Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Discussion
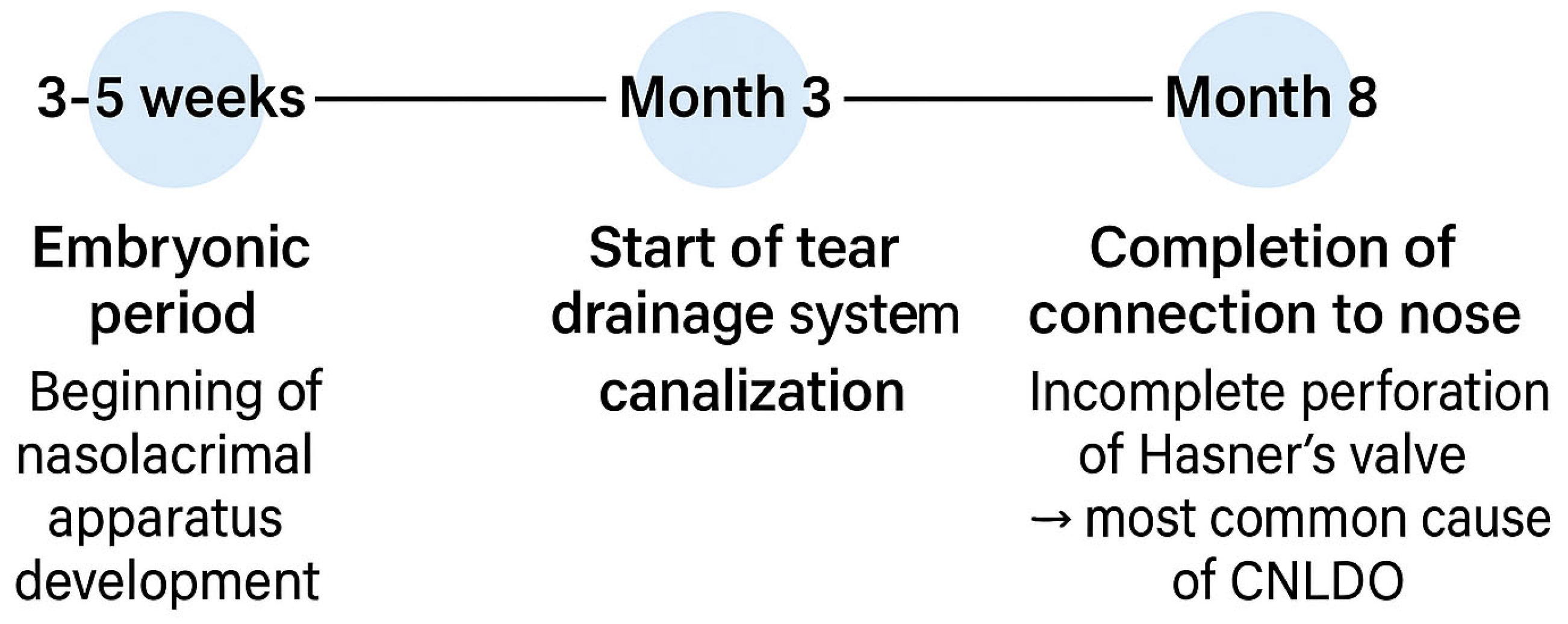
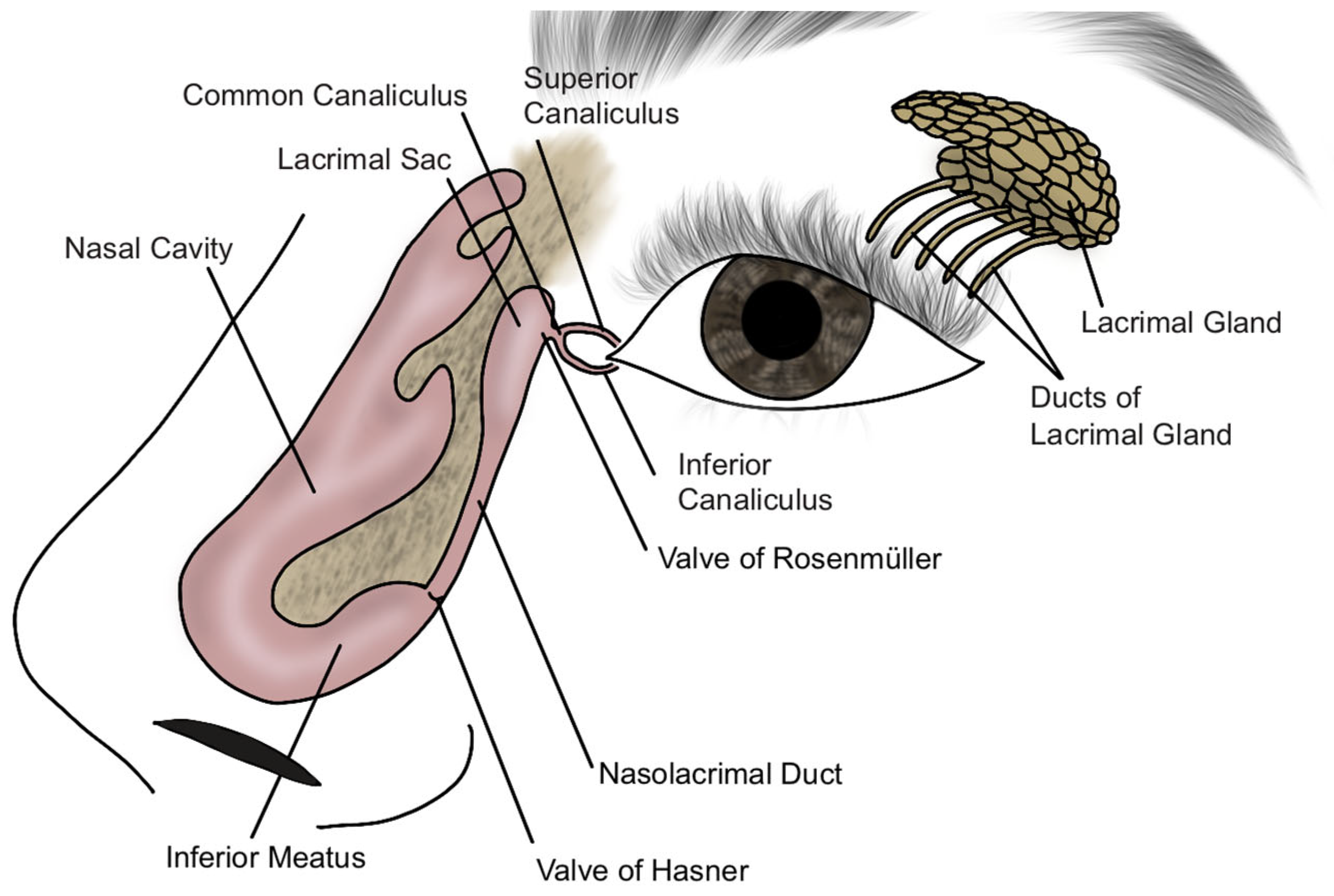
3.1. Etiopathogenesis and Epidemiology
3.1.1. Etiopathogenesis
3.1.2. Epidemiology
3.2. Symptoms and Diagnosis
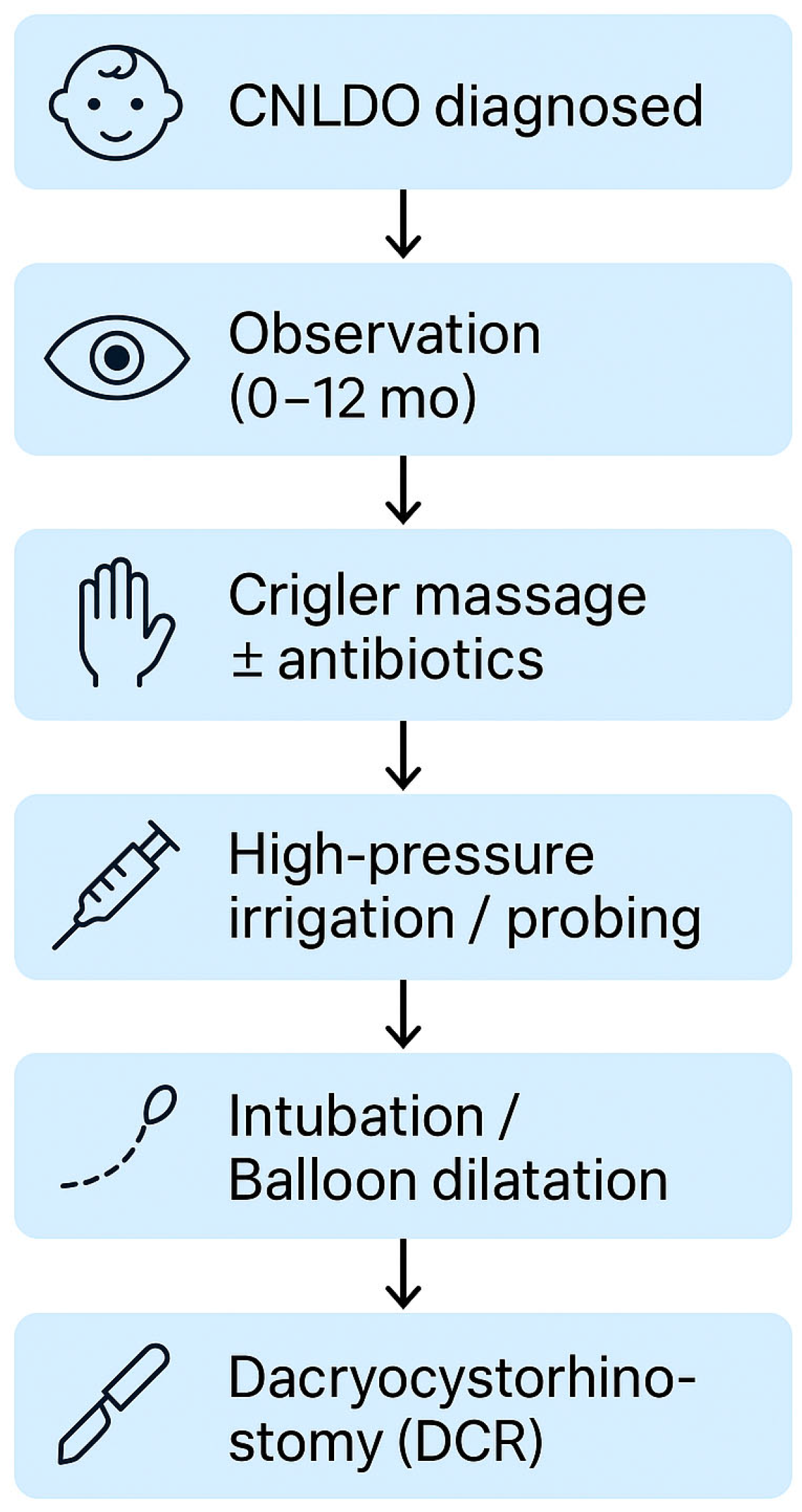
3.3. Treatment
3.3.1. Conservative Treatment
3.3.2. High Pressure Irrigation
3.3.3. Probing and Duct Intubation
3.3.4. Balloon Dilatation
3.3.5. Dacryocystorhinostomy
- External DCR:
- Involves a skin incision over the anterior lacrimal crest, preparation of the osteotomy and suturing of the lacrimal sac to the nasal mucosa to create a sac-nasal mucosal anastomosis;
- Reported success rates are high, reaching approximately 96% in children;
- Endoscopic Endonasal DCR:
- Performed without external incisions, minimizing visible scarring and preserving the medial canthus;
- Nasal endoscopy facilitates identification and correction of intranasal abnormalities during surgery;
- Success rates range from 82% to 94%, approaching those of external DCR [37];
- This approach is preferred by many pediatric ophthalmologists due to its cosmetic and functional advantages [38];
- It allows for precise visualization and management of the obstruction through nasal endoscopy, making it particularly suitable for complex or refractory cases.
3.3.6. Other Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNLDO | Congenital nasolacrimal duct obstruction |
| NLD | Nasolacrimal duct |
| FDDT | Fluorescein dye disappearance test |
| DCR | Dacryocystorhinostomy |
| DE | Dacryoendoscopy |
References
- Schnall, B.M. Pediatric nasolacrimal duct obstruction. Curr. Opin. Ophthalmol. 2013, 24, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.; Stern, M. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Tiffany, J.M.; Gouveia, S.M.; Yokoi, N.; Voon, L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Guo, D.; Liu, R.; Tu, M.; Chen, Z.; Zheng, Y.; Liu, C.; Liang, L. Obstruction of the Tear Drainage Altered Lacrimal Gland Structure and Function. Invest. Ophthalmol. Vis. Sci. 2023, 64, 13. [Google Scholar] [CrossRef]
- Paulsen, F. Anatomy and physiology of efferent tear ducts. Ophthalmologe 2008, 105, 339–345. [Google Scholar] [CrossRef]
- Heichel, J. Congenital Nasolacrimal Duct Obstruction—Early Diagnosis and Graded Therapeutic Approach as Key Points for Successful Management. Semin. Ophthalmol. 2024, 39, 510–520. [Google Scholar] [CrossRef]
- Abu Serhan, H.; AlSamhori, J.F.; Siddiq, A.; Hassan, A.R.; Irshaidat, S.; Abu Serhan, L.; Alawadhi, A.; Abdelaal, A.; Al-Thawabieh, W. Preferred Practice Patterns of Congenital Nasolacrimal Duct Obstruction in Jordan. Clin. Ophthalmol. 2023, 17, 2309–2322. [Google Scholar] [CrossRef]
- Li, Y.; Wei, M.; Liu, X.; Zhang, L.; Song, X.; Xiao, C. Dacryoendoscopy-assisted incision of Hasner’s valve under nasoendoscopy for membranous congenital nasolacrimal duct obstruction after probing failure: A retrospective study. BMC Ophthalmol. 2021, 21, 182. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsumura, N.; Miyazaki, C.; Kamao, T.; Yokoi, N.; Fujimoto, M.; Hayami, M.; Iwasaki, A.; Mimura, M.; Murata, A.; et al. Congenital nasolacrimal duct obstruction: Clinical guideline. Jpn. J. Ophthalmol. 2024, 68, 367–388. [Google Scholar] [CrossRef]
- Olitsky, S.E. Update on congenital nasolacrimal duct obstruction. Int. Ophthalmol. Clin. 2014, 54, 1–7. [Google Scholar] [CrossRef]
- Nakamura, J.; Ohno, T.; Mizuki, Y.; Takeuchi, M.; Mizuki, N.; Matsumura, N. Diversity in Lacrimal Pathway Morphology Among Patients with Congenital Nasolacrimal Duct Obstruction. Clin. Ophthalmol. 2024, 18, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Paulsen, F. Syndromic and Nonsyndromic Systemic Associations of Congenital Lacrimal Drainage Anomalies: A Major Review. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Honavar, S.; Prakash, V.; Rao, G. Outcome of probing for congenital nasolacrimal duct obstruction in older children. Am. J. Ophthalmol. 2000, 130, 42–48. [Google Scholar] [CrossRef]
- Vagge, A.; Desideri, L.F.; Nucci, P.; Serafino, M.; Giannaccare, G.; Lembo, A.; Traverso, C.E. Congenital Nasolacrimal Duct Obstruction (CNLDO): A Review. Diseases 2018, 6, 96. [Google Scholar] [CrossRef]
- Landau Prat, D.; Tadros, S.; Revere, K.; Katowitz, J.; Katowitz, W. Management of congenital nasolacrimal duct obstruction in down syndrome. Eye 2022, 37, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, K.; Murgova, S.; Duhlenski, B.; Hristova, I. Congenital Nasolacrimal Duct Obstruction: Epidemiology and Risk Factors. J. IMAB Annu. Proceeding (Sci. Pap.) 2019, 25, 2317–2322. [Google Scholar] [CrossRef]
- Al Tamimi, E.; AlHammad, F.; Yassin, S.; AlBadri, K.; AlJarudi, S.; AlShawaf, M.; Khandekar, R. Unilateral Congenital Nasolacrimal Duct Obstruction, Is It An Amblyogenic Factor? Middle East. Afr. J. Ophthalmol. 2018, 25, 156. [Google Scholar] [CrossRef]
- Kilic, D.; Aydin, I.; Sirem, M.R.; Birgin, H.; Guven, S. Congenital Nasolacrimal Duct Obstruction and Refractive Amblyopia Risk Factors: Effect of Age at the Time of Probing. Beyoglu Eye J. 2022, 7, 30. [Google Scholar] [CrossRef]
- Bektas, F.M.; Guclu, E.S.; Argin, M.A. Investigation of Refractive Errors in Congenital Nasolacrimal Duct Obstruction. Beyoglu Eye J. 2024, 9, 220–227. [Google Scholar] [CrossRef]
- Sodhi, G.; Liu, E.; Renz, J.; Heher, K.; Kapadia, M. Infections of the Eyelids, Orbit, and Ocular Adnexa. In The Infected Eye; Springer: Cham, Switzerland, 2016; pp. 163–175. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kakizaki, H.; Chan, W.; Selva, D. Management of congenital nasolacrimal duct obstruction: Review Article. Acta Ophthalmol. 2009, 88, 506–513. [Google Scholar] [CrossRef]
- Kim, J.S.; Jin, S.W.; Hur, M.C.; Kwon, Y.H.; Ryu, W.Y.; Jeong, W.J.; Ahn, H.B. The Clinical Characteristics and Surgical Outcomes of Epiblepharon in Korean Children: A 9-Year Experience. J. Ophthalmol. 2014, 2014, 156501. [Google Scholar] [CrossRef] [PubMed]
- Ozturker, C.; Purevdorj, B.; Karabulut, G.O.; Seif, G.; Fazil, K.; Khan, Y.A.; Kaynak, P. A Comparison of Transcanalicular, Endonasal, and External Dacryocystorhinostomy in Functional Epiphora: A Minimum Two-Year Follow-Up Study. J. Ophthalmol. 2022, 2022, 3996854. [Google Scholar] [CrossRef] [PubMed]
- Repka, M. Timing of Simple Probing for Congenital Nasolacrimal Duct Obstruction: Not So Simple. JAMA Ophthalmol. 2018, 136, 1286–1287. [Google Scholar] [CrossRef] [PubMed]
- Irfan, S. Is Surgical Intervention Avoidable in Congenital Nasolacrimal Duct Obstruction (CNLDO)? Indian. J. Clin. Exp. Ophthalmol. 2020, 6, 501–510. [Google Scholar] [CrossRef]
- Bansal, O.; Bothra, N.; Sharma, A.; Walvekar, P.; Ali, M. Congenital nasolacrimal duct obstruction update study (CUP study): Paper I—Role and outcomes of Crigler’s lacrimal sac compression. Eye 2020, 35, 1600–1604. [Google Scholar] [CrossRef]
- Lekskul, A.; Preechaharn, P.; Jongkhajornpong, P.; Wuthisiri, W. Age-Specific Outcomes of Conservative Approach and Probing for Congenital Nasolacrimal Duct Obstruction. Clin. Ophthalmol. 2022, 16, 1821–1828. [Google Scholar] [CrossRef]
- Srivastava, P.; Srivastava, N. A non-surgical conservative management of congenital dacryostenosis. Int. J. Community Med. Public Health 2023, 10, 1459–1463. [Google Scholar] [CrossRef]
- Kashkouli, M.B. Re: Treatment of Congenital Nasolacrimal Duct Obstruction With High-Pressure Irrigation Under Topical Anesthesia. Ophthalmic. Plast Reconstr. Surg. 2006, 22, 240–241. [Google Scholar] [CrossRef]
- Örge, F.H.; Boente, C.S. The Lacrimal System. Pediatr. Clin. N. Am. 2014, 61, 529–539. [Google Scholar] [CrossRef]
- Petris, C.; Liu, D. Probing for congenital nasolacrimal duct obstruction. Cochrane Database Syst. Rev. 2017, 2017, CD011109. [Google Scholar] [CrossRef]
- Świerczyńska, M.; Tobiczyk, E.; Rodak, P.; Barchanowska, D.; Filipek, E. Success rates of probing for congenital nasolacrimal duct obstruction at various ages. BMC Ophthalmol. 2020, 20, 403. [Google Scholar] [CrossRef] [PubMed]
- Farat, J.; Schellini, S.; Dib, R.; Santos, F.; Sousa, R.; Jorge, E. Probing for congenital nasolacrimal duct obstruction: A systematic review and meta-analysis of randomized clinical trials. Arq. Bras. Oftalmol. 2021, 84, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Okumuş, S.; Öner, V.; Durucu, C.; Coşkun, E.; Aksoy, Ü.; Durucu, E.; Şahin, L.; Erbağcı, I. Nasolacrimal duct intubation in the treatment of congenital nasolacrimal duct obstruction in older children. Eye 2016, 30, 85–88. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abushnein, A.; Yasir, M.; Yosif, W. Mono-canalicular Lacrimal Stent Intubation for Congenital Nasolacrimal Duct Obstruction Treatment. Open Ophthalmol. J. 2024, 18, e18743641298283. [Google Scholar] [CrossRef]
- Jafarizadeh, A.; Manouchehri, V.; Sobhi, N.; Mousavi, F.; Tondro Anamag, F. Probing and Nasolacrimal Intubation Outcomes in Children Over 18 Months of Age with Congenital Nasolacrimal Duct Obstruction. Heliyon 2024, 10, e36245. [Google Scholar] [CrossRef]
- Moreira, C.; Correia, I.; Cunha, I.; Sousa, H.; Barros, E. Endonasal endoscopic dacryocystorhinostomy in the paediatric population. Rhinol. Online 2019, 2, 99–102. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Zhang, C.-Y.; Liu, W.; Wu, Q.; Yu, G.; Li, L.; Wei, W.-B. Endoscopic dacryocystorhinostomy to treat congenital nasolacrimal canal dysplasia: A retrospective analysis in 40 children. BMC Ophthalmol. 2019, 19, 244. [Google Scholar] [CrossRef]
- Freitag, S.; Aakalu, V.; Foster, J.; McCulley, T.; Tao, J.; Vagefi, M.R.; Yen, M.T.; Kim, S.J.; Wladis, E.J. Use of Mitomycin C in Dacryocystorhinostomy. Ophthalmology 2023, 130. [Google Scholar] [CrossRef]
- Marcet, M.; Phelps, P.; Cowling, B.; Selva, D. Antimetabolites as an adjunct to dacryocystorhinostomy for nasolacrimal duct obstruction: Protocols. Cochrane Database Syst. Rev. 2016, 2016, CD012309. [Google Scholar] [CrossRef]
- Urban, B.; Samsel, A.; Filipek, E.; Niwald, A.M.; Krzyżanowska-Berkowska, P.; Bakunowicz-Łazarczyk, A. Management in congenital nasolacrimal duct obstruction—Guidelines of the Polish Ophthalmological Society. Klin. Ocz./Acta Ophthalmol. Pol. 2020, 122, 17–20. [Google Scholar] [CrossRef]
- Iskandar, K.; Marchin, L.; Kodjikian, L.; Rocher, M.; Roques, C. Highlighting the Microbial Contamination of the Dropper Tip and Cap of In-Use Eye Drops, the Associated Contributory Factors, and the Risk of Infection: A Past-30-Years Literature Review. Pharmaceutics 2022, 14, 2176. [Google Scholar] [CrossRef] [PubMed]
- Golash, V.; Kaur, H.; Athwal, S.; Chakartash, R.; Laginaf, M.; Khandwala, M. Management of congenital nasolacrimal duct obstruction: Results of a national survey of paediatric and oculoplastic ophthalmologists. Eye 2021, 35, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Swol, J.; Myers, W.; Nguyen, S.; Eiseman, A. Revision dacryocystorhinostomy: Systematic review and meta-analysis. Orbit 2022, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alagöz, G.; Serin, D.; Çelebi, S.; Kükner, S.; Elçioglu, M.; Güngel, H. Treatment of Congenital Nasolacrimal Duct Obstruction with High-Pressure Irrigation Under Topical Anesthesia. Ophthalmic Plast. Reconstr. Surg. 2005, 21, 423–426. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, S.-M.; Kim, G.; Joo, E.-Y.; Song, M.; Sa, H.-S. Efficacy and Safety of Inhalation Sedation during Office Probing for Congenital Nasolacrimal Duct Obstruction. J. Clin. Med. 2021, 10, 1800. [Google Scholar] [CrossRef]
- Pierre Filho Pde, T.P.; Pierre, L.L. Bilateral congenital dacryocystocele complicated with acute dacryocystitis. Rev. Bras. Oftalmol. 2023, 82, e0005. [Google Scholar] [CrossRef]
- Pensiero, S.; Diplotti, L.; Visalli, G.; Ronfani, L.; Giangreco, M.; Barbi, E. Minimally-Invasive Surgical Approach to Congenital Dacryostenosis: Proposal for a New Protocol. Front. Pediatr. 2021, 9, 569262. [Google Scholar] [CrossRef]
- Kashkouli, M.B.; Karimi, N.; Khademi, B. Surgical management of congenital nasolacrimal duct obstruction; one procedure for all versus all procedures for one. Curr. Opin. Ophthalmol. 2019, 30, 364–371. [Google Scholar] [CrossRef]
- Al-Faky, Y. Nasal endoscopy in the management of congenital nasolacrimal duct obstruction. Saudi J. Ophthalmol. 2013, 28, 6–11. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Sutherland, M.; Haddow, K.; Blaikie, A. Success rates of endoscopic-assisted probing for congenital nasolacrimal duct obstruction in children. J. Laryngol. Otol. 2013, 127, 794–798. [Google Scholar] [CrossRef]
- Shahgholi, S.; Zand, A.; Jamshidian-Tehrani, M.; Bahremani, E.; Aghajani, A.; Rafizadeh, S. Comparative efficacy of probing with or without intubation, and/or inferior turbinate fracture in simple congenital nasolacrimal duct obstruction: A randomized clinical trial. Sci. Rep. 2024, 14, 20324. [Google Scholar] [CrossRef]
- Wladis, E.J.; Aakalu, V.K.; Yen, M.T.; Bilyk, J.R.; Sobel, R.K.; Mawn, L.A. Balloon Dacryoplasty for Congenital Nasolacrimal Duct Obstruction: A Report by the American Academy of Ophthalmology. Ophthalmology 2018, 125, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Naik, M.N.; Honavar, S.G. Balloon dacryoplasty: Ushering the new and routine era in minimally invasive lacrimal surgeries. Int. Ophthalmol. 2013, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wu, Q.; Fan, Y.; Cao, W.; Lin, Q.; Yu, G. Comparison of balloon catheter dilatation and silicon intubation as the secondary treatment for congenital nasolacrimal duct obstruction after failed primary probing. Zhonghua Yan Ke Za Zhi 2016, 52, 123–128. [Google Scholar] [CrossRef]
- Goldich, Y.; Barkana, Y.; Zadok, D.; Avni, I.; Pras, E.; Mezer, E.; Morad, Y. Balloon catheter dilatation versus probing as primary treatment for congenital dacryostenosis. Br. J. Ophthalmol. 2010, 95, 634–636. [Google Scholar] [CrossRef]
- Penttilä, E.; Smirnov, G.; Tuomilehto, H.; Kaarniranta, K.; Seppä, J. Endoscopic Dacryocystorhinostomy as Treatment for Lower Lacrimal Pathway Obstructions in Adults: Review Article. Allergy Rhinol. (Providence) 2015, 6, 12–19. [Google Scholar] [CrossRef]
- Vinciguerra, A.; Nonis, A.; Resti, A.G.; Bussi, M.; Trimarchi, M. Impact of Post-Surgical Therapies on Endoscopic and External Dacryocystorhinostomy: Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2020, 34, 846–856. [Google Scholar] [CrossRef]
- Heichel, J.; Struck, H.-G.; Fiorentzis, M.; Hammer, T.; Bredehorn-Mayr, T. A Case Series of Dacryoendoscopy in Childhood: A Diagnostic and Therapeutic Alternative for Complex Congenital Nasolacrimal Duct Obstruction Even in the First Year of Life. Adv. Ther. 2017, 34, 1221–1232. [Google Scholar] [CrossRef]
- Wong, N.T.Y.; Aljufairi, F.M.A.A.; Lai, K.K.H.; Chin, J.K.Y.; Tham, C.C.Y.; Pang, C.P.; Chong, K.K.L. Dacryoendoscopy in patients with lacrimal outflow obstruction: A systematic review. Int. Ophthalmol. 2025, 45, 90. [Google Scholar] [CrossRef]
- Eisenbach, N.; Karni, O.; Sela, E.; Nemet, A.; Dror, A.; Levy, E.; Kassif, Y.; Ovadya, R.; Ronen, O.; Marshak, T. Conjunctivodacryocystorhinostomy (CDCR) success rates and complications in endoscopic vs non-endoscopic approaches: A systematic review. Int. Forum Allergy Rhinol. 2021, 11, 174–194. [Google Scholar] [CrossRef]
- Wali, U.; Sabt, B.; Al Badaai, Y.; Al-Mujaini, A. Transcanalicular laser-assisted dacryocystorhinostomy: First report from Oman. Indian. J. Ophthalmol. 2018, 66, 170–172. [Google Scholar] [CrossRef]
| Method | Target Age | Success Rate (%) | Invasiveness | Anesthesia | Remarks | Study, Year |
|---|---|---|---|---|---|---|
| Observation | 0–12 months | ~90% | None | Not applicable | High effectiveness in infants | Repka, 2018 [24]; Irfan, 2020 [25] |
| Crigler Massage | 0–12 months | >85% | Low | No | Can be performed at home by parents | Bansal et al., 2021 [26]; Lekskul et al., 2022 [27]; Srivastava et al., 2023 [28] |
| High-Pressure Irrigation | >6 months | 80–90% | Low | Topical | Quick outpatient procedure | Kashkouli, 2006 [29] |
| Probing | 6–16 months | 80–95% | Moderate | Depends on age | May require repetition; performed under general/local anesthesia | Örge et al., 2014 [30]; Petris et al., 2017 [31]; Świerczyńska et al., 2020 [32]; Farat et al., 2021 [33] |
| Intubation | >12 months | 85–96% | Moderate | General | Effective in recurrent or resistant cases | Okumuş et al., 2016 [34]; Abushnein et al., 2024 [35]; Jafarizadeh et al., 2024 [36] |
| DCR * (external/endoscopic) | >1 year (preferred) | 82–96% | High | General | Final-line treatment for complex or refractory obstructions | Heichel, 2024 [6]; Moreira et al., 2019 [37]; Cui et al., 2019 [38]; Freitag et al., 2023 [39]; Phelps et al., 2020 [40] |
| Age Range | Indications for Probing |
|---|---|
| 0–6 months | - Mucocele - Lacrimal sac abscess - Significant dacryocystocele with chronic purulent discharge - Bilateral dacryocystocele witch airway obstruction |
| 6–10 months | - Recurrent infectious dacryocystitis |
| 8–12 months | - Persistent CNLDO without recurrent infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczyk, K.; Biedka, K.; Estreicher, A.; Wesołowski, M.; Bulski, J.; Sobaś, A.; Ziobro, O.; Maj, F.; Sornat, K.; Klasa, A.; et al. Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies. J. Clin. Med. 2025, 14, 3716. https://doi.org/10.3390/jcm14113716
Błaszczyk K, Biedka K, Estreicher A, Wesołowski M, Bulski J, Sobaś A, Ziobro O, Maj F, Sornat K, Klasa A, et al. Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies. Journal of Clinical Medicine. 2025; 14(11):3716. https://doi.org/10.3390/jcm14113716
Chicago/Turabian StyleBłaszczyk, Katarzyna, Kamil Biedka, Agata Estreicher, Michał Wesołowski, Jakub Bulski, Aleksandra Sobaś, Oliwia Ziobro, Filip Maj, Karol Sornat, Anna Klasa, and et al. 2025. "Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies" Journal of Clinical Medicine 14, no. 11: 3716. https://doi.org/10.3390/jcm14113716
APA StyleBłaszczyk, K., Biedka, K., Estreicher, A., Wesołowski, M., Bulski, J., Sobaś, A., Ziobro, O., Maj, F., Sornat, K., Klasa, A., Karwacki, J., & Sebzda, T. (2025). Congenital Nasolacrimal Duct Obstruction: Natural Course, Diagnosis and Therapeutic Strategies. Journal of Clinical Medicine, 14(11), 3716. https://doi.org/10.3390/jcm14113716






