Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Procedures
2.3. Outcomes
2.4. Measurements
2.4.1. Nasal Symptoms
2.4.2. Questionnaires
2.4.3. Olfactory Function Testing
2.4.4. Nasal Endoscopy
2.4.5. Computed Tomography Analysis of CRSwNPs
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
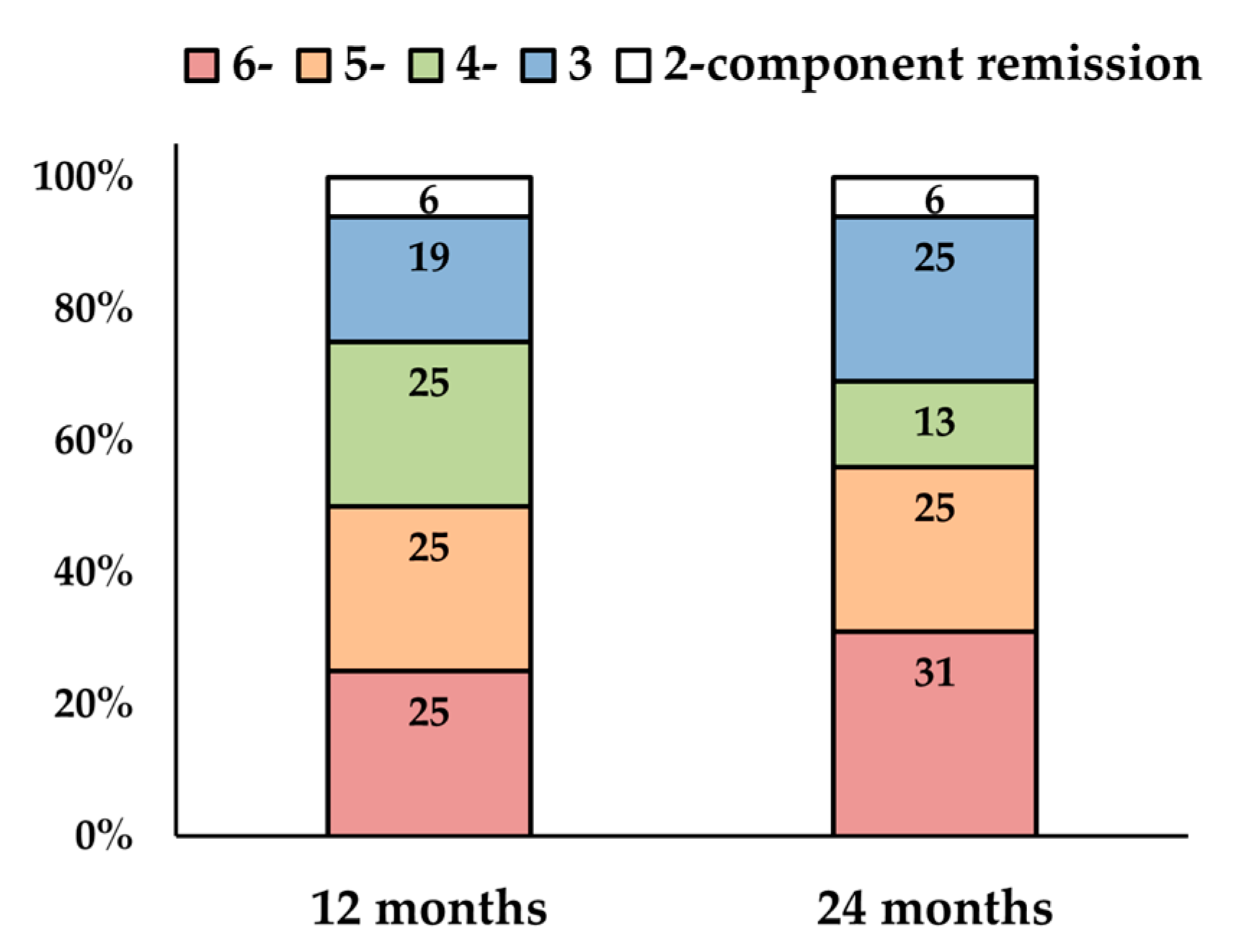
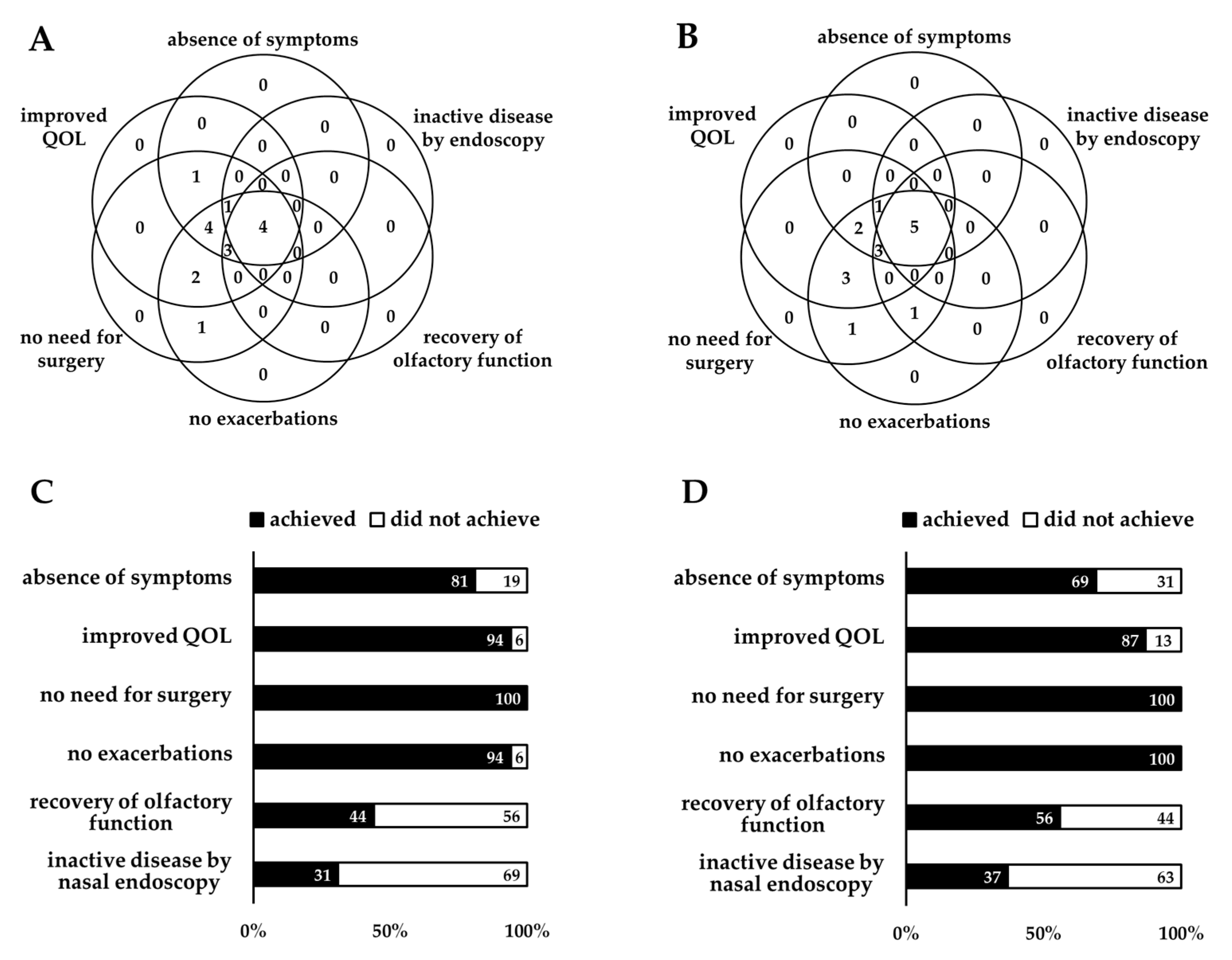
3.2. CRSwNPs Remission Rates
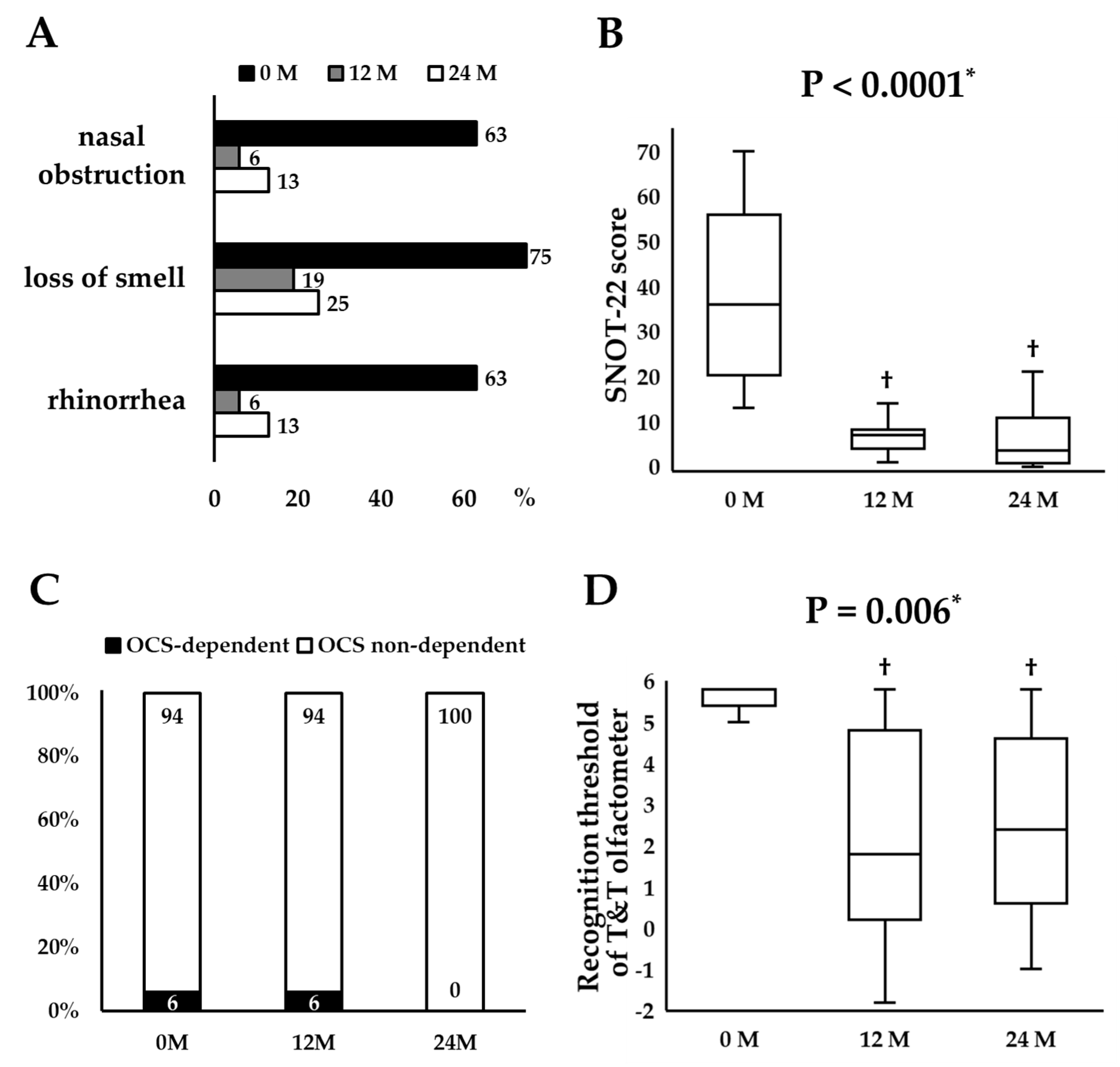
3.3. Longitudinal Changes in Remission Components
3.4. Predictors of CRSwNPs Remission
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACQ-7 | 7-item Asthma Control Questionnaire |
| ANOVA | analysis of variance |
| CRSwNPs | chronic rhinosinusitis with nasal polyps |
| CT | computed tomography |
| EPOS | European Position Paper on Rhinosinusitis and Nasal Polyps |
| ESS | endoscopic sinus surgery |
| EUFOREA | European Forum for Research and Education in Allergy and Airway diseases |
| ICSs | inhaled corticosteroids |
| Ig | immunoglobulin |
| IU | international unit |
| LKS | Lund–Kennedy Endoscopy Scale score |
| LMS | Lund–Mackay computed tomography score |
| n | number |
| N-ERD | non-steroidal anti-inflammatory drug-exacerbated respiratory disease |
| NPS | nasal polyp score |
| OCSs | oral corticosteroids |
| QOL | quality of life |
| SNOT-22 | the 22-item Sinonasal Outcome Test |
References
- Menzies-Gow, A.; Bafadhel, M.; Busse, W.W.; Casale, T.B.; Kocks, J.W.H.; Pavord, I.D.; Szefler, S.J.; Woodruff, P.G.; de Giorgio-Miller, A.; Trudo, F.; et al. An expert consensus framework for asthma remission as a treatment goal. J. Allergy Clin. Immunol. 2020, 145, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicenter, randomized, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, S.; Matsune, S.; Takeno, S.; Asako, M.; Takeuchi, M.; Fujita, H.; Takahashi, Y.; Amin, N.; Deniz, Y.; Rowe, P.; et al. The Effect of Dupilumab on Intractable Chronic Rhinosinusitis with Nasal Polyps in Japan. Laryngoscope 2021, 131, E1770–E1777. [Google Scholar] [CrossRef]
- Maspero, J.F.; Katelaris, C.H.; Busse, W.W.; Castro, M.; Corren, J.; Chipps, B.E.; Peters, A.T.; Pavord, I.D.; Ford, L.B.; Sher, L.; et al. Dupilumab Efficacy in Uncontrolled, Moderate-to-Severe Asthma with Self-Reported Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 527–539.e9. [Google Scholar] [CrossRef]
- Gurnell, M.; Radwan, A.; Bachert, C.; Lugogo, N.; Cho, S.H.; Nash, S.; Zhang, H.; Khan, A.H.; Jacob-Nara, J.A.; Rowe, P.J.; et al. Dupilumab Reduces Asthma Disease Burden and Recurrent SCS Use in Patients with CRSwNP and Coexisting Asthma. J. Asthma Allergy 2024, 17, 1–8. [Google Scholar] [CrossRef]
- De Corso, E.; Pasquini, E.; Trimarchi, M.; La Mantia, I.; Pagella, F.; Ottaviano, G.; Garzaro, M.; Pipolo, C.; Torretta, S.; Seccia, V.; et al. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): A multicentric observational Phase IV real-life study (DUPIREAL). Allergy 2023, 78, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, G.; Saccardo, T.; Roccuzzo, G.; Bernardi, R.; Chicco, A.D.; Pendolino, A.L.; Scarpa, B.; Mairani, E.; Nicolai, P. Effectiveness of Dupilumab in the Treatment of Patients with Uncontrolled Severe CRSwNP: A “Real-Life” Observational Study in Naïve and Post-Surgical Patients. J. Pers. Med. 2022, 12, 1526. [Google Scholar] [CrossRef]
- Galletti, C.; Ragusa, M.; Sireci, F.; Ciodaro, F.; Barbieri, M.A.; Giunta, G.; Grigaliute, E.; Immordino, A.; Lorusso, F.; Dispenza, F.; et al. Dupilumab in chronic rhinosinusitis with nasal polyps: Real life data in a multicentric Sicilian experience. Am. J. Otolaryngol. 2024, 45, 104106. [Google Scholar] [CrossRef]
- Tajiri, T.; Suzuki, M.; Nishiyama, H.; Ozawa, Y.; Kurokawa, R.; Ito, K.; Fukumitsu, K.; Mori, Y.; Kanemitsu, Y.; Fukuda, S.; et al. Efficacy of dupilumab for severe chronic rhinosinusitis with nasal polyps and asthma: A prospective study. Ann. Allergy Asthma Immunol. 2024, 133, 550–558. [Google Scholar] [CrossRef]
- Seys, S.F.; Schneider, S.; de Kinderen, J.; Reitsma, S.; Cavaliere, C.; Tomazic, P.V.; Morgenstern, C.; Mortuaire, G.; Wagenmann, M.; Bettio, G.; et al. Real-world effectiveness of dupilumab in a European cohort of chronic rhinosinusitis with nasal polyps (CHRINOSOR). J. Allergy Clin. Immunol. 2024, 155, 451–460. [Google Scholar] [CrossRef]
- Oykhman, P.; Paramo, F.A.; Bousquet, J.; Kennedy, D.W.; Brignardello-Petersen, R.; Chu, D.K. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: A systematic review and network meta-analysis. J. Allergy Clin. Immunol. 2022, 149, 1286–1295. [Google Scholar] [CrossRef]
- Al-Ahmad, M.; Ali, A.; Talat, W.; Dawood, H.A.; Imam, O. Long-term effects of dupilumab on chronic rhinosinusitis with nasal polyps: A step towards clinical remission. World Allergy Organ. J. 2025, 18, 101024. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.J.; Elzinga, H.E.; van der Lans, R.J.L.; Fokkens, W.J.; Reitsma, S. Almost Half of the Dupilumab-Treated Patients with Severe Chronic Rhinosinusitis with Nasal Polyps Achieve Remission in One Year. Allergy 2025, 80, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; De Corso, E.; Backer, V.; Bernal-Sprekelsen, M.; Bjermer, L.; von Buchwald, C.; Chaker, A.; Diamant, Z.; Gevaert, P.; Han, J.; et al. EPOS2020/EUFOREA expert opinion on defining disease states and therapeutic goals in CRSwNP. Rhinology 2024, 62, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Caminati, M.; De Corso, E.; Ottaviano, G.; Pipolo, C.; Schiappoli, M.; Seccia, V.; Spinelli, F.R.; Savarino, E.V.; Gisondi, P.; Senna, G. Remission in Type 2 Inflammatory Diseases: Current Evidence, Unmet Needs, and Suggestions for Defining Remission in Chronic Rhinosinusitis with Nasal Polyps. Curr. Allergy Asthma Rep. 2024, 24, 11–23. [Google Scholar] [CrossRef]
- Lund, V.J.; Kennedy, D.W. Quantification for staging sinusitis. The Staging and Therapy Group. Ann. Otol. Rhinol. Laryngol. Suppl. 1995, 167, 17–21. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022, 205, 17–35. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; O’Byrne, P.M.; Guyatt, G.H.; Ferrie, P.J.; King, D.R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 1999, 14, 902–907. [Google Scholar] [CrossRef]
- Miwa, T.; Ikeda, K.; Ishibashi, T.; Kobayashi, M.; Kondo, K.; Matsuwaki, Y.; Ogawa, T.; Shiga, H.; Suzuki, M.; Tsuzuki, K.; et al. Clinical practice guidelines for the management of olfactory dysfunction—Secondary publication. Auris Nasus Larynx 2019, 46, 653–662. [Google Scholar] [CrossRef]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitis. Rhinology 1993, 31, 183–184. [Google Scholar] [PubMed]
- Bachert, C.; Han, J.K.; Wagenmann, M.; Hosemann, W.; Lee, S.E.; Backer, V.; Mullol, J.; Gevaert, P.; Klimek, L.; Prokopakis, E.; et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J. Allergy Clin. Immunol. 2021, 147, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Matsuda, T.; Hashiba, M.; Baba, S. A study of the relationship between the T&T olfactometer and the University of Pennsylvania Smell Identification Test in a Japanese population. Am. J. Rhinol. 1998, 12, 353–358. [Google Scholar]
- Tsetsos, N.; Goudakos, J.K.; Daskalakis, D.; Konstantinidis, I.; Markou, K. Monoclonal antibodies for the treatment of chronic rhinosinusitis with nasal polyposis: A systematic review. Rhinology 2018, 56, 11–21. [Google Scholar] [CrossRef]
- Niimi, A.; Fukunaga, K.; Taniguchi, M.; Nakamura, Y.; Tagaya, E.; Horiguchi, T.; Yokoyama, A.; Yamaguchi, M.; Nagata, M. Executive summary: Japanese guidelines for adult asthma (JGL) 2021. Allergol. Int. 2023, 72, 207–226. [Google Scholar] [CrossRef]
- Oishi, K.; Hamada, K.; Murata, Y.; Matsuda, K.; Ohata, S.; Yamaji, Y.; Asami-Noyama, M.; Edakuni, N.; Kakugawa, T.; Hirano, T.; et al. A Real-World Study of Achievement Rate and Predictive Factors of Clinical and Deep Remission to Biologics in Patients with Severe Asthma. J. Clin. Med. 2023, 12, 2900. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J. Executive summary of EPOS 2020 including integrated care pathways. Rhinology 2020, 58, 82–111. [Google Scholar] [CrossRef]
- Mullol, J.; Azar, A.; Buchheit, K.M.; Hopkins, C.; Bernstein, J.A. Chronic Rhinosinusitis with Nasal Polyps: Quality of Life in the Biologics Era. J. Allergy Clin. Immunol. Pract. 2022, 10, 1434–1453.e9. [Google Scholar] [CrossRef]
- Soler, Z.M.; Nash, S.; Lane, A.P.; Patel, Z.M.; Lee, S.E.; Fokkens, W.J.; Corbett, M.; Jacob-Nara, J.A.; Sacks, H. Reduced sense of smell in patients with severe chronic rhinosinusitis and its implications for diagnosis and management: A narrative review. Adv. Ther. 2024, 41, 4384–4395. [Google Scholar] [CrossRef]
- Fujieda, S.; Matsune, S.; Takeno, S.; Ohta, N.; Asako, M.; Bachert, C.; Inoue, T.; Takahashi, Y.; Fujita, H.; Deniz, Y.; et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy 2022, 77, 186–196. [Google Scholar] [CrossRef] [PubMed]
| n = 16 | |
|---|---|
| Age, years | 57 (37–77) |
| Females, n (%) | 9 (56) |
| Body mass index, kg/m2 | 23.0 (17.9–28.9) |
| Smoking status, never/ex, n | 11/5 |
| Duration of CRSwNPs, years | 7 (2–48) |
| Number of patients who underwent ESS, n (%) Number of previous ESS, times | 11 (69) 1 (0–2) |
| Comorbidities, n (%) | |
| Allergic rhinitis | 11 (69) |
| N-ERD | 4 (25) |
| Atopic dermatitis | 3 (19) |
| Concomitant medications, n (%) | |
| Oral corticosteroids | 1 (6) |
| Intranasal corticosteroids | 9 (56) |
| Leukotriene receptor antagonists | 15 (94) |
| Antihistamines | 16 (100) |
| SNOT-22 total scores | 36 (13–70) |
| ACQ-7 total scores | 1.6 (0.1–2.9) |
| T&T olfactometer | |
| Detection thresholds | 5.7 (−1.6 to 5.8) |
| Recognition thresholds | 5.8 (0.6–5.8) |
| Bilateral NPS | 6 (2−8) |
| LMS | 13 (6–23) |
| Blood eosinophil counts, /μL | 583 (363–1159) |
| Blood neutrophil counts, /μL | 4294 (2420–6316) |
| Serum total IgE, IU/mL | 462 (141–16,000) |
| Characteristics at Baseline | Remission (n = 4) | Non-Remission (n = 12) | p Value |
|---|---|---|---|
| Age, years | 51 (38–64) | 56.5 (37–77) | 0.67 † |
| Females, n (%) | 2 (50) | 7 (58) | 0.77 * |
| Body mass index, kg/m2 | 22.7 (17.9–28.9) | 23.0 (18.8–26.3) | 0.95 † |
| Smoking status, never/ex, n | 4/0 | 7/5 | 0.12 * |
| Duration of CRSwNPs, years | 5 (4–17) | 11.5 (2–48) | 0.22 † |
| History of ESS, yes (%) | 3 (75) | 8 (67) | 0.76 * |
| Number of previous ESS, times | 1 (0–1) | 1 (0–2) | 0.79 † |
| Comorbidities, n (%) | |||
| Allergic rhinitis | 2 (50) | 9 (75) | 0.35 * |
| N-ERD | 1 (25) | 3 (25) | 0.99 * |
| Atopic dermatitis | 2 (50) | 1 (8) | 0.06 * |
| Concomitant medications, n (%) | |||
| Oral corticosteroids | 0 (0) | 1 (8) | 0.55 * |
| SNOT-22 total scores | 40.5 (18–68) | 36 (13–70) | 0.67 † |
| ACQ-7 total scores | 1.8 (1.4–2.3) | 1.4 (0.1–2.9) | 0.43 † |
| T&T olfactometer | |||
| Detection thresholds | 0 (−1.6 to 5.8) | 5.8 (4.6–5.8) | 0.06 † |
| Recognition thresholds | 3.6 (0.6–5.8) | 5.8 (5.4–5.8) | 0.01 † |
| Bilateral NPS | 4.5 (2–6) | 6 (2–8) | 0.46 † |
| LMS | 7.5 (6–22) | 14.5 (6–23) | 0.22 † |
| Blood eosinophil counts, /μL | 504 (426–780) | 657 (363–1159) | 0.50 † |
| Blood neutrophil counts, /μL | 4170 (2420–6084) | 4294 (2695–6316) | 0.86 † |
| Serum total IgE, IU/mL | 289 (259–3470) | 547 (141–16,000) | 0.43 † |
| Characteristics at Baseline | Remission (n = 5) | Non-Remission (n = 11) | p Value |
|---|---|---|---|
| Age, years | 43 (38–64) | 57 (37–77) | 0.31 † |
| Females, n (%) | 3 (60) | 6 (55) | 0.84 * |
| Body mass index, kg/m2 | 24.9 (17.9–28.9) | 22.3 (18.8–25.4) | 0.57 † |
| Smoking status, never/ex, n | 5/0 | 6/5 | 0.07 * |
| Duration of CRSwNPs, years | 4.5 (2–17) | 16 (6–48) | 0.04 † |
| History of ESS, yes (%) | 4 (80) | 7 (64) | 0.51 * |
| Number of previous ESS, times | 1 (0–1) | 1 (0–2) | 0.90 * |
| Comorbidities, n (%) | |||
| Allergic rhinitis | 3 (60) | 8 (73) | 0.61 * |
| N-ERD | 2 (40) | 2 (18) | 0.35 * |
| Atopic dermatitis | 2 (40) | 1 (9) | 0.14 * |
| Concomitant medications, n (%) | |||
| Oral corticosteroids | 0 (0) | 1 (9) | 0.49 * |
| SNOT-22 total scores | 59 (18–68) | 33 (13–70) | 0.36 † |
| ACQ-7 total scores | 1.9 (1.4–2.3) | 1.2 (0.1–2.9) | 0.33 † |
| T&T olfactometer | |||
| Detection thresholds | 1 (−1.6 to 5.8) | 5.8 (4.6–5.8) | 0.20 † |
| Recognition thresholds | 5.0 (0.6–5.8) | 5.8 (5.4–5.8) | 0.045 † |
| Bilateral NPS | 6 (2–6) | 6 (2–8) | 0.64 † |
| LMS | 9 (6–22) | 14 (6–23) | 0.61 † |
| Blood eosinophil counts, /μL | 528 (426–851) | 639 (363–1159) | 0.91 † |
| Blood neutrophil counts, /μL | 4431 (2420–6084) | 4158 (2695–6316) | 0.91 † |
| Serum total IgE, IU/mL | 317 (259–3470) | 485 (141–16,000) | 0.82 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajiri, T.; Suzuki, M.; Nishiyama, H.; Ozawa, Y.; Amakusa, Y.; Suzuki, T.; Ito, K.; Mori, Y.; Fukumitsu, K.; Fukuda, S.; et al. Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study. J. Clin. Med. 2025, 14, 3654. https://doi.org/10.3390/jcm14113654
Tajiri T, Suzuki M, Nishiyama H, Ozawa Y, Amakusa Y, Suzuki T, Ito K, Mori Y, Fukumitsu K, Fukuda S, et al. Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study. Journal of Clinical Medicine. 2025; 14(11):3654. https://doi.org/10.3390/jcm14113654
Chicago/Turabian StyleTajiri, Tomoko, Motohiko Suzuki, Hirono Nishiyama, Yoshiyuki Ozawa, Yuki Amakusa, Tatsuro Suzuki, Keima Ito, Yuta Mori, Kensuke Fukumitsu, Satoshi Fukuda, and et al. 2025. "Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study" Journal of Clinical Medicine 14, no. 11: 3654. https://doi.org/10.3390/jcm14113654
APA StyleTajiri, T., Suzuki, M., Nishiyama, H., Ozawa, Y., Amakusa, Y., Suzuki, T., Ito, K., Mori, Y., Fukumitsu, K., Fukuda, S., Kanemitsu, Y., Uemura, T., Ohkubo, H., Takemura, M., Ito, Y., Oguri, T., & Niimi, A. (2025). Dupilumab-Induced Remission in Chronic Rhinosinusitis with Nasal Polyps and Comorbid Asthma: A 24-Month Study. Journal of Clinical Medicine, 14(11), 3654. https://doi.org/10.3390/jcm14113654





