Effects of Pregnancy on Liver and Kidney Cyst Growth Rates in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
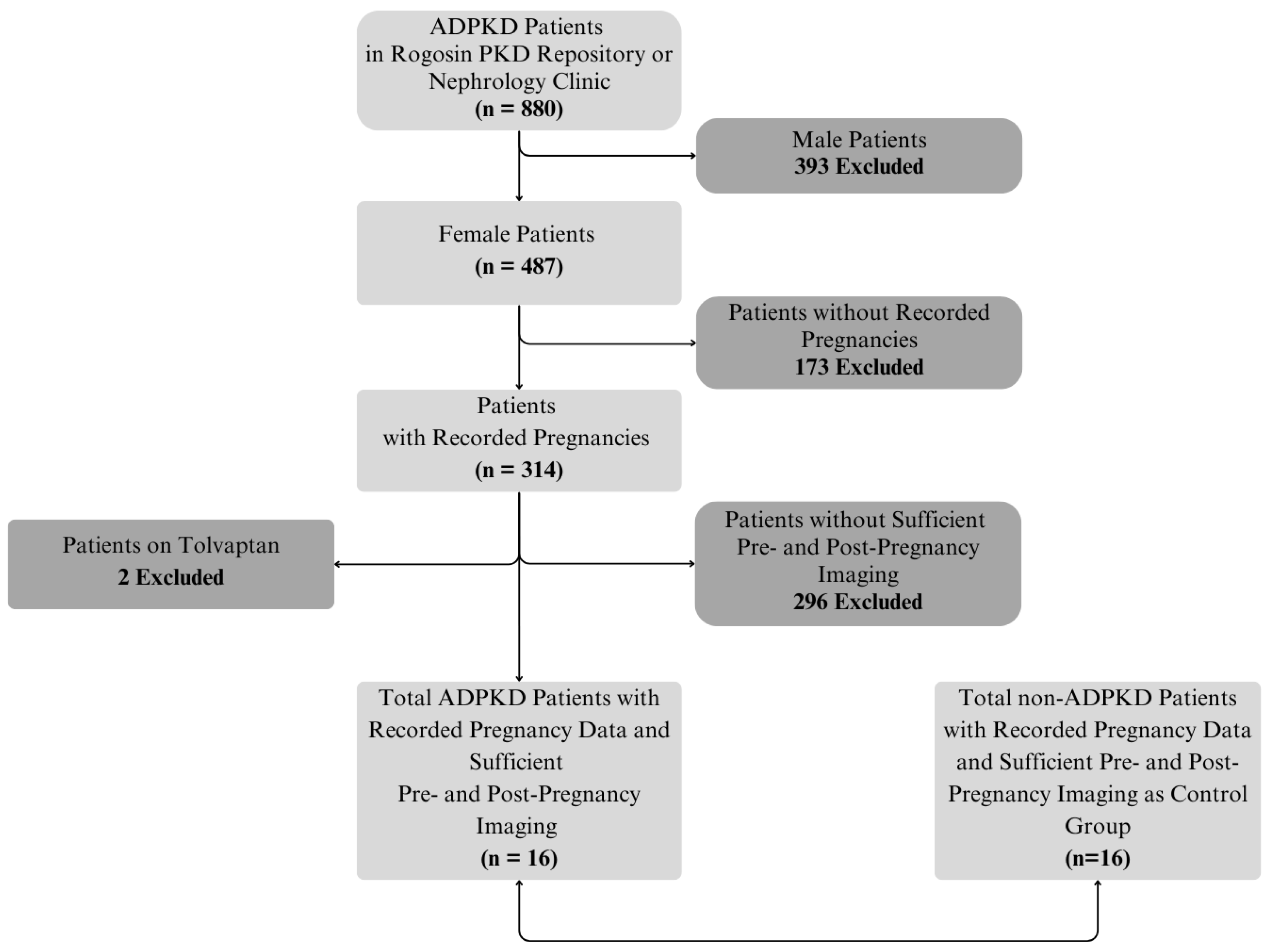
2.1. Subjects and Data Extraction
2.2. MRI Acquisition
2.3. Image Analysis and Calculation of Growth Rates
2.4. Effect of Gravida
2.5. Effect of In Vitro Fertilization (IVF)
2.6. Comparison to Pregnancies in Women Without ADPKD
2.7. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Interobserver Agreement
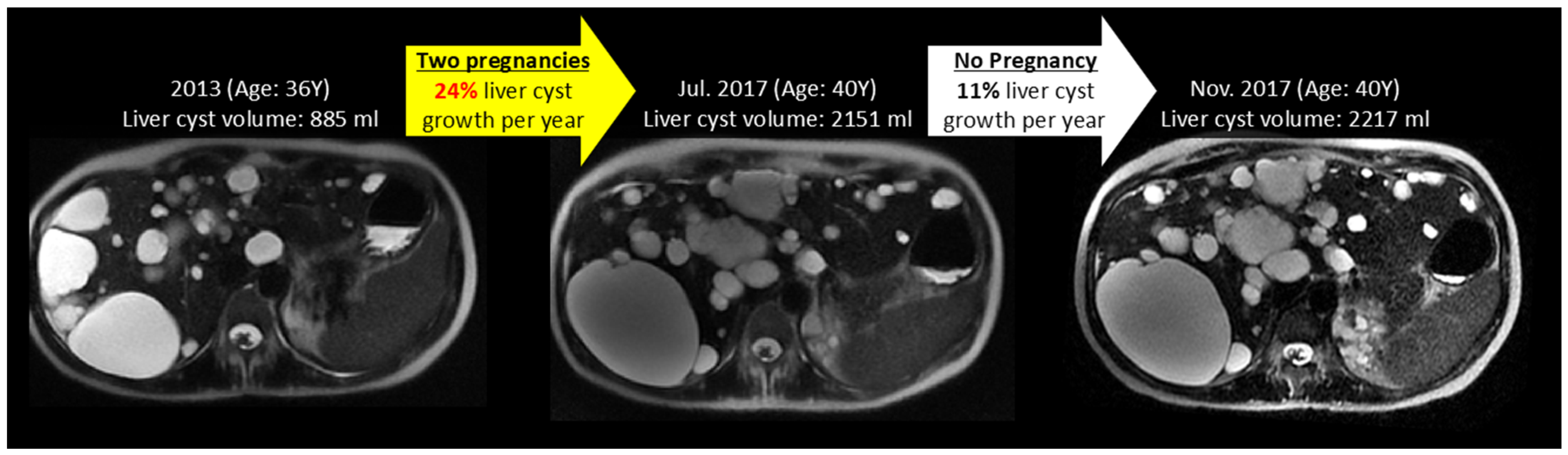
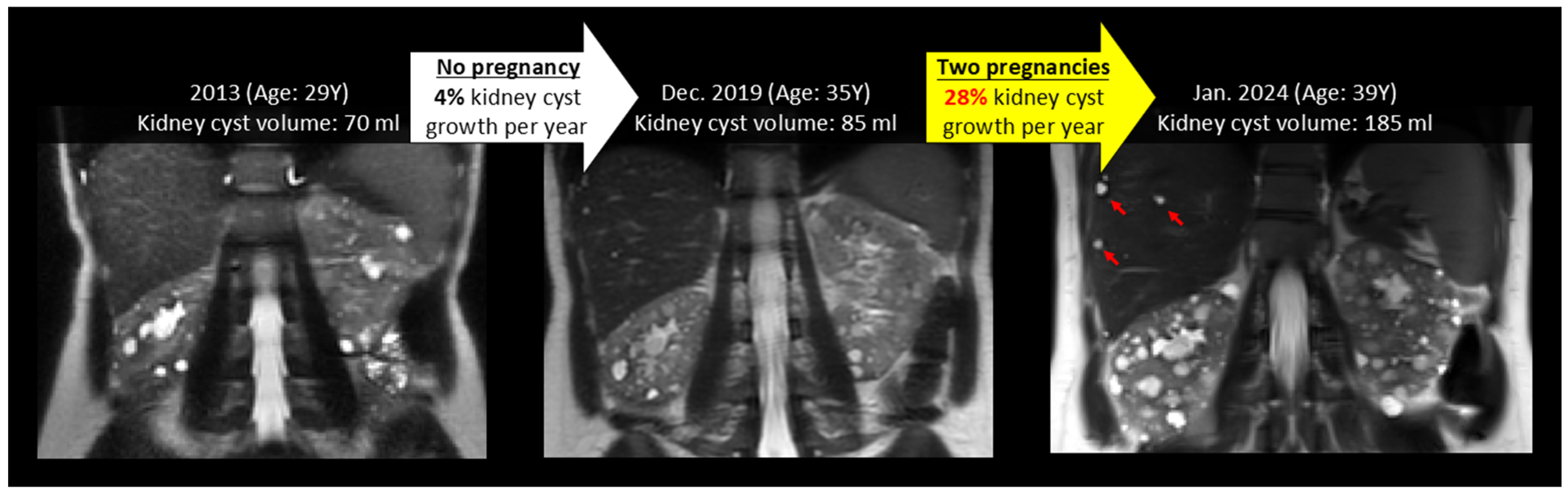
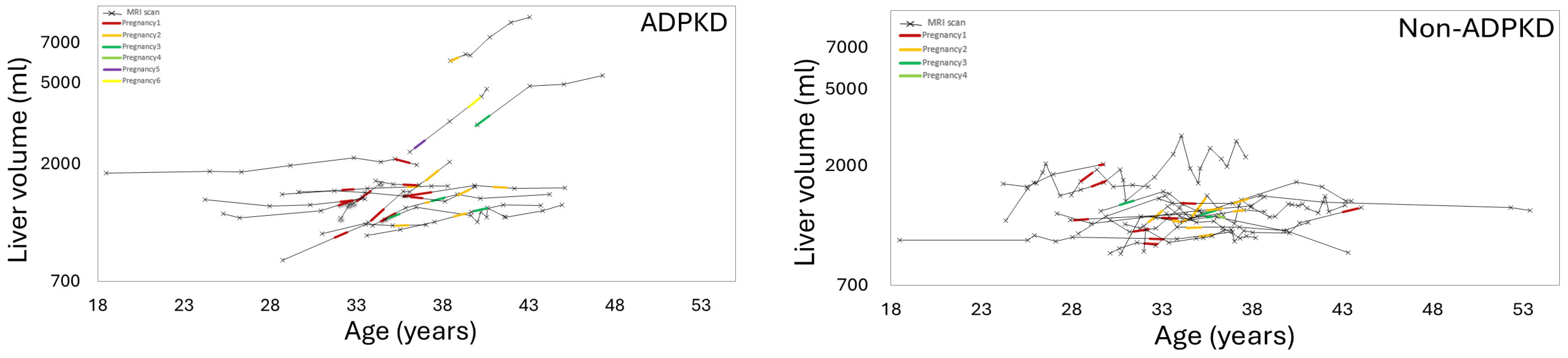
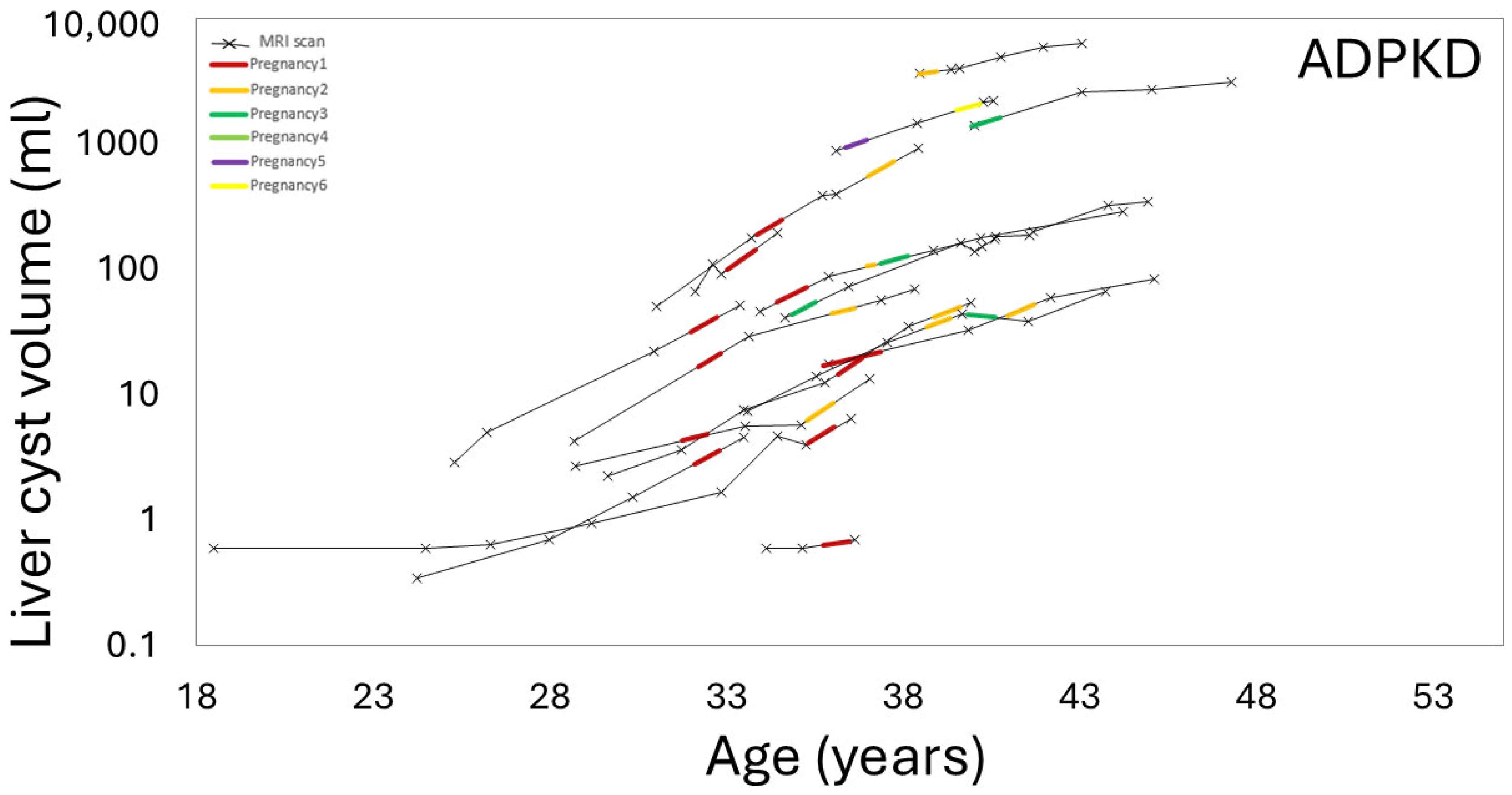
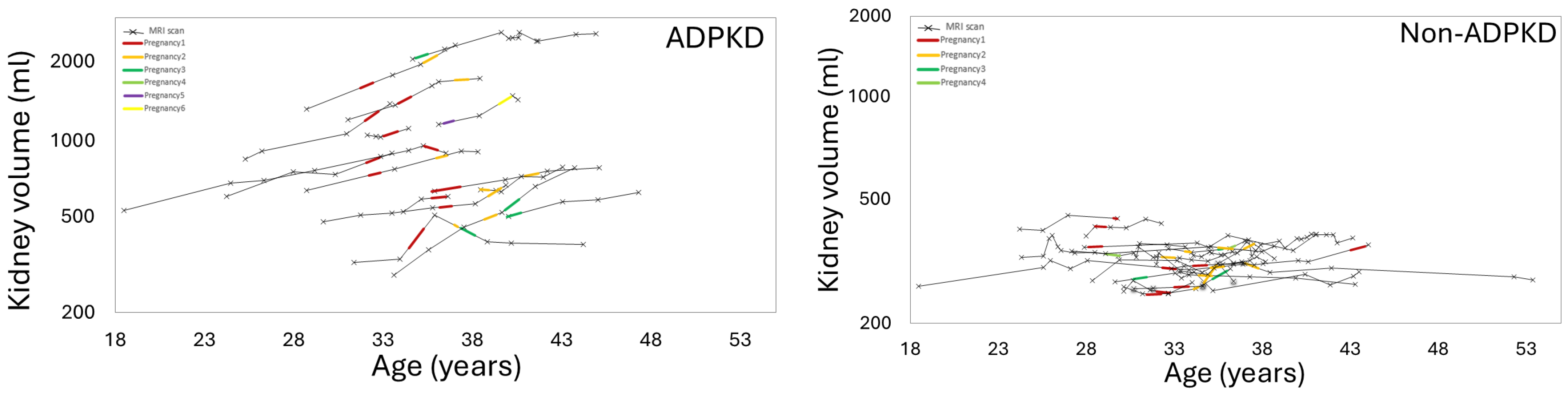
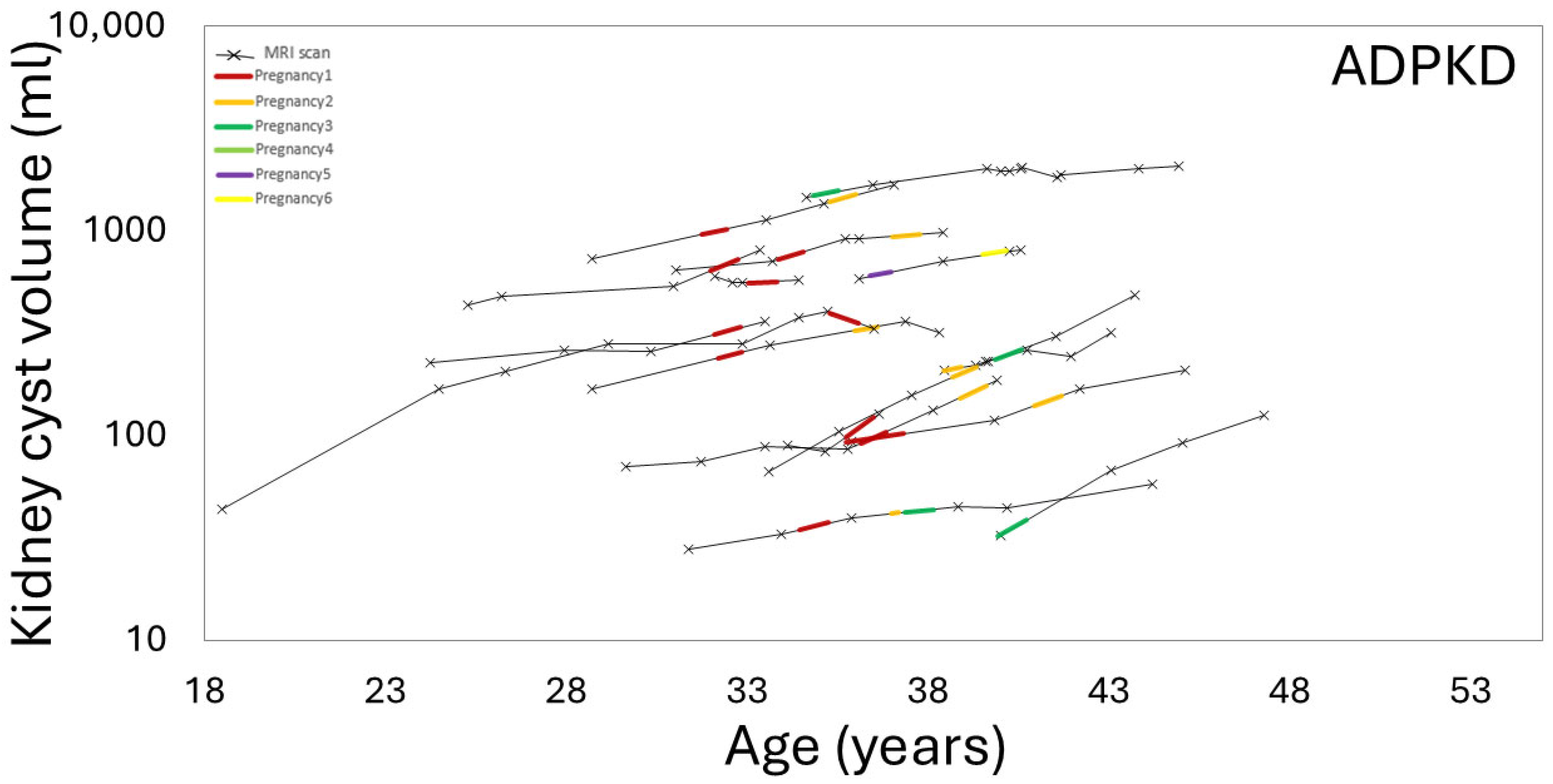
3.3. Effect of Pregnancy on Liver, Kidney, Cyst Growth
3.4. Effect of Gravida and Cumulative Pregnancy Effect on Kidney, Liver, Cysts Growth Rates
3.5. Effect of IVF on Liver, Kidney, and Cyst Growth Rates
3.6. Effect of Pregnancy on Organ Growth in Non-ADPKD Subjects
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADPKD | Autosomal dominant polycystic kidney disease |
| TKV | Total kidney volume |
| Ht-TKV | Height-adjusted total kidney volume |
| Ht-liver cyst volume | Height-adjusted liver cyst volume |
| Ht-total kidney cyst volume | Height-adjusted total kidney cyst volume |
| MRI | Magnetic Resonance Imaging |
| IVF | In vitro fertilization |
| MIC | Mayo imaging classification |
| HIPAA | Health Insurance Portability and Accountability Act |
| BMI | Body mass index |
| SSFSE | Single shot fast spin echo |
| SSFP | Steady-state free precession |
| SD | Standard deviation |
| PLD | Polycystic liver disease |
| LFT | Liver function test |
| GA | Gestational age |
References
- Aung, T.T.; Bhandari, S.K.; Chen, Q.; Malik, F.T.; Willey, C.J.; Reynolds, K.; Jacobsen, S.J.; Sim, J.J. Autosomal Dominant Polycystic Kidney Disease Prevalence among a Racially Diverse United States Population, 2002 through 2018. Kidney360 2021, 2, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Somlo, S. Cyst growth, polycystins, and primary cilia in autosomal dominant polycystic kidney disease. Kidney Res. Clin. Pract. 2014, 33, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Audrézet, M.P.; Chen, J.M.; Hourmant, M.; Morin, M.P.; Perrichot, R.; Charasse, C.; Whebe, B.; Renaudineau, E.; Jousset, P.; et al. Type of PKD1 mutation influences renal outcome in ADPKD. J. Am. Soc. Nephrol. 2013, 24, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Cornec-Le Gall, E.; Alam, A.; Perrone, R.D. Autosomal dominant polycystic kidney disease. Lancet 2019, 393, 919–935. [Google Scholar] [CrossRef]
- Aiello, V.; Ciurli, F.; Conti, A.; Cristalli, C.P.; Lerario, S.; Montanari, F.; Sciascia, N.; Vischini, G.; Fabbrizio, B.; Di Costanzo, R.; et al. DNAJB11 Mutation in ADPKD Patients: Clinical Characteristics in a Monocentric Cohort. Genes 2023, 15, 3. [Google Scholar] [CrossRef]
- Senum, S.R.; Li, Y.S.M.; Benson, K.A.; Joli, G.; Olinger, E.; Lavu, S.; Madsen, C.D.; Gregory, A.V.; Neatu, R.; Kline, T.L.; et al. Monoallelic IFT140 pathogenic variants are an important cause of the autosomal dominant polycystic kidney-spectrum phenotype. Am. J. Hum. Genet. 2022, 109, 136–156. [Google Scholar] [CrossRef]
- Besse, W.; Chang, A.R.; Luo, J.Z.; Triffo, W.J.; Moore, B.S.; Gulati, A.; Hartzel, D.N.; Mane, S.; Torres, V.E.; Somlo, S.; et al. ALG9 Mutation Carriers Develop Kidney and Liver Cysts. J. Am. Soc. Nephrol. 2019, 30, 2091–2102. [Google Scholar] [CrossRef]
- Bae, K.T.; Zhu, F.; Chapman, A.B.; Torres, V.E.; Grantham, J.J.; Guay-Woodford, L.M.; Baumgarten, D.A.; King, B.F., Jr.; Wetzel, L.H.; Kenney, P.J.; et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin. J. Am. Soc. Nephrol. 2006, 1, 64–69. [Google Scholar] [CrossRef]
- Al Sayyab, M.; Chapman, A. Pregnancy in Autosomal Dominant Polycystic Kidney Disease. Adv. Kidney Dis. Health 2023, 30, 454–460. [Google Scholar] [CrossRef]
- Aapkes, S.E.; Bernts, L.H.P.; Barten, T.R.M.; van den Berg, M.; Gansevoort, R.T.; Drenth, J.P.H. Estrogens in polycystic liver disease: A target for future therapies? Liver Int. 2021, 41, 2009–2019. [Google Scholar] [CrossRef]
- Porter, L.E.; Elm, M.S.; Van Thiel, D.H.; Dugas, M.C.; Eagon, P.K. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology 1983, 84, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.Q.; Vesco, K.K.; Purnell, J.Q.; Francisco, M.; Goddard, E.; Guan, X.; DeBarber, A.; Leo, M.C.; Baetscher, E.; Rooney, W.; et al. Pregnancy and weaning regulate human maternal liver size and function. Proc. Natl. Acad. Sci. USA 2021, 118, e2107269118. [Google Scholar] [CrossRef] [PubMed]
- Aapkes, S.E.; Bernts, L.H.P.; van den Berg, M.; Gansevoort, R.T.; Drenth, J.P.H. Tamoxifen for the treatment of polycystic liver disease: A case report. Medicine 2021, 100, e26797. [Google Scholar] [CrossRef] [PubMed]
- van Aerts, R.M.M.; van de Laarschot, L.F.M.; Banales, J.M.; Drenth, J.P.H. Clinical management of polycystic liver disease. J. Hepatol. 2018, 68, 827–837. [Google Scholar] [CrossRef]
- Neijenhuis, M.K.; Kievit, W.; Verheesen, S.M.; D’Agnolo, H.M.; Gevers, T.J.; Drenth, J.P. Impact of liver volume on polycystic liver disease-related symptoms and quality of life. United Eur. Gastroenterol. J. 2018, 6, 81–88. [Google Scholar] [CrossRef]
- Torres, V.E.; Ahn, C.; Barten, T.R.M.; Brosnahan, G.; Cadnapaphornchai, M.A.; Chapman, A.B.; Cornec-Le Gall, E.; Drenth, J.P.H.; Gansevoort, R.T.; Harris, P.C.; et al. KDIGO 2025 clinical practice guideline for the evaluation, management, and treatment of autosomal dominant polycystic kidney disease (ADPKD): Executive summary. Kidney Int. 2025, 107, 234–254. [Google Scholar] [CrossRef]
- Pei, Y.; Obaji, J.; Dupuis, A.; Paterson, A.D.; Magistroni, R.; Dicks, E.; Parfrey, P.; Cramer, B.; Coto, E.; Torra, R.; et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009, 20, 205–212. [Google Scholar] [CrossRef]
- He, X.; Hu, Z.; Dev, H.; Romano, D.J.; Sharbatdaran, A.; Raza, S.I.; Wang, S.J.; Teichman, K.; Shih, G.; Chevalier, J.M.; et al. Test Retest Reproducibility of Organ Volume Measurements in ADPKD Using 3D Multimodality Deep Learning. Acad. Radiol. 2024, 31, 889–899. [Google Scholar] [CrossRef]
- Gabow, P.A.; Johnson, A.M.; Kaehny, W.D.; Manco-Johnson, M.L.; Duley, I.T.; Everson, G.T. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology 1990, 11, 1033–1037. [Google Scholar] [CrossRef]
- Everson, G.T. Hepatic Cysts in Autosomal Dominant Polycystic Kidney Disease. Am. J. Kidney Dis. 1993, 22, 520–525. [Google Scholar] [CrossRef]
- Chebib, F.T.; Jung, Y.; Heyer, C.M.; Irazabal, M.V.; Hogan, M.C.; Harris, P.C.; Torres, V.E.; El-Zoghby, Z.M. Effect of genotype on the severity and volume progression of polycystic liver disease in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transpl. 2016, 31, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T.; Tao, C.; Feldman, R.; Yu, A.S.L.; Torres, V.E.; Perrone, R.D.; Chapman, A.B.; Brosnahan, G.; Steinman, T.I.; Braun, W.E.; et al. Volume Progression and Imaging Classification of Polycystic Liver in Early Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Sherstha, R.; McKinley, C.; Russ, P.; Scherzinger, A.; Bronner, T.; Showalter, R.; Everson, G.T. Postmenopausal estrogen therapy selectively stimulates hepatic enlargement in women with autosomal dominant polycystic kidney disease. Hepatology 1997, 26, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Abebe, K.; Torres, V.E.; Chapman, A.B.; Bae, K.T.; Tao, C.; Sun, H.; Perrone, R.D.; Steinman, T.I.; Braun, W.; et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin. Gastroenterol. Hepatol. 2015, 13, 155–164.e6. [Google Scholar] [CrossRef]
- Petrone, M.; Catania, M.; De Rosa, L.I.; Degliuomini, R.S.; Kola, K.; Lupi, C.; Brambilla Pisoni, M.; Salvatore, S.; Candiani, M.; Vezzoli, G.; et al. Role of Female Sex Hormones in ADPKD Progression and a Personalized Approach to Contraception and Hormonal Therapy. J. Clin. Med. 2024, 13, 1257. [Google Scholar] [CrossRef]
- Campbell, R.E.; Edelstein, C.L.; Chonchol, M. Overview of ADPKD in Pregnancy. Kidney Int. Rep. 2025, 10, 1011–1019. [Google Scholar] [CrossRef]
- Zheng, B.; Gitomer, B.; Nowak, K.; You, Z.; Chonchol, M. Pregnancy and Its Association with Total Kidney Volume in Nulliparous Women with Autosomal Dominant Polycystic Kidney Disease: TH-PO444. J. Am. Soc. Nephrol. 2023, 34, 214. [Google Scholar] [CrossRef]
| ADPKD (n = 16) | Non-ADPKD (n = 16) | p-Value | |
|---|---|---|---|
| Age at the latest scan (yr) | 40 ± 5 | 39 ± 5 | 0.78 |
| Age at the first pregnancy (yr) | 35 ± 2 | 31 ± 5 | 0.04 |
| Race | 0.45 | ||
| White | 12 (75%) | 10 (63%) | |
| Asian | 3 (19%) | 2 (12%) | |
| Others or Declined | 1 (6%) | 4 (25%) | |
| Height (m) | 1.64 ± 0.08 | 1.62 ± 0.08 | 0.49 |
| Weight (kg) | 69 ± 11 | 63 ± 12 | 0.16 |
| BMI (kg/m2) | 25 (22, 27) | 23 (21, 26) | 0.27 |
| Underwent IVF (# of patients) | 9 (56%) | 2 (12%) | 0.01 |
| CKD stage at the time of the latest MRI | 0.008 | ||
| Stage G1 | 6 (37%) | 14 (87%) | |
| Stage G2 | 8 (50%) | 2 (13%) | |
| Stage G3a | 2 (13%) | 0 (0%) | |
| Stage G3b | 0 (0%) | 0 (0%) | |
| Aspartate Aminotransferase (mg/dL) | 19 (17, 22) | 24 (19, 27) | 0.16 |
| Alanine Aminotransferase (mg/dL) | 15 (12, 19) | 17 (14, 30) | 0.26 |
| Gravida number | 2 (2, 3) | 2 (1, 2) | 0.52 |
| Scans per study period | |||
| During a period including pregnancy | 42 | 41 | N/A |
| During a period of non-pregnancy | 60 | 122 | N/A |
| Interval (days) | |||
| Between MRI exams | 693 (393, 906) | 330 (180, 526) | <0.001 |
| During the period including pregnancy(ies) | 810 (675, 1092 *) | 715 (480, 1219) | 0.52 |
| During the period of non-pregnancy | 578 (320, 848) | 259 (145, 439) | <0.001 |
| Between the nearest pre-pregnancy MRI and delivery | 489 (325, 790) | 493 (375, 774) | 0.99 |
| Between delivery and the nearest post-delivery MRI | 254 (173, 399) | 266 (95, 393) | 0.51 |
| Annual Growth Rate (%/yr) | ||||
|---|---|---|---|---|
| During a Period Including Pregnancy | During Non-Pregnancy Period | p-Value | ||
| Liver volume | ADPKD | 6 [2, 7] | 0.3 [−0.4, 2] | 0.04 |
| Non-ADPKD | 3 (1, 10) | 1 (−2, 3) | 0.06 | |
| p-value | 0.78 | 0.93 | ||
| Total kidney volume | ADPKD | 6 [4, 8] | 3 [0.8, 5] | 0.14 |
| Non-ADPKD | 0.1 (−0.8, 2) | 0.5 (−0.4, 1) | 0.95 | |
| p-value | 0.002 | 0.11 | ||
| Liver cyst volume | ADPKD | 34 ± 16 | 23 ± 17 | 0.005 |
| Kidney cyst volume | ADPKD | 12 ± 11 | 4 ± 9 | 0.05 |
| Annual Growth Rate (%/yr) | 1st Pregnancy (n = 11) | 2nd Pregnancy (n = 7 *) | ≥3rd Pregnancy (n = 6 **) | p-Value |
|---|---|---|---|---|
| Liver volume | 5 ± 6 | 4 ± 6 | 9 ± 5 | 0.21 |
| Liver cyst volume | 38 ± 17 | 36 ± 19 | 20 ± 15 | 0.11 |
| Total kidney volume | 6 ± 7 | 6 ± 6 | 5 ± 7 | 0.92 |
| Kidney cyst volume | 11 ± 11 | 15 ± 12 | 12 ± 8 | 0.75 |
| Annual Growth Rate (%/yr) | Pre 1st Pregnancy (n = 9) | Post 1st Pregnancy (n = 4) | Post 2nd Pregnancy (n = 3) | Post ≥ 3rd Pregnancies (n = 5) | p-Value |
|---|---|---|---|---|---|
| Liver volume | −0.3 (−1, 1) | 1 ± 2 | 4 ± 6 | 0.3 ± 2 | 0.52 |
| Liver cyst volume | 37 ± 29 | 20 ± 20 | 13 ± 9 | 15 ± 9 | 0.13 |
| Total kidney volume | 4 ± 4 | 8 ± 4 | 2 ± 3 | −0.3 ± 7 | 0.10 |
| Kidney cyst volume | 2 ± 7 | 15 ± 12 | 8 ± 20 | 10 ± 9 | 0.22 |
| Annual Growth Rate (%/yr) | IVF-Induced Pregnancy * (5 Pregnancies from 4 ADPKD Subjects) | Naturally Conceived Pregnancy (21 Pregnancies from 12 ADPKD Subjects) | p-Value |
|---|---|---|---|
| Liver volume | |||
| During a period including pregnancy | 3 ± 7 | 6 ± 6 | 0.33 |
| During non-pregnancy | 0.1 (−1.3, 7) | 0.2 (−0.4, 3) | 0.75 |
| Liver cyst volume | |||
| During a period including pregnancy | 38 ± 22 | 29 ± 17 | 0.53 |
| During non-pregnancy | 7 (0.8, 27) | 21 (10, 31) | 0.37 |
| Total kidney volume | |||
| During a period including pregnancy | 4 ± 6 | 6 ± 7 | 0.41 |
| During non-pregnancy | 5 ± 6 | 3 ± 5 | 0.63 |
| Total kidney cyst volume | |||
| During a period including pregnancy | 9 (2, 12) | 12 (8, 16) | 0.41 |
| During non-pregnancy | 2 ± 13 | 8 ± 11 | 0.27 |
| Annual Growth Rate (%/yr) | |||
|---|---|---|---|
| During a Period Including Pregnancy (n = 16) | During Non-Pregnancy Period (n = 16) | p-Value | |
| Liver volume | 3 (1, 10) | 1 (−2, 3) | 0.06 |
| Total kidney volume | 0.1 (−0.8, 2) | 0.5 (−0.4, 1) | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazojoo, V.; Davoudi, V.; Blumenfeld, J.D.; Zhu, C.; Malha, L.; Lo, G.C.; Chevalier, J.M.; Shimonov, D.; Sharbatdaran, A.; Dev, H.; et al. Effects of Pregnancy on Liver and Kidney Cyst Growth Rates in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. J. Clin. Med. 2025, 14, 3688. https://doi.org/10.3390/jcm14113688
Bazojoo V, Davoudi V, Blumenfeld JD, Zhu C, Malha L, Lo GC, Chevalier JM, Shimonov D, Sharbatdaran A, Dev H, et al. Effects of Pregnancy on Liver and Kidney Cyst Growth Rates in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. Journal of Clinical Medicine. 2025; 14(11):3688. https://doi.org/10.3390/jcm14113688
Chicago/Turabian StyleBazojoo, Vahid, Vahid Davoudi, Jon D. Blumenfeld, Chenglin Zhu, Line Malha, Grace C. Lo, James M. Chevalier, Daniil Shimonov, Arman Sharbatdaran, Hreedi Dev, and et al. 2025. "Effects of Pregnancy on Liver and Kidney Cyst Growth Rates in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study" Journal of Clinical Medicine 14, no. 11: 3688. https://doi.org/10.3390/jcm14113688
APA StyleBazojoo, V., Davoudi, V., Blumenfeld, J. D., Zhu, C., Malha, L., Lo, G. C., Chevalier, J. M., Shimonov, D., Sharbatdaran, A., Dev, H., Raza, S. I., Hu, Z., He, X., RoyChoudhury, A., & Prince, M. R. (2025). Effects of Pregnancy on Liver and Kidney Cyst Growth Rates in Autosomal Dominant Polycystic Kidney Disease: A Pilot Study. Journal of Clinical Medicine, 14(11), 3688. https://doi.org/10.3390/jcm14113688








