Efficacy and Safety of Intranasal Etripamil for Paroxysmal Supraventricular Tachycardia: Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Eligibility Criteria
- Randomized controlled trials (RCTs);
- Inclusion of patients with PSVT;
- Administration of etripamil (70 mg) via the intranasal route;
- English language publication’
- Reporting of primary clinical outcomes, including conversion to a sinus rhythm or recurrence of arrhythmic events.
- Did not include adult human participants;
- Lacked safety data on adverse events (AEs), treatment-emergent serious adverse events (TESAEs), or other prespecified outcomes (nasal discomfort, epistaxis, etc.);
- Did not have a comparator group;
- Did not report the timepoints for conversion to a normal sinus rhythm;
- Had incomplete or unpublished data, abstracts without full peer-reviewed publications, or registered protocols without results.
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study and Patient Characteristics
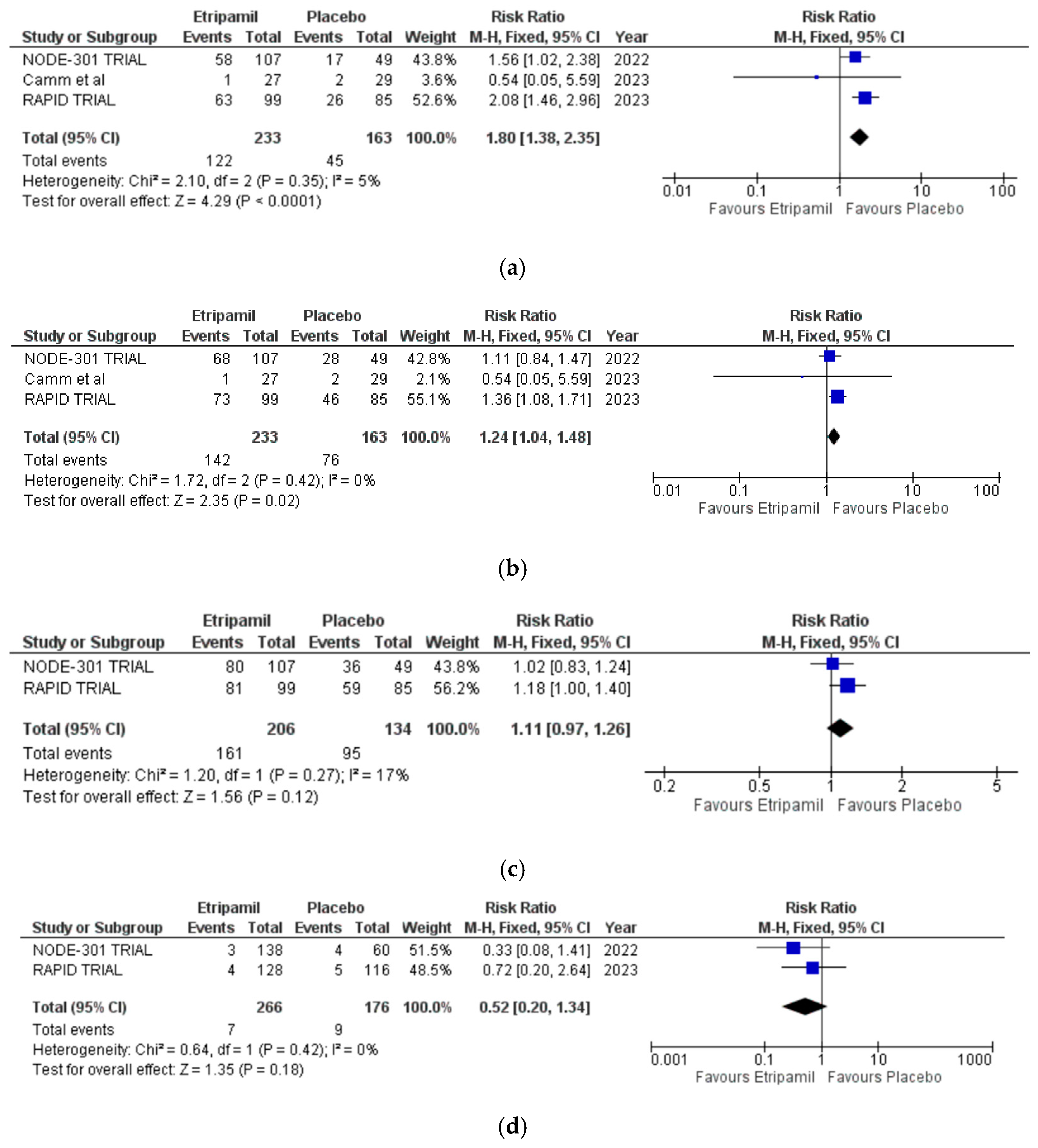
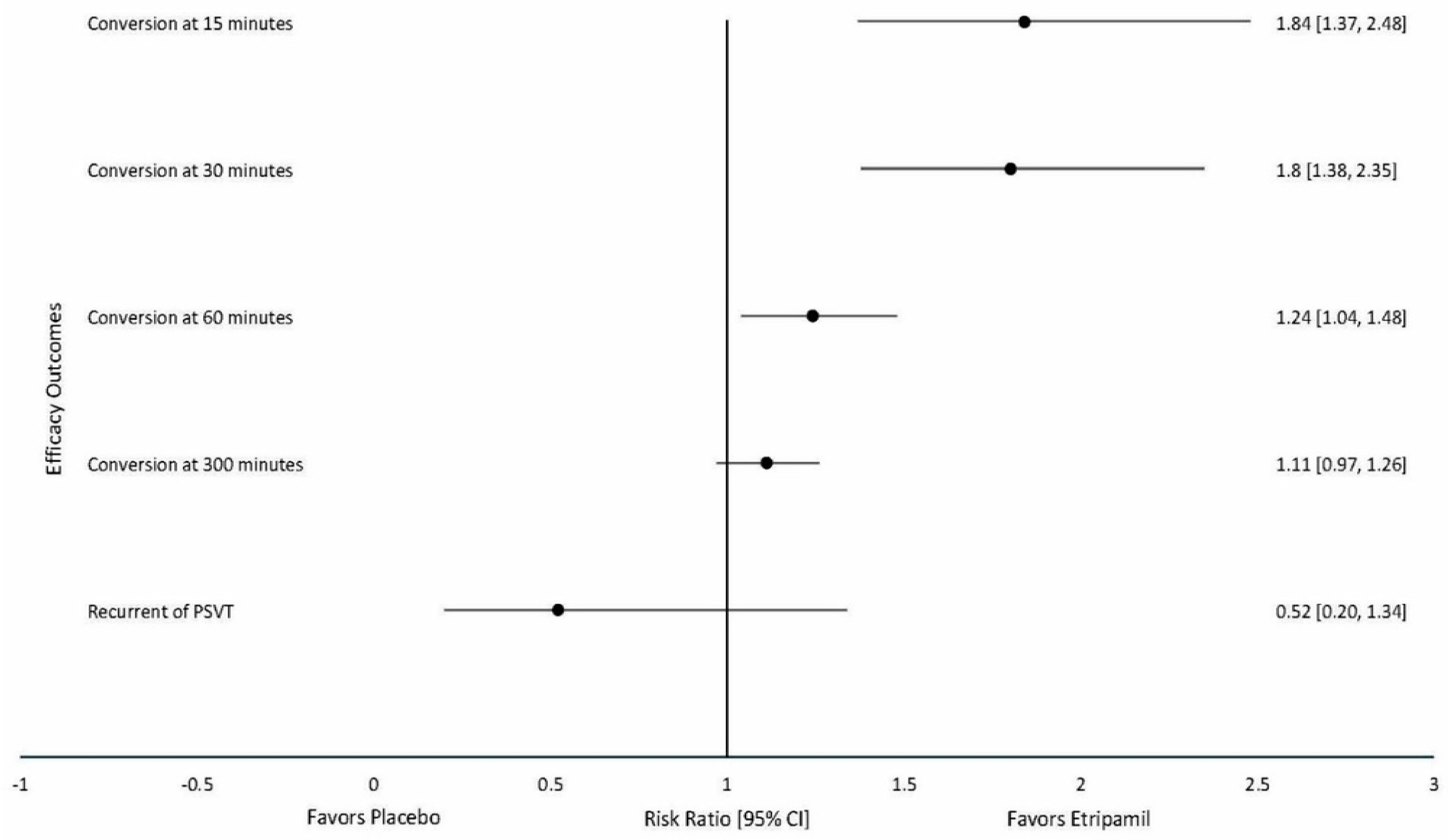
3.3. Primary Clinical Outcomes
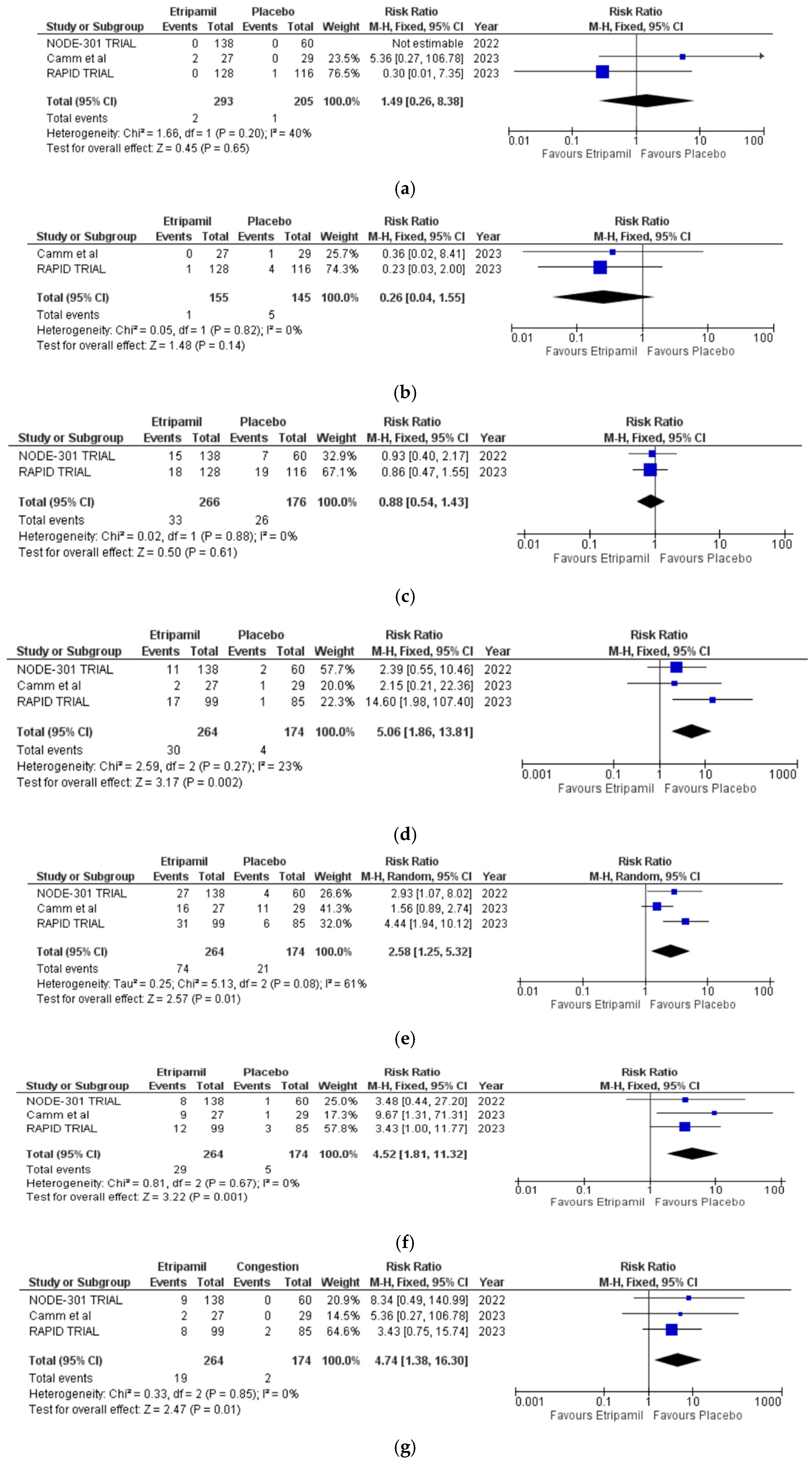
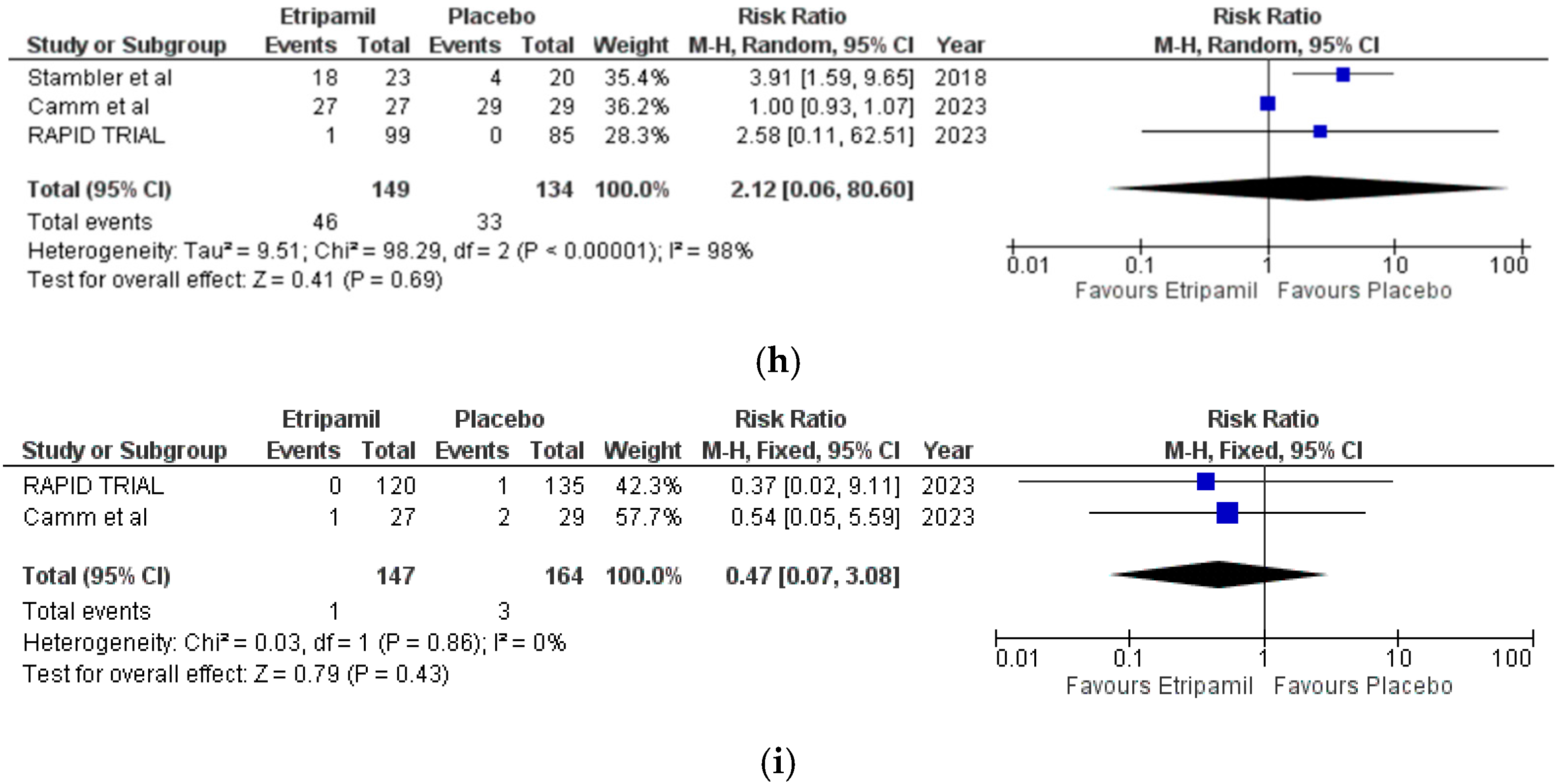
3.4. Secondary Outcomes
3.5. Risk of Assessment
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSVT | Paroxysmal supraventricular tachycardia |
| MeSH | Medical subject headings |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| AV node | Atrioventricular node |
| AVNRT | Atrioventricular reentrant tachycardia |
| IV | Intravenous |
| BMI | Body mass index |
| NSVT | Non-sustained ventricular tachycardia |
| AEs | Adverse events |
| TESAEs | Treatment-emergent severe adverse events |
| AF | Atrial fibrillation |
| CCBs | Calcium channel blockers |
References
- Peng, G.; Zei, P.C. Diagnosis and management of paroxysmal supraventricular tachycardia. JAMA 2024, 331, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Rehorn, M.; Sacks, N.C.; Emden, M.R.; Healey, B.; Preib, M.T.; Cyr, P.L.; Pokorney, S.D. Prevalence and incidence of patients with paroxysmal supraventricular tachycardia in the United States. J. Cardiovasc. Electrophysiol. 2021, 32, 2199–2206. [Google Scholar] [CrossRef]
- Yetkin, E.; Ozturk, S.; Cuglan, B.; Turhan, H. Clinical presentation of paroxysmal supraventricular tachycardia: Evaluation of usual and unusual symptoms. Cardiovasc. Endocrinol. Metab. 2020, 9, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, Y.; Quintanilla Rodriguez, B.S.; Ahmed, I.; Pothineni, N.V.K. Paroxysmal Supraventricular Tachycardia; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507699/ (accessed on 28 February 2024).
- Weintraub, S.; Frishman, W.H. A novel calcium channel blocker: Etripamil: What is the future of intranasal drug delivery in the treatment of cardiac arrhythmias? Cardiol. Rev. 2021, 29, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. Eur. Heart J. 2020, 41, 655–720. [Google Scholar] [CrossRef]
- Page, R.L.; Joglar, J.A.; Caldwell, M.A.; Calkins, H.; Conti, J.B.; Deal, B.J.; Estes, N.M.; Field, M.E.; Goldberger, Z.D.; Hammill, S.C.; et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia. Circulation 2016, 133, e471–e505. [Google Scholar] [CrossRef]
- Calkins, H.; Yong, P.; Miller, J.M.; Olshansky, B.; Carlson, M.; Saul, J.P.; Huang, S.K.S.; Liem, L.B.; Klein, L.S.; Moser, S.A.; et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: Final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation 1999, 99, 262–270. [Google Scholar] [CrossRef]
- IP, J.; Coutu, B.; Noseworthy, P.; Parody, M.L.; Rafii, F.; Sears, S.F.; Singh, N.; Stambler, B.S.; Bharucha, D.B.; Camm, A.J. Etripamil Nasal Spray for Recurrent Paroxysmal Supraventricular Tachycardia Conversion. J. Am. Coll. Cardiol. 2024, 83, 2032–2034. [Google Scholar] [CrossRef]
- Raja, J.M.; Cave, B.; Jefferies, J.L.; Khouzam, R.N. Etripamil: Intranasal calcium channel blocker: A novel noninvasive modality in the treatment of paroxysmal supraventricular tachycardia. Curr. Probl. Cardiol. 2019, 44, 258–267. [Google Scholar] [CrossRef]
- Faisaluddin, M.; Ashish, K.; Hajra, A.; Mondal, S.; Bandyopadhyay, D. Etripamil: Self-management of supraventricular tachycardia is not far away? Int. J. Cardiol. Heart Vasc. 2019, 22, 82–83. [Google Scholar] [CrossRef]
- Stambler, B.S.; Plat, F.; Sager, P.T.; Shardonofsky, S.; Wight, D.; Potvin, D.; Pandey, A.S.; Ip, J.E.; Coutu, B.; Mondésert, B.; et al. First Randomized, Multicenter, Placebo-Controlled Study of Self-Administered Intranasal Etripamil for Acute Conversion of Spontaneous Paroxysmal Supraventricular Tachycardia (NODE-301). Circ. Arrhythm. Electrophysiol. 2022, 15, e010915. [Google Scholar] [CrossRef]
- Roberts, L.; Savard, S.; Wardell, S.; Woods, R. Self-administered intranasal etripamil: A new treatment to keep SVT out of the ED? Can. J. Emerg. Med. 2025, 27, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.; Gupta, D. Intranasal etripamil for rapid treatment of paroxysmal supraventricular tachycardia. Future Cardiol. 2024, 20, 163–170. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 371, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.5; Cochrane: Los Angeles, CA, USA, 2024; Available online: www.training.cochrane.org/handbook (accessed on 2 May 2025).
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. Cochrane Bias Methods Group Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. ROBVIS: An R Package and Web Application for Visualizing Risk-of-Bias Assessments Using RoB 2; Version 1.0; University of York: York, UK, 2020; Available online: https://www.riskofbias.info (accessed on 2 May 2025).
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Stambler, B.S.; Dorian, P.; Sager, P.T.; Wight, D.; Douville, P.; Potvin, D.; Shamszad, P.; Haberman, R.J.; Kuk, R.S.; Lakkireddy, D.R.; et al. Etripamil Nasal Spray for Rapid Conversion of Supraventricular Tachycardia to Sinus Rhythm. J. Am. Coll. Cardiol. 2018, 72, 489–497. [Google Scholar] [CrossRef]
- Milestone Pharmaceuticals Inc. Efficacy and Safety of Etripamil for the Termination of Spontaneous PSVT (NODE-301). NLM identifier: NCT03464019. Available online: https://clinicaltrials.gov/ct2/show/NCT03464019 (accessed on 2 May 2025).
- Alings, M.; Stambler, B.S.; Camm, A.J. Self-administered intranasal etripamil using a symptom-prompted, repeat-dose regimen for atrioventricular-nodal-dependent supraventricular tachycardia (RAPID): A multicenter, randomised trial. Lancet 2023, 402, 118–128. [Google Scholar] [CrossRef]
- Camm, A.J.; Piccini, J.P.; Alings, M.; Dorian, P.; Gosselin, G.; Guertin, M.-C.; Ip, J.E.; Kowey, P.R.; Mondésert, B.; Prins, F.J.; et al. Multicenter, Phase 2, Randomized Controlled Study of the Efficacy and Safety of Etripamil Nasal Spray for the Acute Reduction of Rapid Ventricular Rate in Patients With Symptomatic Atrial Fibrillation (ReVeRA-201). Circ. Arrhythm. Electrophysiol. 2023, 16, 639–650. [Google Scholar] [CrossRef]
- Smith, G. Management of supraventricular tachycardia using the Valsalva manoeuvre: A historical review and summary of published evidence. Eur. J. Emerg. Med. 2012, 19, 346–352. [Google Scholar] [CrossRef]
- Matthews, G.D.K.; Grace, A.A. Unmasking Adenosine: The Purinergic Signalling Molecule Critical to Arrhythmia Pathophysiology and Management. Arrhythmia Electrophysiol. Rev. 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Abuelazm, M.; Kambalapalli, S.; Saleh, O.; Elzeftawy, M.A.; Albakri, K.; Gowaily, I.; Abdelazeem, B. The Efficacy and Safety of Etripamil Nasal Spray for Acute Paroxysmal Supraventricular Tachycardia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2023, 23, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Delaney, B.; Loy, J.; Kelly, A.M. The relative efficacy of adenosine versus verapamil for the treatment of stable paroxysmal supraventricular tachycardia in adults: A meta-analysis. Eur. J. Emerg. Med. 2011, 18, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Stambler, B.S.; Plat, F.; Sager, P.T.; Lubkov, V.; Shardonofsky, S.; Wight, D.; Chen, M.; Camm, A.J. Rationale for and design of a multicenter, placebo-controlled, phase 3 study to assess efficacy and safety of intranasal etripamil for the conversion of paroxysmal supraventricular tachycardia. Am. Heart J. 2022, 253, 20–29. [Google Scholar] [CrossRef]
- Song, D.; Jha, M.; Shah, V.; Hum, A.; Sacher, A.; Ahmed, F.; Jain, H.; ElDawud, D.; Ahmed, A.A.; Mal, M.; et al. The safety and efficacy of etripamil in paroxysmal supraventricular tachycardia (PSVT). J. Am. Coll. Cardiol. 2025, 85 (Suppl. S12), 226. [Google Scholar] [CrossRef]
| Study | Country | Population Size | Male | Age (Years) | BMI (kg/m2) | Outcomes |
|---|---|---|---|---|---|---|
| Stambler et al., 2018 [20] | Canada USA | 104 | 45 (43.3) | 52.2 ± 13.13 a | 29.35 ± 8.98 a | Etripamil nasal spray rapidly terminated induced SVT with a high conversion rate. |
| NODE-301 TRIAL [21] | Canada USA | 196 | 67 (34.1) | 56.8 ± 13.9 a | 29.2 ± 7.6 | Etripamil self-administration during PSVT was safe and well tolerated. Although the primary 5 h efficacy endpoint was not met, the pooled analysis indicated an etripamil treatment effect in terminating PSVT. |
| RAPID TRIAL [22] | Belgium Canada France Germany Hungary Netherlands Poland Spain USA | 184 | 53 (28.8) | 54 ± 12 | NR | Intranasal etripamil was well tolerated, safe, and superior to a placebo for the rapid conversion of atrioventricular nodal-dependent paroxysmal supraventricular tachycardia to a sinus rhythm. |
| Camm et al., 2023 [23] | Canada Netherlands | 56 | 34 (60.7) | 64.6 ± 10.47 | NR | Intranasal etripamil (70 mg) reduced arrhythmic events and improved symptom relief and treatment satisfaction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, M.; Song, D.; Kung, A.; Lo, S.; Sacher, A.; Ang, S.P.; Akthar, A.; Jain, H.; Ahmed, R.; Bates, M.; et al. Efficacy and Safety of Intranasal Etripamil for Paroxysmal Supraventricular Tachycardia: Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2025, 14, 3720. https://doi.org/10.3390/jcm14113720
Jha M, Song D, Kung A, Lo S, Sacher A, Ang SP, Akthar A, Jain H, Ahmed R, Bates M, et al. Efficacy and Safety of Intranasal Etripamil for Paroxysmal Supraventricular Tachycardia: Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2025; 14(11):3720. https://doi.org/10.3390/jcm14113720
Chicago/Turabian StyleJha, Mayank, David Song, Andrew Kung, Sam Lo, Alexander Sacher, Song P. Ang, Aasim Akthar, Hritvik Jain, Raheel Ahmed, Matthew Bates, and et al. 2025. "Efficacy and Safety of Intranasal Etripamil for Paroxysmal Supraventricular Tachycardia: Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 14, no. 11: 3720. https://doi.org/10.3390/jcm14113720
APA StyleJha, M., Song, D., Kung, A., Lo, S., Sacher, A., Ang, S. P., Akthar, A., Jain, H., Ahmed, R., Bates, M., Lee, S., & Goldbarg, S. (2025). Efficacy and Safety of Intranasal Etripamil for Paroxysmal Supraventricular Tachycardia: Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 14(11), 3720. https://doi.org/10.3390/jcm14113720







