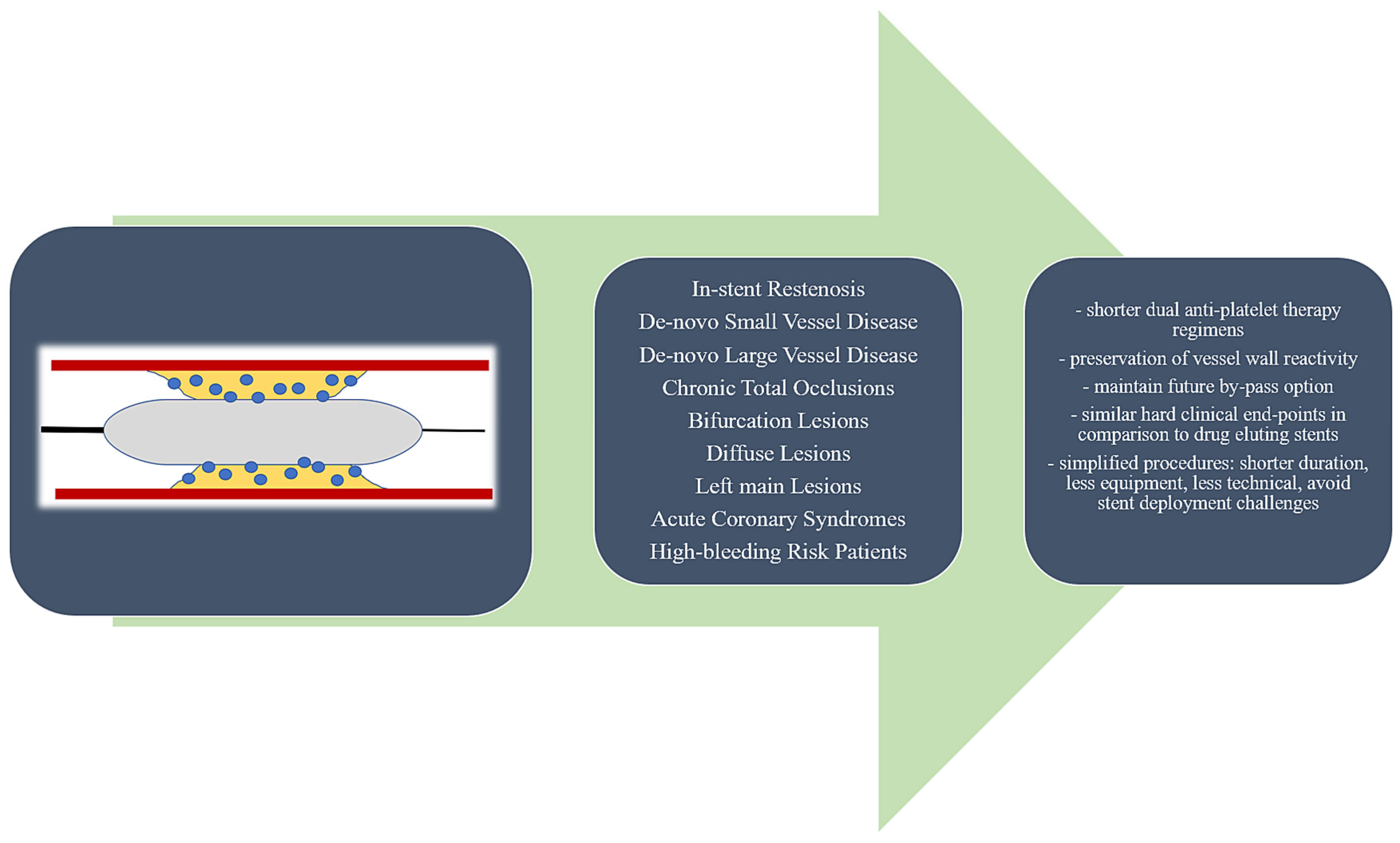
Drug-Coated Balloons in All-Comer Population—Are We There Yet?
Abstract
1. Introduction
2. Dcb in In-Stent Restenosis
2.1. Guideline Recommendations
2.2. Evidence from Major Trials
2.3. DES ISR vs. BMS ISR—Same but Different?
3. Dcb in De Novo Small Vessels
4. Dcb in De Novo Large Vessels
4.1. The “Leave Nothing Behind” Concept—Rationale, Advantages, and Pitfalls
4.2. Evidence from Major Trials
5. DCB in Diffuse Lesions—Alone or as Part of Hybrid Strategy
6. DCB in Left Main (LM)
7. DCB in Acute Coronary Syndromes
8. Dcb in High-Bleeding-Risk (Hbr) Patients
9. DCB in Chronic Total Occlusion
10. DCB in Bifurcation Lesions
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lazar, F.L.; Onea, H.L.; Olinic, D.M.; Cortese, B.A. 2024 scientific update on the clinical performance of drug-coated balloons. AsiaIntervention 2024, 10, 15–25. [Google Scholar] [CrossRef] [PubMed]
- van Werkum Heestermans, A.A.; Zomer, A.C.; Kelder, J.C.; Suttorp, M.J.; Rensing, B.J.; Koolen, J.J.; Brueren, B.R.; Dambrink, J.H.; Hautvast, R.W.; Verheugt, F.W.; et al. Predictors of coronary stent thrombosis: The Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 2009, 53, 1399–1409. [Google Scholar] [CrossRef]
- Cortese, B.; Sanchez-Jimenez, E.; Lazar, L. Coronary stent failure: Role of a blended approach with drug-coated balloons for complex lesions. Minerva Cardiol. Angiol. 2024, 72, 266–280. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention 2019, 14, 1435–1534. [Google Scholar] [CrossRef]
- Latib, A.; Agostoni, P.; Dens, J.; Patterson, M.; Lancellotti, P.; Tam, F.C.C.; Schotborgh, C.; Kedhi, E.; Stella, P.; Shen, C.; et al. PREVAIL Study Investigators. Paclitaxel Drug-Coated Balloon for the Treatment of De Novo Small-Vessel and Restenotic Coronary Artery Lesions: 12-Month Results of the Prospective, Multicenter, Single-Arm PREVAIL Study. J. Invasive Cardiol. 2021, 33, E863–E869. [Google Scholar] [CrossRef]
- Jensen, C.J.; Richardt, G.; Tölg, R.; Erglis, A.; Skurk, C.; Jung, W.; Neumann, F.J.; Stangl, K.; Brachmann, J.; Fischer, D.; et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: The BIOLUX randomised controlled trial. EuroIntervention 2018, 14, 1096–1103. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; ESC Scientific Document Group. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Giacoppo, D.; Alvarez-Covarrubias, H.A.; Koch, T.; Cassese, S.; Xhepa, E.; Kessler, T.; Wiebe, J.; Joner, M.; Hochholzer, W.; Laugwitz, K.L.; et al. Coronary artery restenosis treatment with plain balloon, drug-coated balloon, or drug-eluting stent: 10-year outcomes of the ISAR-DESIRE 3 trial. Eur. Heart J. 2023, 44, 1343–1357. [Google Scholar] [CrossRef]
- Kufner, S.; Cassese, S.; Valeskini, M.; Neumann, F.J.; Schulz-Schüpke, S.; Hoppmann, P.; Fusaro, M.; Schunkert, H.; Laugwitz, K.L.; Kastrati, A.; et al. ISAR-DESIRE 3 Investigators. Long-Term Efficacy and Safety of Paclitaxel-Eluting Balloon for the Treatment of Drug-Eluting Stent Restenosis: 3-Year Results of a Randomized Controlled Trial. JACC Cardiovasc. Interv. 2015, 8, 877–884. [Google Scholar] [CrossRef]
- Elgendy, I.Y.; Mahmoud, A.N.; Elgendy, A.Y.; Mojadidi , M.K.; Elbadawi, A.; Eshtehardi, P.; Perez-Vizcayno, M.J.; Wayangankar, S.A.; Jneid, H.; Anderson, R.D.; et al. Drug-Eluting Balloons Versus Everolimus-Eluting Stents for In-Stent Restenosis: A Meta-Analysis of Randomized Trials. Cardiovasc. Revasc. Med. 2019, 20, 612–618. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.P.M.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients With Coronary Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Rittger, H.; Waliszewski, M.; Brachmann, J.; Hohenforst-Schmidt, W.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Schlundt, C.; et al. Long-Term Outcomes After Treatment With a Paclitaxel-Coated Balloon Versus Balloon Angioplasty: Insights From the PEPCAD-DES Study (Treatment of Drug-eluting Stent [DES] In-Stent Restenosis With SeQuent Please Paclitaxel-Coated Percutaneous Transluminal Coronary Angioplasty [PTCA] Catheter). JACC Cardiovasc. Interv. 2015, 8, 1695–1700. [Google Scholar] [PubMed]
- Zhu, Y.; Liu, K.; Kong, X.; Nan, J.; Gao, A.; Liu, Y.; Han, H.; Li, H.; Zhu, H.; Zhang, J.; et al. Comparison of Drug-Coated Balloon Angioplasty vs. Drug-Eluting Stent Implantation for Drug-Eluting Stent Restenosis in the Routine Clinical Practice: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 766088. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.K.; Lee, S.Y. Differential Effects of Drug-Coated Balloon Angioplasty for In-Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2679–2681. [Google Scholar] [CrossRef]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef]
- Cortese, B.; Testa, G.; Rivero, F.; Erriquez, A.; Alfonso, F. Long-Term Outcome of Drug-Coated Balloon vs Drug-Eluting Stent for Small Coronary Vessels: PICCOLETO-II 3-Year Follow-Up. JACC Cardiovasc. Interv. 2023, 16, 1054–1061. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; BASKET-SMALL 2 Investigators; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef]
- Fezzi, S.; Giacoppo, D.; Fahrni, G.; Latib, A.; Alfonso, F.; Colombo, A.; Mahfoud, F.; Scheller, B.; Jeger, R.; Cortese, B.; et al. Individual patient data meta-analysis of paclitaxel-coated balloons vs. drug-eluting stents for small-vessel coronary artery disease: The ANDROMEDA study. Eur. Heart J. 2025, 21, ehaf002. [Google Scholar] [CrossRef]
- di Palma, G.; Sanchez-Jimenez, E.F.; Lazar, L.; Cortese, B. Should paclitaxel be considered an old generation DCB? The limus era. Rev. Cardiovasc. Med. 2021, 22, 1323–1330. [Google Scholar] [CrossRef]
- El Khoury, A.; Lazar, L.; Cortese, B. The fate of coronary dissections left after sirolimus-coated balloon angioplasty: A prespecified subanalysis of the EASTBOURNE study. Catheter. Cardiovasc. Interv. 2023, 102, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.S.; Alfonso, F.; Latib, A.; Ong, P.J.; International DCB Consensus Group; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.F.; Ge, Z.; Kong, X.Q.; Chen, X.; Han, L.; Qian, X.S.; Zuo, G.F.; Wang, Z.M.; Wang, J.; ULTIMATE III Investigators; et al. Intravascular Ultrasound vs Angiography-Guided Drug-Coated Balloon Angioplasty: The ULTIMATE III Trial. JACC Cardiovasc. Interv. 2024, 17, 1519–1528. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.; Wang, J.; Li, Z.; Shi, Y.; Wang, G.; Yan, B.; Wang, Z.; Shi, H.; Zhao, N.; et al. Optical coherence tomography-guided drug coated balloon in non-small de novo coronary artery lesions: A prospective clinical research. Am. J. Transl. Res. 2021, 13, 11617–11624. [Google Scholar]
- Zhao, K.; Guo, Q.; Zhao, Z.; Tang, H.; You, R.; Peng, L.; Rao, L.; Li, M. Clinical value of drug-coated balloon versus second-generation drug-eluting stent for de novo lesions in large coronary arteries: Insights from the real world. BMC Cardiovasc. Disord. 2024, 24, 697. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Ji, F.; Zhang, W.; Yang, C.; Xu, F.; Wang, F. A Non-inferiority, Randomized Clinical Trial Comparing Paclitaxel-Coated Balloon Versus New-Generation Drug-Eluting Stents on Angiographic Outcomes for Coronary De Novo Lesions. Cardiovasc. Drugs Ther. 2022, 36, 655–664. [Google Scholar] [CrossRef]
- Hu, F.W.; Chang, S.; Li, Q.; Zhu, Y.X.; Wang, X.Y.; Cheng, Y.W.; Zhou, Q.H.; Liu, B.; Iqbal, J.; Tang, X.X.; et al. Long-Term Clinical Outcomes After Percutaneous Coronary Intervention With Drug-Coated Balloon-Only Strategy in de novo Lesions of Large Coronary Arteries. Front. Cardiovasc. Med. 2022, 9, 882303. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, X.T.; Chen, R.R. Comparison of Efficacy and Safety Between Drug-Coated Balloons Versus Drug-Eluting Stents in the Treatment of De Novo Coronary Lesions in Large Vessels: A Study-Level Meta-Analysis of Randomized Control Trials. Cardiovasc. Drugs Ther. 2024, 38, 1375–1384. [Google Scholar] [CrossRef]
- Leone, P.P.; Oliva, A.; Regazzoli, D.; Gitto, M.; Novelli, L.; Cozzi, O.; Stefanini, G.G.; Rossi, M.L.; Sticchi, A.; Tartaglia, F.; et al. Immediate and follow-up outcomes of drug-coated balloon angioplasty in de novo long lesions on large coronary arteries. EuroIntervention 2023, 19, e923–e925. [Google Scholar] [CrossRef]
- Basavarajaiah, S.; Latib, A.; Shannon, J.; Naganuma, T.; Sticchi, A.; Bertoldi, L.; Costopoulos, C.; Figini, F.; Carlino, M.; Chieffo, A.; et al. Drug-eluting balloon in the treatment of in-stent restenosis and diffuse coronary artery disease: Real-world experience from our registry. J. Interv. Cardiol. 2014, 27, 348–355. [Google Scholar] [CrossRef]
- Basavarajaiah, S.; Athukorala, S.; Kalogeras, K.; Panoulas, V.; Loku Waduge, B.H.; Bhatia, G.; Watkin, R.; Pulikal, G.; Lee, K.; Ment, J.; et al. Mid-term clinical outcomes from use of Sirolimus coated balloon in coronary intervention; data from real world population. Catheter. Cardiovasc. Interv. 2021, 98, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qiao, S.; Cui, J.; Yuan, J.; Yang, W.; Liu, R.; Wang, T.; Guan, H.; Tian, T.; Zhu, F.; et al. Drug-eluting stent and drug-coated balloon for the treatment of de novo diffuse coronary artery disease lesions: A retrospective case series study. Clin. Cardiol. 2023, 46, 1511–1518. [Google Scholar] [CrossRef]
- Costopoulos, C.; Latib, A.; Naganuma, T.; Sticchi, A.; Figini, F.; Basavarajaiah, S.; Carlino, M.; Chieffo, A.; Montorfano, M.; Naim, C.; et al. The role of drug-eluting balloons alone or in combination with drug-eluting stents in the treatment of de novo diffuse coronary disease. JACC Cardiovasc. Interv. 2013, 6, 1153–1159. [Google Scholar] [CrossRef]
- Iwańczyk, S.; Lazar, F.L.; Onea, H.L.; Pesenti, N.; Wańha, W.; Woźniak, P.; Gościniak, W.; Prof, M.L.; Cortese, B. Sirolimus-coated balloon versus drug-eluting stent for complex coronary lesions. A propensity matched comparison. Int. J. Cardiol. 2024, 415, 132436. [Google Scholar] [CrossRef]
- Buono, A.; Pellicano, M.; Regazzoli, D.; Donahue, M.; Tedeschi, D.; Loffi, M.; Zimbardo, G.; Reimers, B.; Danzi, G.; DEBlasio, G.; et al. Procedural and one-year outcomes following drug-eluting stent and drug-coated balloon combination for the treatment of de novo diffuse coronary artery disease: The HYPER Study. Minerva Cardioangiol. 2024, 72, 163–171. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Wickramarachchi, U.; Richardson, P.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; Ryding, A.; et al. Long-term safety of paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease: The SPARTAN DCB study. Clin. Res. Cardiol. 2021, 110, 220–227. [Google Scholar] [CrossRef]
- Yang, X.; Lu, W.; Pan, L.; Han, Z.; Pan, S.; Wang, X.; Zhu, Y.; Shan, Y.; Peng, M.; Qin, P.; et al. Long-term outcomes of drug-coated balloons in patients with diffuse coronary lesions. Front. Cardiovasc. Med. 2022, 9, 935263. [Google Scholar] [CrossRef]
- Pan, L.; Lu, W.; Han, Z.; Pan, S.; Wang, X.; Shan, Y.; Wang, X.; Zheng, X.; Li, R.; Zhou, Y.; et al. Clinical Outcomes of Drug-Coated Balloon in Coronary Patients with and without Diabetes Mellitus: A Multicenter, Propensity Score Study. J. Diabetes Res. 2021, 2021, 5495219. [Google Scholar] [CrossRef]
- Stone, G.W.; Kappetein, A.P.; Sabik, J.F.; Pocock, S.J.; Morice, M.C.; Puskas, J.; Kandzari, D.E.; Karmpaliotis, D.; Brown, W.M.; EXCEL Trial Investigators; et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N. Engl. J. Med. 2019, 381, 1820–1830, Erratum in N. Engl. J. Med. 2020, 382, 1078. [Google Scholar] [CrossRef]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.A.; Trovik, T.; Kellerth, T.; Kalinauskas, G.; NOBLE investigators; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020, 395, 191–199. [Google Scholar] [CrossRef]
- Erdoğan, E.; Li, Z.; Zhu, Y.X.; Tufaro, V.; Feng, S.L.; Li, Q.; Liang, L.; Chang, S.; Bu, L.T.; Liu, B.; et al. DCB combined with provisional DES implantation in the treatment of De Novo Medina 0,1,0 or 0,0,1 left main coronary bifurcation lesions: A proof-of-concept study. Anatol. J. Cardiol. 2022, 26, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tao, H.; Han, X.; Lu, Y.; Xue, X.; Feng, R.; Lv, F.; Liu, Y.; Jin, H.; Li, L.; et al. Improved Outcomes of Combined Main Branch Stenting and Side Branch Drug-Coated Balloon versus Two-Stent Strategy in Patients with Left Main Bifurcation Lesions. J. Interv. Cardiol. 2022, 2022, 8250057. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lu, W.; Han, Z.; Pan, S.; Wang, X.; Shan, Y.; Peng, M.; Qin, X.; Sun, G.; Zhang, P.; et al. Drug-coated balloon in the treatment of coronary left main true bifurcation lesion: A patient-level propensity-matched analysis. Front. Cardiovasc. Med. 2022, 9, 1028007. [Google Scholar] [CrossRef]
- Uskela, S.; Eranti, A.; Kärkkäinen, J.M.; Rissanen, T.T. Drug-coated balloon-only strategy for percutaneous coronary intervention of de novo left main coronary artery disease: The importance of proper lesion preparation. Front. Med. 2023, 17, 75–84. [Google Scholar] [CrossRef]
- Gunawardena, T.D.; Corballis, N.; Merinopoulos, I.; Wickramarachchi, U.; Reinhold, J.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; et al. Drug-Coated Balloon vs. Drug-Eluting Stents for De Novo Unprotected Left Main Stem Disease: The SPARTAN-LMS Study. J. Cardiovasc. Dev. Dis. 2023, 10, 84. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Lu, Y.; Zhou, S.; Zhang, Y.; Zhao, J.; Yang, H.; Xing, J.; Feng, R.; Xue, X.; et al. The Drug Coated Balloon-Only Strategy for Treatment of de Novo Left Main Coronary Artery Bifurcation Lesion: Stentless Strategy. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221118489. [Google Scholar] [CrossRef]
- Kitani, S.; Igarashi, Y.; Tsuchikane, E.; Nakamura, S.; Koshida, R.; Habara, M.; Tan, M.; Shimoji, K.; Takaya, T.; Kijima, M. Long-Term Clinical Outcomes of Drug-Coated Balloon Following Directional Coronary Atherectomy for Bifurcated or Ostial Lesions in the DCA/DCB Registry. Catheter. Cardiovasc. Interv. 2025, 105, 273–279. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawamori, H.; Toba, T.; Kakizaki, S.; Nakamura, K.; Fujimoto, D.; Sasaki, S.; Fujii, H.; Hamana, T.; Osumi, Y.; et al. Clinical impact of optical coherence tomography findings after drug-coated balloon treatment for patients with acute coronary syndromes. Int. J. Cardiol. 2023, 387, 131149. [Google Scholar] [CrossRef]
- Scheller, B.; Ohlow, M.A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Böhm, M.; et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: The randomised PEPCAD NSTEMI trial. EuroIntervention 2020, 15, 1527–1533. [Google Scholar] [CrossRef]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc. Interv. 2019, 12, 1691–1699. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Reinhold, J.; Wickramarachchi, U.; Maart, C.; Gilbert, T.; Richardson, P.; Sulfi, S. Assessment of Paclitaxel Drug-Coated Balloon Only Angioplasty in STEMI. JACC Cardiovasc. Interv. 2023, 16, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Mangner, N.; Farah, A.; Ohlow, M.A.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Linke, A.; Stachel, G.; Markovic, S.; BASKET-SMALL 2 Investigators; et al. Safety and Efficacy of Drug-Coated Balloons Versus Drug-Eluting Stents in Acute Coronary Syndromes: A Prespecified Analysis of BASKET-SMALL 2. Circ. Cardiovasc. Interv. 2022, 15, e011325. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, C.; Giangiacomi, F.; Merinopoulos, I.; Gherbesi, E.; Faggiano, A.; Pasero, G.; Barbieri, L.; Tumminello, G.; Colombo, F.; Mircoli, L.; et al. Drug coated balloon angioplasty for de novo coronary lesions in large vessels: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 4921. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Hafez, A.; Atta, K.; Elsayed, H.; Abdelaziz, M.; Elaraby, A.; Kadhim, H.; Mechi, A.; Ezzat, M.; Fadel, A.; et al. Drug-coated balloons versus drug-eluting stents in patients with acute myocardial infarction undergoing percutaneous coronary intervention: An updated meta-analysis with trial sequential analysis. BMC Cardiovasc Disord. 2023, 23, 605, Erratum in BMC Cardiovasc. Disord. 2024, 24, 36. [Google Scholar] [CrossRef]
- Palmerini, T.; Bacchi Reggiani, L.; Della Riva, D.; Romanello, M.; Feres, F.; Abizaid, A.; Gilard, M.; Morice, M.C.; Valgimigli, M.; Hong, M.K.; et al. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J. Am. Coll. Cardiol. 2017, 69, 2011–2022. [Google Scholar] [CrossRef]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; MASTER DAPT Investigators; et al. Dual Antiplatelet Therapy after PCI in Patients at High Bleeding Risk. N. Engl. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietilä, M.; Minkkinen, M.J.; DEBUT trial investigators; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239. [Google Scholar] [CrossRef]
- Scheller, B.; Rissanen, T.T.; Farah, A.; Ohlow, M.A.; Mangner, N.; Wöhrle, J.; Möbius-Winkler, S.; Weilenmann, D.; Leibundgut, G.; BASKET-SMALL 2 Investigators; et al. Drug-Coated Balloon for Small Coronary Artery Disease in Patients With and Without High-Bleeding Risk in the BASKET-SMALL 2 Trial. Circ. Cardiovasc. Interv. 2022, 15, e011569. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zhang, H.P.; Li, C.; Fu, Y.; He, K.Z.; Liu, X.M.; Wang, H.J.; Xia, K.; Xu, L.; Zhong, J.C.; et al. Comparisons of drug-eluting balloon versus drug-eluting stent for the treatment of cancer patients presenting with acute myocardial infarction. Eur. J. Med. Res. 2023, 28, 334. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.P.; Costa, F.; Jeppsson, A.; Jüni, P.; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2018, 39, 213–260. [Google Scholar]
- Räsänen, A.; Kärkkäinen, J.M.; Eranti, A.; Eränen, J.; Rissanen, T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy combined with single antiplatelet treatment in patients at high bleeding risk: Single center experience of a novel concept. Catheter. Cardiovasc. Interv. 2023, 101, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Serruys, P.W. Single-Antiplatelet Treatment After Coronary Angioplasty With Drug-Coated Balloon. J. Am. Heart Assoc. 2023, 12, e028413. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.F.; Sapontis, J.; Kirtane, A.J.; Karmpaliotis, D.; Kalra, S.; Jones, P.G.; Lombardi, W.L.; Grantham, J.A.; McCabe, J.M. Prevalence, predictors, and health status implications of periprocedural complications during coronary chronic total occlusion angioplasty. EuroIntervention 2018, 14, e1199–e1206. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zheng, Z.; Ma, Q.; Shi, Y.; Liu, J. Efficacy and safety of drug-coated balloons in chronic total coronary occlusion recanalization: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2024, 24, 324. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Lu, W.; Pan, L.; Han, Z.; Pan, S.; Shan, Y.; Wang, X.; Zheng, X.; Li, R.; et al. Long-term outcomes of less drug-eluting stents by the use of drug-coated balloons in de novo coronary chronic total occlusion intervention: A multicenter observational study. Front. Cardiovasc. Med. 2023, 10, 1045859. [Google Scholar] [CrossRef]
- Köln, P.J.; Scheller, B.; Liew, H.B.; Rissanen, T.T.; Ahmad, W.A.; Weser, R.; Hauschild, T.; Nuruddin, A.A.; Clever, Y.P.; Ho, H.H.; et al. Treatment of chronic total occlusions in native coronary arteries by drug-coated balloons without stenting-A feasibility and safety study. Int. J. Cardiol. 2016, 225, 262–267. [Google Scholar] [CrossRef]
- Onishi, T.; Onishi, Y.; Kobayashi, I.; Umezawa, S.; Niwa, A. Drug-coated balloon angioplasty for de novo small vessel disease including chronic total occlusion and bifurcation in real-world clinical practice. Cardiovasc. Interv. Ther. 2019, 34, 139–148. [Google Scholar] [CrossRef]
- Funatsu, A.; Nakamura, S.; Inoue, N.; Nanto, S.; Nakamura, M.; Iwabuchi, M.; Ando, K.; Asano, R.; Habara, S.; Saito, S.; et al. A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin. Res. Cardiol. 2017, 106, 824–832. [Google Scholar] [CrossRef]
- Kwon, O.; Lee, P.H.; Lee, S.W.; Kweon, J.; Lee, J.Y.; Lee, K.; Kang, D.Y.; Ahn, J.M.; Park, D.W.; Kang, S.J.; et al. Fate of lumen size in distal coronary segment following successful chronic total occlusion recanalization. J. Cardiol. 2021, 77, 65–71. [Google Scholar] [CrossRef]
- Shafey, W.E.D.H.E. Pattern of vascular remodeling of distal reference segment after recanalization of chronic total occlusion, long-term angiographic follow-up. Egypt. Heart J. 2017, 69, 161–163. [Google Scholar] [CrossRef]
- Pellegrini, D.; Donahue, M.; Regazzoli, D.; Tedeschi, D.; Loffi, M.; Pellicano, M.; De Blasio, G.; Tespili, M.; Guagliumi, G.; Ielasi, A.; et al. Drug-coated balloon combined with drug-eluting stent for the treatment of coronary bifurcation lesions: Insights from the HYPER study. Eur. Heart J. Suppl. 2023, 25, C79–C83. [Google Scholar] [CrossRef] [PubMed]
- Corballis, N.H.; Paddock, S.; Gunawardena, T.; Merinopoulos, I.; Vassiliou, V.S.; Eccleshall, S.C. Drug coated balloons for coronary artery bifurcation lesions: A systematic review and focused meta-analysis. PLoS ONE 2021, 16, e0251986. [Google Scholar] [CrossRef]
- Megaly, M.; Rofael, M.; Saad, M.; Shishehbor, M.; Brilakis, E.S. Outcomes With Drug-Coated Balloons for Treating the Side Branch of Coronary Bifurcation Lesions. J. Invasive Cardiol. 2018, 30, 393–399. [Google Scholar] [PubMed]
- Ke, D.; He, X.; Chen, C.; Lin, C.; Luo, Y.; Fan, L.; Li, S.; Zheng, X.; Chen, L. Randomized Pilot Study to Compare DCB-Based versus DST-Based Strategies for the Treatment of True or Complex Coronary Bifurcation Lesions. Rev. Cardiovasc. Med. 2023, 24, 99. [Google Scholar] [CrossRef]
- Kleber, F.X.; Rittger, H.; Ludwig, J.; Schulz, A.; Mathey, D.G.; Boxberger, M.; Degenhardt, R.; Scheller, B.; Strasser, R.H. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: Results of the randomized multicenter PEPCAD-BIF trial. Clin. Res. Cardiol. 2016, 105, 613–621. [Google Scholar] [CrossRef]
- Her, A.Y.; Ann, S.H.; Singh, G.B.; Kim, Y.H.; Okamura, T.; Garg, S.; Koo, B.K.; Shin, E.S. Serial Morphological Changes of Side-Branch Ostium after Paclitaxel-Coated Balloon Treatment of De Novo Coronary Lesions of Main Vessels. Yonsei Med. J. 2016, 57, 606–613. [Google Scholar] [CrossRef]
- Lazar, F.L.; Prvulović, Đ.; Onea, H.L.; Cortese, B. The role of drug-coated balloons for coronary bifurcation management: Results from the prospective EASTBOURNE-BIF study. Minerva Cardiol. Angiol. 2024, 72, 346–354. [Google Scholar] [CrossRef]

| Study | Population | Endpoint | Results |
|---|---|---|---|
| PICCOLETO II [15] | 232 patients (DCB vs. DES) | 6-month LLL | 0.04 vs. 0.17 mm; p = 0.001 for non-inferiority; p = 0.03 for superiority 3-year follow-up: MACE: 20.8% vs. 10.8% [p = 0.046] Acute vessel occlusion: 4% vs. 0% [p = 0.042] All cause death: 4% vs. 3.9%; p = 0.98 MI: 6.9% vs. 2%; p = 0.14 Cardiac death: 1% vs. 1.9%; p = 0.56 TLR: 14.8% vs. 8.8%; p = 0.18 |
| BASKET SMALL 2 [17] | 758 patients (DCB vs. DES) | MACE | 3-year follow-up: stent thrombosis (DCB vs. DES): Kaplan–Meier estimate 1% vs. 2%; HR 0.33, 95% CI 0.07–1.64; p = 0.18) major bleedings (DCB vs. DES): Kaplan–Meier estimate 2% vs. 4%, HR 0.43, 95% CI 0.17–1.13; p = 0.088 |
| RESTORE SVD [18] | 230 patients (DCB vs. DES) | DS % | DS%: (29.6 ± 2.0% versus 24.1 ± 2.0%; the 1-sided 97.5% upper confidence limit of the difference was 10.9%) TLF (1 year): 4.4% vs. 2.6%, p = 0.72 |
| ANDROMEDA [19] | 1154 patients (1360 lesions) | n/a | MACE (DCB vs. DES): hazard ratio (HR) 0.67, 95% confidence interval (CI) 0.47–0.96 |
| Study | Angiographic Scenario | Main Findings |
| Isar-Desire 3 [8,9] | in-stent restenosis |
|
| Zhu Y et al. [13] | in-stent restenosis | lower TLR rates for DES implantation (risk ratio = 1.53, 95% CI 1.15–2.04, p = 0.003) |
| PICCOLETO II [15] | small vessel disease | lower LLL rates for DCB angioplasty similar hard clinical endpoints at 3-year follow-up (Table 1) |
| BASKET SMALL 2 [17] | small vessel disease | similar MACE at 3-year follow-up |
| RESTORE SVD [18] | small vessel disease | similar TLF and DS% rates at 1-year follow-up |
| ANDROMEDA [19] | small vessel disease | similar TLF rates at 3-year follow-up, but with a lower MACE rate compared with DES |
| Yu X et al. [26] | de novo large vessel disease | similar 12-month MACE rates (2.44% vs. 6.33%; p = 0.22) |
| Sun B et al. [28] | de novo large vessel disease | lower LLL in the DCB group (MD −0.13, 95% CI −0.22 to −0.05, p = 0.003, I2 = 60%) |
| Costopoulos et al. [33] | hybrid approach | similar MACE (20.8 vs. 22.7%; p = 0.74) similar TLR (9.6 vs. 9.3%; p = 0.84) |
| Yang et al. [37] | hybrid approach | similar DS% (31.96 ± 17.21 vs. 30.67 ± 18.80%, p = 0.622) lower LLL in the DCB group (0.06 ± 0.61 vs. 0.41 ± 0.64 mm, p < 0.001) At 3-year follow-up: similar MACE (11.3 vs. 13.7%, p = 0.32) and TLR (7.3 vs. 8.3%; p = 0.63) |
| Pan L et al. [38] | hybrid approach diabetic vs. non-diabetic patients | higher TLF (5.36 vs. 2.77%, p = 0.025) and TLR (5.36 vs. 2.77%, p = 0.025) rates in diabetic patients similar risk of MACE (OR: 1.580, 95% CI: 0.912–2.735) or any revascularization (OR: 1.534, 95% CI: 0.983–2.393; p = 0.058). |
| Liu et al. [42] | left main hybrid approach vs. DES | lower LLL for a hybrid strategy in the SB (−0.17 vs. 0.43 mm; p < 0.001), as well as in the LM stem (0.09 vs. 0.17 mm; p = 0.03) |
| Pan et al. [43] | left main | lower TLF (7.56 vs. 14.36%, p = 0.025) and TLR (2.91 vs. 9.42%, p = 0.007) lower LLL (0.13 ± 0.42 vs. 0.42 ± 0.62 mm, p < 0.001). |
| SPARTAN-LMS [45] | left main | similar CD (4.9 vs. 6.5%, p = 0.786), TVMI (0 vs. 6.5%) or TLR (7.3 vs. 8.3%, p = 0.86) |
| PEPCAD NSTEMI [49] | acute coronary syndromes | similar TLF (3.8 vs. 6.6%, p = 0.53) and MACE rates (6.7 vs. 14.2%, p = 0.11) |
| REVELATION Trial [50] | acute coronary syndromes | similar 9-month FFR (0.92 ± 0.05 vs. 0.91 ± 0.06, p = 0.27) |
| Abdelaziz A [54] | acute coronary syndromes | similar MACE (OR 0.89, 95% CI 0.57–1.4, p = 0.63) similar rates of CD (OR 0.59, 95% CI 0.22–1.56, p = 0.29), MI (OR 0.88, 95% CI 0.34–2.29, p = 0.79) and LLL (MD = −0.6, 95% CI −0.3–0.19, p = 0.64) |
| Rasanen et al. [61] | high-bleeding-risk patients | low rates of MACE (4.1% stable CAD; 12.1% ACS), TLR (0% stable CAD; 3.0% ACS) |
| Zhao Y et al. [64] | chronic total occlusion | lower MACE (13% vs. 21.5%) similar TVR (7.1% vs. 7.9%) and TLR rates |
| Ke D et al. [74] | bifurcation lesions | lower LLL vs. DES (main-vessel: 0.05 ± 0.24 mm vs. 0.25 ± 0.35 mm, p = 0.013; side-branch: –0.02 ± 0.19 mm vs. 0.11 ± 0.15 mm, p = 0.005) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, F.-L.; Onea, H.L.; Homorodean, C.; Bitea, I.C.; Lazar, D.R.; Ober, M.C.; Tataru, D.; Olinic, M.; Spinu, M.; Kacso, T.P.; et al. Drug-Coated Balloons in All-Comer Population—Are We There Yet? J. Clin. Med. 2025, 14, 3608. https://doi.org/10.3390/jcm14103608
Lazar F-L, Onea HL, Homorodean C, Bitea IC, Lazar DR, Ober MC, Tataru D, Olinic M, Spinu M, Kacso TP, et al. Drug-Coated Balloons in All-Comer Population—Are We There Yet? Journal of Clinical Medicine. 2025; 14(10):3608. https://doi.org/10.3390/jcm14103608
Chicago/Turabian StyleLazar, Florin-Leontin, Horea Laurentiu Onea, Calin Homorodean, Ioan Cornel Bitea, Diana Raluca Lazar, Mihai Claudiu Ober, Dan Tataru, Maria Olinic, Mihail Spinu, Teodor Paul Kacso, and et al. 2025. "Drug-Coated Balloons in All-Comer Population—Are We There Yet?" Journal of Clinical Medicine 14, no. 10: 3608. https://doi.org/10.3390/jcm14103608
APA StyleLazar, F.-L., Onea, H. L., Homorodean, C., Bitea, I. C., Lazar, D. R., Ober, M. C., Tataru, D., Olinic, M., Spinu, M., Kacso, T. P., & Olinic, D.-M. (2025). Drug-Coated Balloons in All-Comer Population—Are We There Yet? Journal of Clinical Medicine, 14(10), 3608. https://doi.org/10.3390/jcm14103608









