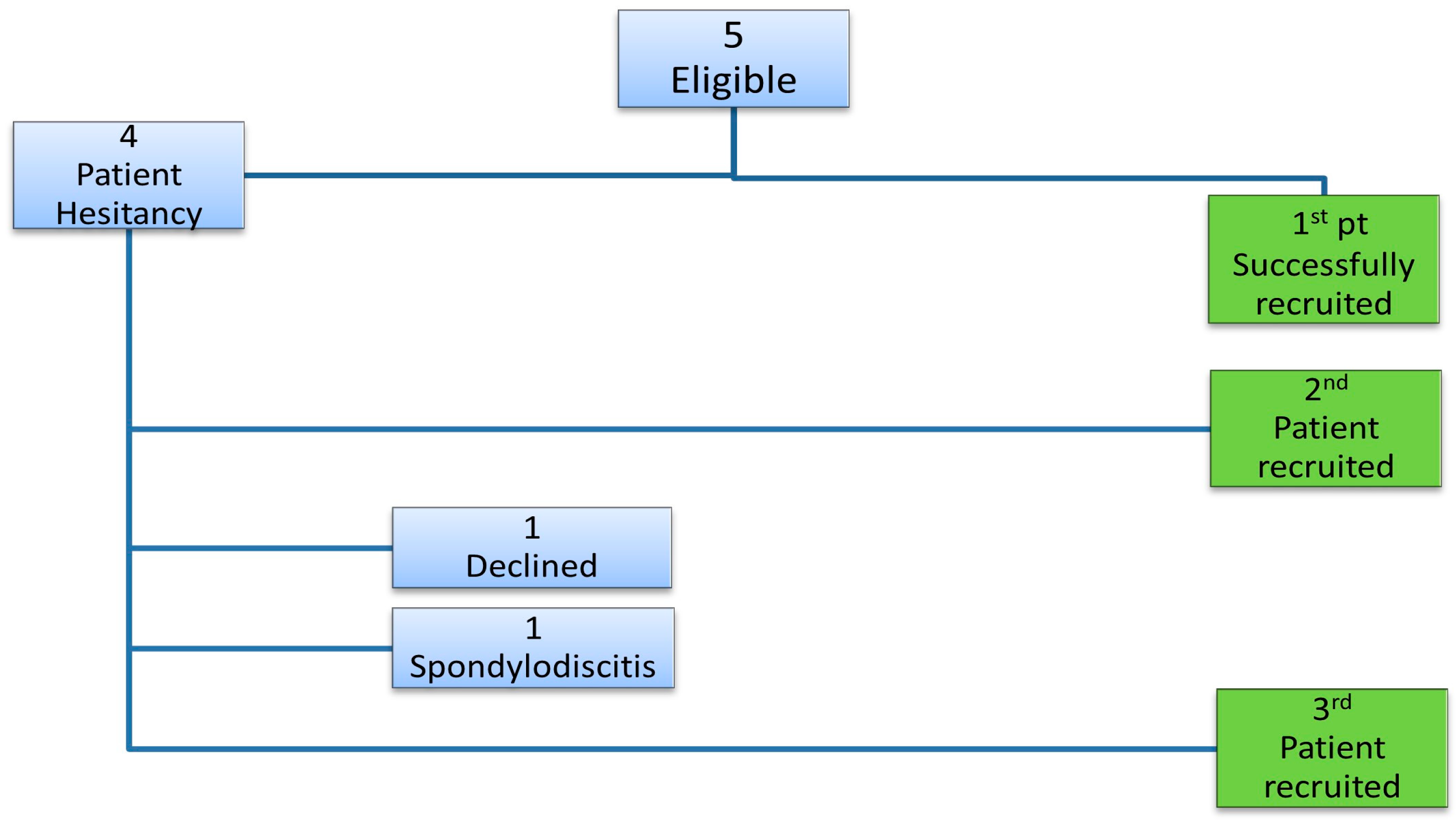
3. Results
4. Discussion
4.1. Recruitment Barriers and Strategies
4.2. Family Support
4.3. Ethical Considerations
- Autonomy was upheld by ensuring participants provided informed consent after a thorough understanding of the risks and benefits of this study [34,36]. Trial participants in our study underwent a comprehensive process that included counselling sessions with the PI prior to obtaining consent. This included detailed conversations about the study procedures, potential outcomes, and any associated risk. This approach ensured that patients’ decisions were based on their complete understanding of this study.
- Beneficence was reflected in the trial design, which aimed to maximize potential benefits, such as improving neurological function through innovative therapies [36]. On top of that, the team ensured that participants were emotionally healthy and had strong family support to assist them throughout the trial before recruiting them.
- Non-maleficence was prioritized by meticulously assessing risks, particularly given the invasive nature of spinal cord stimulation (SCS), and by maintaining participant safety as the trial’s foremost concern [34]. The strict eligibility criteria (Appendix A) established in our study were crucial in upholding the principle of non-maleficence, as we excluded patients suffering from severe spasticity and osteoporosis, safeguarding them against potential deterioration of their conditions.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
References
- Wan, K.R.; Ng, Z.Y.V.; Wee, S.K.; Fatimah, M.; Lui, W.; Phua, M.W.; So, Q.Y.R.; Maszczyk, T.K.; Premchand, B.; Saffari, S.E.; et al. Recovery of Volitional Motor Control and Overground Walking in Participants with Chronic Clinically Motor Complete Spinal Cord Injury: Restoration of Rehabilitative Function with Epidural Spinal Stimulation (RESTORES) Trial-A Preliminary Study. J. Neurotrauma 2024, 41, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Kaufman, S.; Alpaugh, E.S.; Lawson, J.; Apgar, T.L.; Imani, F.; Khademi, S.-H.; Cornett, E.M.; Kaye, A.D. Burst Spinal Cord Stimulation in the Management of Chronic Pain: Current Perspectives. Anesthesiol. Pain Med. 2022, 12, e126416. [Google Scholar] [CrossRef] [PubMed]
- Sammartino, F.; MacDonell, J.; North, R.B.; Krishna, V.; Poree, L. Disease applications of spinal cord stimulation: Chronic nonmalignant pain. Prog. Brain Res. 2015, 218, 63–85. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A.; Atkinson, D.; Harkema, S.J. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 2017, 7, 13476. [Google Scholar] [CrossRef] [PubMed]
- Boakye, M.; Ball, T.; Dietz, N.; Sharma, M.; Angeli, C.; Rejc, E.; Kirshblum, S.; Forrest, G.; Arnold, F.W.; Harkema, S. Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: A case series and technical note. Surg. Neurol. Int. 2023, 14, 87. [Google Scholar] [CrossRef]
- Rejc, E.; Angeli, C.A.; Ichiyama, R.M. Editorial: Advances in Spinal Cord Epidural Stimulation for Motor and Autonomic Functions Recovery After Severe Spinal Cord Injury. Front. Syst. Neurosci. 2022, 16, 820913. [Google Scholar] [CrossRef]
- Pasha, A.S.; Silbert, R. Challenges and Opportunities in Modernizing Clinical Trial Recruitment. J. Law Med. Ethics 2023, 51, 314–321. [Google Scholar] [CrossRef]
- Briel, M.; Elger, B.S.; McLennan, S.; Schandelmaier, S.; von Elm, E.; Satalkar, P. Exploring reasons for recruitment failure in clinical trials: A qualitative study with clinical trial stakeholders in Switzerland, Germany, and Canada. BMJ Open 2021, 11, e043224. [Google Scholar] [CrossRef]
- Lim, P.A.C.; Tow, A.M. Recovery and regeneration after spinal cord injury: A review and summary of recent literature. Ann. Acad. Med. Singap. 2007, 36, 49–57. [Google Scholar] [CrossRef]
- Huang, H.; Bach, J.R.; Sharma, H.S.; Chen, L.; Wu, P.; Sarnowska, A.; Otom, A.; Xue, M.; Saberi, H.; He, X. The 2023 yearbook of Neurorestoratology. J. Neurorestoratology 2024, 12, 100136. [Google Scholar] [CrossRef]
- Huang, H.; Bach, J.R.; Sharma, H.S.; Saberi, H.; Jeon, S.R.; Guo, X.; Shetty, A.; Hawamdeh, Z.; Sharma, A.; von Wild, K.; et al. The 2022 yearbook of Neurorestoratology. J. Neurorestoratology 2023, 11, 100054. [Google Scholar] [CrossRef]
- Darrow, D.P.; Balser, D.Y.; Freeman, D.; Pelrine, E.; Krassioukov, A.; Phillips, A.; Netoff, T.; Parr, A.; Samadani, U. Effect of epidural spinal cord stimulation after chronic spinal cord injury on volitional movement and cardiovascular function: Study protocol for the phase II open label controlled E-STAND trial. BMJ Open 2022, 12, e059126. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Field-Fote, E.C.; Tefertiller, C.; van Nes, I.; Trumbower, R.; Kalsi-Ryan, S.; Purcell, M.; Janssen, T.W.J.; Krassioukov, A.; Morse, L.R.; et al. Non-invasive spinal cord electrical stimulation for arm and hand function in chronic tetraplegia: A safety and efficacy trial. Nat. Med. 2024, 30, 1276–1283. Available online: https://www.nature.com/articles/s41591-024-02940-9#Abs1 (accessed on 30 May 2024). [CrossRef]
- Shams, R.; Drasites, K.P.; Zaman, V.; Matzelle, D.; Shields, D.C.; Garner, D.P.; Sole, C.J.; Haque, A.; Banik, N.L. The pathophysiology of osteoporosis after spinal cord injury. Front. Endocrinol. 2021, 12, 652467. [Google Scholar] [CrossRef]
- Adler, R.A. Spinal cord injury-induced osteoporosis: Pathogenesis and emerging therapies. Curr. Osteoporos. Rep. 2019, 17, 422–431. [Google Scholar]
- Lim, S.-W.; Shiue, Y.-L.; Ho, C.-H.; Yu, S.-C.; Kao, P.-H.; Wang, J.-J.; Kuo, J.-R. Anxiety and Depression in Patients with Traumatic Spinal Cord Injury: A Nationwide Population-Based Cohort Study. PLoS ONE 2017, 12, e0169623. [Google Scholar] [CrossRef]
- Côté, M.P.; Murray, M.; Lemay, M.A. Rehabilitation strategies after spinal cord injury: Inquiry into the mechanisms of success and failure. J. Neurotrauma 2017, 34, 1729–1745. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.; Jones, K. How important is resilience among family members supporting relatives with traumatic brain injury or spinal cord injury? Clin. Rehabil. 2012, 27, 367–377. [Google Scholar] [CrossRef]
- Darrow, D.; Balser, D.; Netoff, T.I.; Krassioukov, A.; Phillips, A.; Parr, A.; Samadani, U. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J. Neurotrauma 2019, 36, 377–385. [Google Scholar] [CrossRef]
- Soendergaard, P.L.; Wolffbrandt, M.M.; Biering-Sørensen, F.; Nordin, M.; Schow, T.; Arango-Lasprilla, J.C.; Norup, A. A manual-based family intervention for families living with the consequences of traumatic injury to the brain or spinal cord: A study protocol of a randomized controlled trial. Trials 2019, 20, 646. [Google Scholar] [CrossRef]
- Soendergaard, P.L.; Arango-Lasprilla, J.C.; Wolffbrandt, M.M.; Dornonville de la Cour, F.L.; Biering-Sørensen, F.; Norup, A. Investigating the effectiveness of a family intervention after acquired brain or spinal cord injury: A randomized controlled trial. J. Clin. Med. 2023, 12, 3214. [Google Scholar] [CrossRef] [PubMed]
- Cinefra, M.; Cagnazzo, C.; McMahon, L.; Arizio, F.; Campora, S.; Camisa, R.; Canzanella, G.; Contu, M.; Frati, P.; Sottile, R. The Critical Role of the Clinical Research Coordinator for Clinical Trials: A Survey in Oncology. Med. Access Point Care 2017, 1, e76–e81. [Google Scholar] [CrossRef]
- Gudewicz, I.; Hajtuch, J.; Zaucha, R. Center with or Without a Coordinator? The Coordinator as an Integral Part of a Research Team. Open Access J. Clin. Trials 2024, 16, 1–9. [Google Scholar] [CrossRef]
- Fehlings, M.; Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309. [Google Scholar] [CrossRef]
- World Health Organization. Spinal Cord Injury; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury (accessed on 5 September 2024).
- Ning, G.Z.; Wu, Q.; Li, Y.L.; Feng, S.Q. Epidemiology of traumatic spinal cord injury in Asia: A systematic review. J. Spinal Cord Med. 2012, 35, 229–239. [Google Scholar] [CrossRef]
- Kee, K.M.; Mohamad, N.Z.; Koh, P.P.W.; Yeo, J.P.T.; Ng, Y.S.; Kam, J.C.; Asano, M. Return to work after spinal cord injury: A Singaporean pilot community-based rehabilitation program. Spinal Cord 2020, 58, 1096–1103. [Google Scholar] [CrossRef]
- Taccola, G.; Sayenko, D.; Gad, P.; Gerasimenko, Y.; Edgerton, V.R. And yet it moves: Recovery of volitional control after spinal cord injury. Prog. Neurobiol. 2018, 160, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.C.; Aiyegbusi, O.L.; Ives, J.; Draper, H.; Mercieca-Bebber, R.; Ells, C.; Hunn, A.; Scott, J.A.; Fernandez, C.V.; Dickens, A.P.; et al. Ethical Considerations for the Inclusion of Patient-Reported Outcomes in Clinical Research: The PRO Ethics Guidelines. JAMA 2022, 327, 1910–1919. [Google Scholar] [CrossRef]
- Scheuren, P.S.; Kramer, J.L.K. Next-gen spinal cord injury clinical trials: Lessons learned and opportunities for future success. EBioMedicine 2024, 109, 105381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lynch, J.; Cahalan, R. The impact of spinal cord injury on the quality of life of primary family caregivers: A literature review. Spinal Cord 2017, 55, 964–978. [Google Scholar] [CrossRef]
- Jeyathevan, G.; Craven, B.C.; Cameron, J.I.; Jaglal, S.B. Facilitators and barriers to supporting individuals with spinal cord injury in the community: Experiences of family caregivers and care recipients. Disabil. Rehabil. 2019, 41, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, R.; Loreti, C.; Giovannini, S.; Ricciardi, W.; Padua, L.; Boccia, S. Challenges of Prevention for a Sustainable Personalized Medicine. J. Pers. Med. 2021, 11, 311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maslen, H.; Cheeran, B.; Pugh, J.; Pycroft, L.; Boccard, S.; Prangnell, S.; Green, A.L.; FitzGerald, J.; Savulescu, J.; Aziz, T. Unexpected complications of novel deep brain stimulation treatments: Ethical issues and clinical recommendations. AJOB Neurosci. 2018, 9, 222–224. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar] [PubMed]
- Young, M.J.; Bodien, Y.G.; Edlow, B.L. Ethical Considerations in Clinical Trials for Disorders of Consciousness. Brain Sci. 2022, 12, 211. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Restoration of Rehabilitative Function with Epidural Spinal Stimulation; National Library of Medicine (US): Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT05644171?term=Restoration%20of%20rehabilitative%20function%20with%20epidural%20spinal%20stimulation&rank=1#participation-criteria (accessed on 21 June 2024).
- Tan, J. “I Feel Like a One Million Dollar Man,” Says Paralysed Man Who Can Walk After Spinal Implant. The Straits Times. 2023. Available online: https://www.straitstimes.com/singapore/i-feel-like-a-one-million-dollar-man-says-paralysed-man-who-can-walk-after-spinal-implant (accessed on 21 June 2024).
- Mustaffa, H. Percubaan TTSH, Institut Neurosains, Beri Harapan Pesakit Lumpuh Kembali “Berjalan” [Internet]. Berita Harian. 2023. Available online: https://www.beritaharian.sg/singapura/percubaan-ttsh-institut-neurosains-beri-harapan-pesakit-lumpuh-kembali-berjalan (accessed on 28 May 2025).
- Liu, A. Percubaan TTSH, Institut Neurosains, beri harapan pesakit lumpuh kembali ‘berjalan’ (本地新研究让脊髓受损瘫痪者重新站立). Zaobao. 2023. Available online: https://www.zaobao.com.sg/news/singapore/story20231128-1452968 (accessed on 28 May 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misbaah, F.; Lui, W.L.; Ng, Z.Y.V.; Wee, S.K.; Phua, M.W.; So, R.Q.; Premchand, B.; Susanto, K.; Saffari, S.E.; Justin Ker, R.X.; et al. Recruitment Challenges in Spinal Cord Stimulation Trial for Motor Recovery in Patients with Chronic Complete Spinal Cord Injury. J. Clin. Med. 2025, 14, 3925. https://doi.org/10.3390/jcm14113925
Misbaah F, Lui WL, Ng ZYV, Wee SK, Phua MW, So RQ, Premchand B, Susanto K, Saffari SE, Justin Ker RX, et al. Recruitment Challenges in Spinal Cord Stimulation Trial for Motor Recovery in Patients with Chronic Complete Spinal Cord Injury. Journal of Clinical Medicine. 2025; 14(11):3925. https://doi.org/10.3390/jcm14113925
Chicago/Turabian StyleMisbaah, Fatimah, Wen Li Lui, Zhi Yan Valerie Ng, Seng Kwee Wee, Min Wee Phua, Rosa Q. So, Brian Premchand, Kezia Susanto, Seyed Ehsan Saffari, Rui Xin Justin Ker, and et al. 2025. "Recruitment Challenges in Spinal Cord Stimulation Trial for Motor Recovery in Patients with Chronic Complete Spinal Cord Injury" Journal of Clinical Medicine 14, no. 11: 3925. https://doi.org/10.3390/jcm14113925
APA StyleMisbaah, F., Lui, W. L., Ng, Z. Y. V., Wee, S. K., Phua, M. W., So, R. Q., Premchand, B., Susanto, K., Saffari, S. E., Justin Ker, R. X., Ng, W. H., & Wan, K. R. (2025). Recruitment Challenges in Spinal Cord Stimulation Trial for Motor Recovery in Patients with Chronic Complete Spinal Cord Injury. Journal of Clinical Medicine, 14(11), 3925. https://doi.org/10.3390/jcm14113925






