Open Mouth Posture Syndrome (OMPS): Classification
Abstract
1. Introduction
1.1. From MBS to OMPS
1.1.1. MBS Classification Framework
- Obstructive MB
- Habitual MB
- Anatomical MB
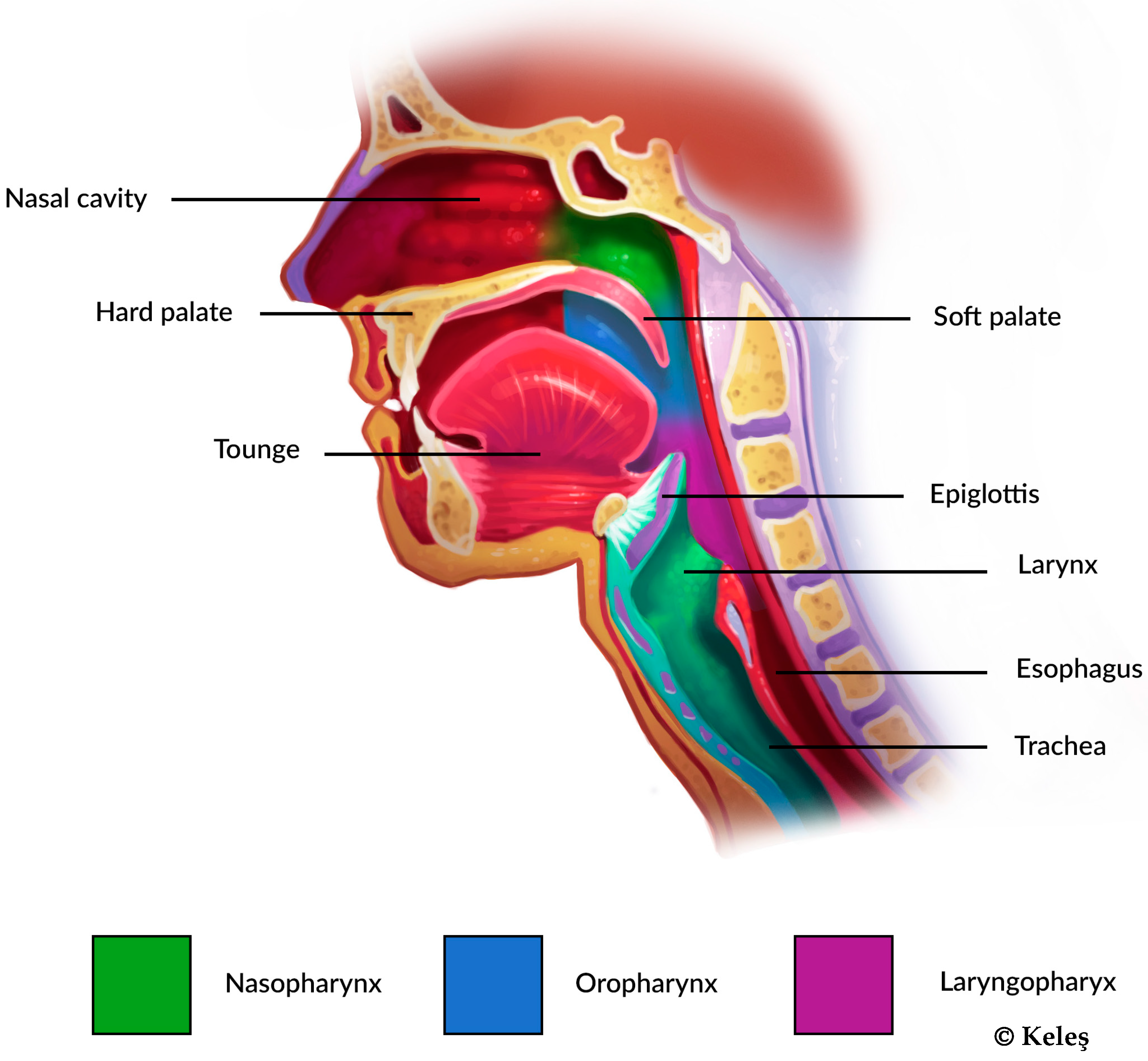
1.1.2. Topography of Obstruction
1.1.3. Sleep-Disordered Breathing
1.1.4. Tongue-Related Issues
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Literature Selection and Processing
2.4. Conceptual Synthesis
3. Results
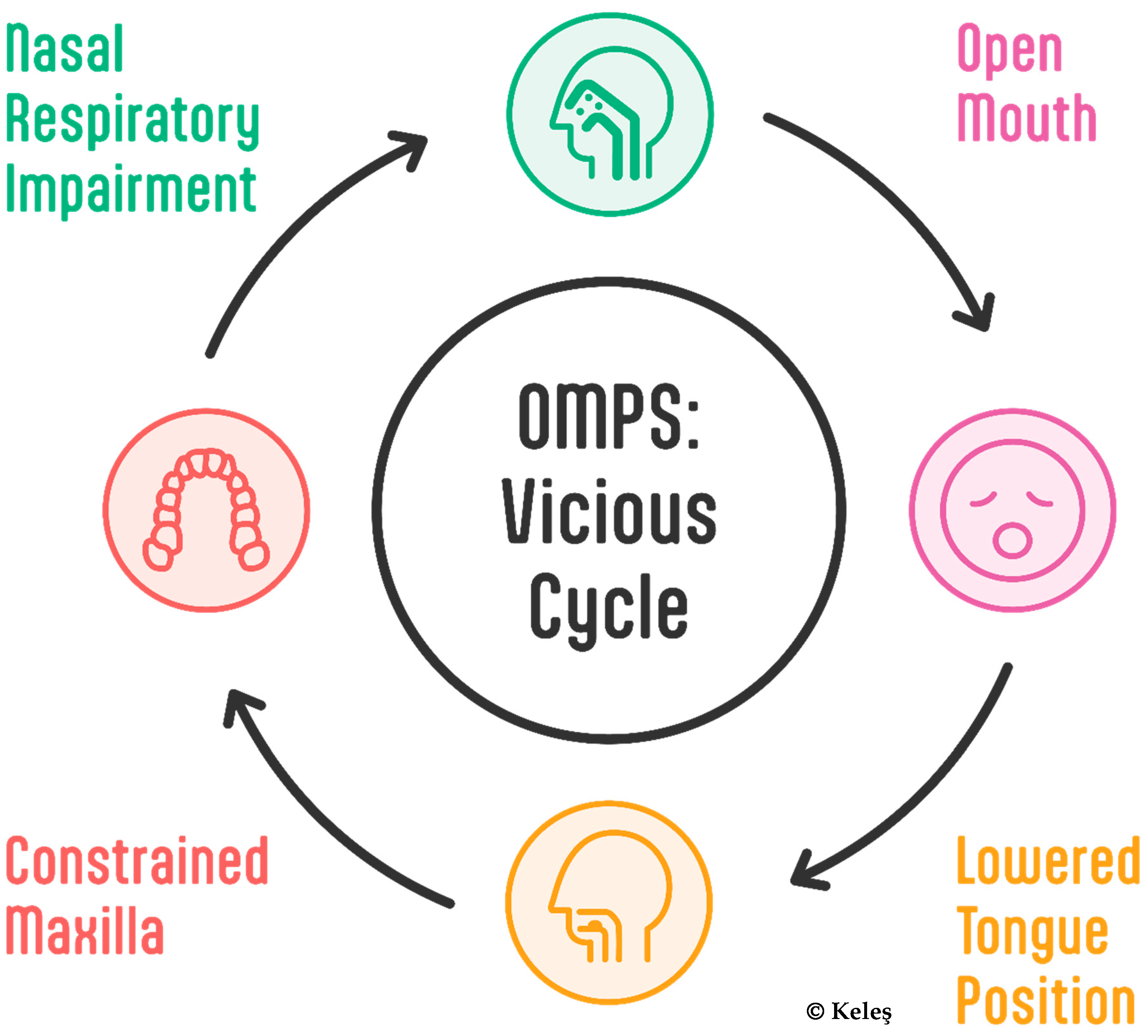
3.1. Common Patterns Across Categories
- Formation Adapts to Usage
- Fundamental biomechanical principles:
- Weak, persistent forces induce gradual tissue adaptation.
- Individual variations significantly impact physiological responses [5].
- Compensatory mechanisms emerge to maintain functional equilibrium [6].
- Typical changes associated with this syndrome:
- Functional Impacts:
- Reduced nasal breathing efficiency increases breathing effort, thus altering breathing patterns and restricting airflow [46];
- Altered perioral muscle activity [68];
- Distinct eating habits [70];
- Compensations include not only the cervical part of the spine but the whole body [71].
- Systemic Effects
- Disrupted sleep patterns [15];
- Daytime consequences (see Quality of Life).
- Key Observations
- All the subcategories show remarkable overlap in their effects on craniofacial development.
- The timing of onset appears to influence severity.
- Early intervention is crucial across all categories.
- Multiple systems are affected regardless of the initial cause.
- Treatment requires a comprehensive approach due to shared impacts.
3.2. OMPS Classification Framework
4. Discussion
- The Need for Classification
- Strategic Significance of OMPS Classification:
- 1.
- Educational advancement
- It provides educators with a comprehensive framework for teaching others about the etiological aspects of malocclusion.
- It enhances our understanding of developmental processes in oral health.
- It bridges gaps between interdisciplinary medical approaches.
- 2.
- Research Imperatives
- This classification enables the systematic investigation of varying risks and symptom expressions.
- It facilitates the analysis of correlations between severity, symptom variability, and etiological factors.
- 3.
- Comprehensive Research Objectives Future research should explore [6]:
- The genetic factors influencing OMPS;
- Environmental determinants of malocclusion;
- The development of validated diagnostic criteria;
- Interdisciplinary treatment protocols.
- Methodological Innovations—The OMPS classification system represents a paradigm shift in understanding oral posture disorders. By delineating subtypes, this framework allows for the following:
- The identification of subtype-specific impacts on craniofacial growth;
- The standardization of data collection for precise analyses (see subsequent article for diagnostics).
- The facilitation of the development of tailored diagnostic and therapeutic protocols (see subsequent article for diagnostics).
- Limitations and future directions—While the classification offers significant insights, it also acknowledges the complexity of OMPS:
- Management requires individualized, interdisciplinary approaches.
- Subsequent research will focus on developing practical diagnostic protocols.
- Ongoing refinement of the classification is anticipated.
- Broader implications—The OMPS classification transcends traditional diagnostic boundaries, offering the following:
- A holistic view of oral posture disorders;
- Interdisciplinary research opportunities;
- Potential for personalized intervention strategies.
5. Conclusions
- Key Contributions:
- A systematic approach to understanding the syndrome complex was developed.
- A quantitative framework for analyzing odds ratios was provided.
- A detailed exploration of severity variations across subtypes was conducted.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Resources
Acknowledgments
Conflicts of Interest
References
- Emslie, R.D.; Massler, M.; Zwemer, J.D. Mouth breathing. I. Etiology and effects; a review. J. Am. Dent. Assoc. 1952, 44, 506–521. [Google Scholar] [CrossRef] [PubMed]
- Triana, B.E.G.; Ali, A.H.; León, I.B.G. Mouth breathing and its relationship to some oral and medical conditions: Physiopathological mechanisms involved. Rev. Habanera Cienc. Médicas 2016, 15, 200–212. [Google Scholar]
- Fantozzi, D.; Doldo, T.; Luzi, C.; Grandini, S.; Marruganti, C. Neurocognitive abilities in children affected by sleep breathing disorders. A systematic review and meta-analysis of case control-studies. Eur. J. Paediatr. Dent. 2023, 1. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, C.A.; Lee, Y.B.; Kim, H.K.; Kang, C.K. EEG signals during mouth breathing in a working memory task. Int. J. Neurosci. 2020, 130, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Harvold, E.P.; Tomer, B.S.; Vargervik, K.; Chierici, G. Primate experiments on oral respiration. Am. J. Orthod. 1981, 79, 359–372. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, T.; Qin, D.; Hua, F.; He, H. The impact of mouth breathing on dentofacial development: A concise review. Front. Public Health 2022, 10, 929165. [Google Scholar] [CrossRef]
- Valcheva, Z.; Arnautska, H.; Dimova, M.; Ivanova, G.; Atanasova, I. The Role of Mouth Breathing on Dentition Development and Formation. J. IMAB Annu. Proceeding Sci. Pap. 2018, 24, 1878–1882. [Google Scholar] [CrossRef]
- Milanesi, J.M.; Berwig, L.C.; Marquezan, M.; Schuch, L.H.; Moraes, A.B.; Silva, A.; Corrêa, E.C.R. Variables associated with mouth breathing diagnosis in children based on a multidisciplinary assessment. Codas 2018, 30, e20170071. [Google Scholar] [CrossRef]
- Junqueira, P.; Marchesan, I.Q.; de Oliveira, L.R.; Ciccone, E.; Haddad, L.; Rizzo, M.C. Speech-language pathology findings in patients with mouth breathing: Multidisciplinary diagnosis according to etiology. Int. J. Orofac. Myol. 2010, 36, 27–32. [Google Scholar] [CrossRef]
- Klein, J.C. Nasal respiratory function and craniofacial growth. Arch. Otolaryngol. Head Neck Surg. 1986, 112, 843–849. [Google Scholar] [CrossRef]
- Singh, G. Textbook of Orthodontics; Jaypee Brothers Medical Pub: New Delhi, India, 2007. [Google Scholar] [CrossRef]
- Galazka, A.; Migacz, E.; Kukwa, A.; Czarnecka, A.; Krzeski, A.; Kukwa, W. Association of breathing patterns and quality of life in patients with nasal obstruction. Otolaryngol. Pol. 2018, 72, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Vig, P.S.; Spalding, P.M.; Lints, R.R. Sensitivity and specificity of diagnostic tests for impaired nasal respiration. Am. J. Orthod. Dentofac. Orthop. 1991, 99, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.F.; Keall, H.; Vig, P.S.; Krause, C.J. Clinical nasal obstruction and objective respiratory mode determination. Ann. Otol. Rhinol. Laryngol. 1988, 97 Pt 1, 397–402. [Google Scholar] [CrossRef]
- Correa, L.; Schmitz, L.; Sakae, T.; Marcelino, T.; Popoaski, C. Evaluation from the quality of life in the oral breathers patients. Arq. Int. Otorrinolaringol. 2014, 16, 074–081. [Google Scholar] [CrossRef]
- Araújo, B.C.L.; de Magalhães Simões, S.; de Gois-Santos, V.T.; Martins-Filho, P.R.S. Association Between Mouth Breathing and Asthma: A Systematic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Kukwa, W.; Guilleminault, C.; Tomaszewska, M.; Kukwa, A.; Krzeski, A.; Migacz, E. Prevalence of upper respiratory tract infections in habitually snoring and mouth breathing children. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 37–41. [Google Scholar] [CrossRef]
- Abreu, R.R.; Rocha, R.L.; Lamounier, J.A.; Guerra, A.F. Etiology, clinical manifestations and concurrent findings in mouth-breathing children. J. Pediatr. 2008, 84, 529–535. [Google Scholar] [CrossRef]
- Foldvary-Schaefer, N.R.; Waters, T.E. Sleep-Disordered Breathing. Continuum 2017, 2, 1093–1116. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cunha, D.A.D.; Albuquerque, R.C.; Costa, R.N.A.; Silva, H.J.D. Sensory Changes in Mouth Breathers: Systematic Review Based on The Prisma Method. Rev. Paul. Pediatr. 2019, 37, 97–103. [Google Scholar] [CrossRef]
- Nobis, W.P.; Schuele, S.; Templer, J.W.; Zhou, G.; Lane, G.; Rosenow, J.M.; Zelano, C. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann. Neurol. 2018, 83, 460–471. [Google Scholar] [CrossRef]
- Dahl, R.; Mygind, N. Anatomy, physiology and function of the nasal cavities in health and disease. Adv. Drug Deliv. Rev. 1998, 29, 3–12. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; De Meyer, M.M.; Claeys, S. Sleep, breathing and the nose. Sleep Med. Rev. 2005, 9, 437–451. [Google Scholar] [CrossRef]
- Sassin, J.F.; Parker, D.C.; Mace, J.W.; Gotlin, R.W.; Johnson, L.C.; Rossman, L.G. Human growth hormone release: Relation to slow-wave sleep and sleep-walking cycles. Science 1969, 165, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Morais-Almeida, M.; Wandalsen, G.F.; Solé, D. Growth and mouth breathers. J. Pediatr. 2019, 95 (Suppl. S1), 66–71. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, Y. Mouth breathing: Adverse effects on facial growth, health, academics, and behavior. Gen. Dent. 2010, 58, 18–25; quiz 26–17, 79–80. [Google Scholar] [PubMed]
- Ribeiro, G.C.; Dos Santos, I.D.; Santos, A.C.; Paranhos, L.R.; César, C.P. Influence of the breathing pattern on the learning process: A systematic review of literature. Braz. J. Otorhinolaryngol. 2016, 82, 466–478. [Google Scholar] [CrossRef]
- Kuroishi, R.C.; Garcia, R.B.; Valera, F.C.; Anselmo-Lima, W.T.; Fukuda, M.T. Deficits in working memory, reading comprehension and arithmetic skills in children with mouth breathing syndrome: Analytical cross-sectional study. Sao Paulo Med. J. 2015, 133, 78–83. [Google Scholar] [CrossRef]
- Bowman, G.F. Some Fallacies in Orthodontia. In The Dental Cosmos, 1887, Vol. 29: A Monthly Record of Dental Science; Forgotten Books: London, UK, 1910; p. 52. [Google Scholar]
- Hunter, S.J.; Gozal, D.; Smith, D.L.; Philby, M.F.; Kaylegian, J.; Kheirandish-Gozal, L. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am. J. Respir. Crit. Care Med. 2016, 194, 739–747. [Google Scholar] [CrossRef]
- Philby, M.F.; Macey, P.M.; Ma, R.A.; Kumar, R.; Gozal, D.; Kheirandish-Gozal, L. Reduced Regional Grey Matter Volumes in Pediatric Obstructive Sleep Apnea. Sci. Rep. 2017, 7, 44566. [Google Scholar] [CrossRef]
- Beebe, D.W. Neurobehavioral morbidity associated with disordered breathing during sleep in children: A comprehensive review. Sleep 2006, 29, 1115–1134. [Google Scholar] [CrossRef]
- Jung, J.Y.; Kang, C.K. Investigation on the Effect of Oral Breathing on Cognitive Activity Using Functional Brain Imaging. Healthcare 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.C.; Casagrande, C.F.; Teixeira, L.P.; Finck, N.S.; de Araujo, M.T. Guidelines proposal for clinical recognition of mouth breathing children. Dent. Press J. Orthod. 2015, 20, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Huseni, S.; Lo, L. A frequent phenotype for paediatric sleep apnoea: Short lingual frenulum. ERJ Open Res. 2016, 2, 00043-2016. [Google Scholar] [CrossRef]
- Pompeia, L.E.; Ilinsky, R.S.; Ortolani, C.L.F.; Faltin, K.J. Ankyloglossia and Its Influence on Growth and Development of the Stomatognathic System. Rev. Paul. Pediatr. 2017, 35, 216–221. [Google Scholar] [CrossRef]
- Yoon, A.J.; Zaghi, S.; Ha, S.; Law, C.S.; Guilleminault, C.; Liu, S.Y. Ankyloglossia as a risk factor for maxillary hypoplasia and soft palate elongation: A functional-morphological study. Orthod. Craniofac. Res. 2017, 20, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yoon, A.; Zaghi, S.; Weitzman, R.; Ha, S.; Law, C.S.; Guilleminault, C.; Liu, S.Y.C. Toward a functional definition of ankyloglossia: Validating current grading scales for lingual frenulum length and tongue mobility in 1052 subjects. Sleep Breath. 2017, 21, 767–775. [Google Scholar] [CrossRef]
- Povoa-Santos, L.; Lacerda-Santos, R.; Alvarenga-Brant, R.; Notaro, S.Q.; Souza-Oliveira, A.C.; Occhi-Alexandre, I.G.P.; Martins-Pfeifer, C.C. Ankyloglossia and malocclusion: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2024, 155, 59–73.e9. [Google Scholar] [CrossRef]
- Martin-Harris, B. Integration of Breathing and Oropharyngeal Swallowing: A Historical Perspective and 13-Year Research Experience. Perspect. Swallowing Swallowing Disord. Dysphagia 2003, 12, 6–12. [Google Scholar] [CrossRef]
- Ashley, F.P.; Usiskin, L.A.; Wilson, R.F.; Wagaiyu, E. The relationship between irregularity of the incisor teeth, plaque, and gingivitis: A study in a group of schoolchildren aged 11–14 years. Eur. J. Orthod. 1998, 20, 65–72. [Google Scholar] [CrossRef]
- Gomez-Gonzalez, C.; Gonzalez-Mosquera, A.; Alkhraisat, M.H.; Anitua, E. Mouth Breathing and Its Impact on Atypical Swallowing: A Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 21. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Lin, Q.; Liu, X.; Cao, J.; Dai, J. Effect of maxillary expansion combined with orofacial myofunctional therapy on the position of the tongue of children with mouth breathing. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2023, 37, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Zaghi, S.; Shamtoob, S.; Peterson, C.; Christianson, L.; Valcu-Pinkerton, S.; Peeran, Z.; Fung, B.; Kwok-Keung Ng, D.; Jagomagi, T.; Archambault, N.; et al. Assessment of posterior tongue mobility using lingual-palatal suction: Progress towards a functional definition of ankyloglossia. J. Oral Rehabil. 2021, 48, 692–700. [Google Scholar] [CrossRef]
- Grabowski, R.; Kundt, G.; Stahl, F. Interrelation between occlusal findings and orofacial myofunctional status in primary and mixed dentition: Part III: Interrelation between malocclusions and orofacial dysfunctions. J. Orofac. Orthop. 2007, 68, 462–476. [Google Scholar] [CrossRef]
- Dzipunova Biljana, T.S.N.; Marina, K.; Biljana, D.O.; Kristina, S.; Irena, S.; Gonxhe, B. The Impact of Mouth Breathing Dysfunction on The Formation of Orthodontic Malocclusions. IOSR J. Dent. Med. Sci. 2021, 20, 11–18. [Google Scholar]
- Pilarski, J.Q.; Leiter, J.C.; Fregosi, R.F. Muscles of Breathing: Development, Function, and Patterns of Activation. Compr. Physiol. 2019, 9, 1025–1080. [Google Scholar] [CrossRef]
- Cordray, H.; Mahendran, G.N.; Tey, C.S.; Nemeth, J.; Raol, N. The Impact of Ankyloglossia Beyond Breastfeeding: A Scoping Review of Potential Symptoms. Am. J. Speech Lang. Pathol. 2023, 32, 3048–3063. [Google Scholar] [CrossRef] [PubMed]
- Harvold, E.P.; Vargervik, K.; Chierici, G. Primate experiments on oral sensation and dental malocclusions. Am. J. Orthod. 1973, 63, 494–508. [Google Scholar] [CrossRef]
- Al Ali, A.; Richmond, S.; Popat, H.; Playle, R.; Pickles, T.; Zhurov, A.I.; Marshall, D.; Rosin, P.L.; Henderson, J.; Bonuck, K. The influence of snoring, mouth breathing and apnoea on facial morphology in late childhood: A three-dimensional study. BMJ Open 2015, 5, e009027. [Google Scholar] [CrossRef]
- Cheng, B.; Mohamed, A.S.; Habumugisha, J.; Guo, Y.; Zou, R.; Wang, F. A Study of the Facial Soft Tissue Morphology in Nasal- and Mouth-Breathing Patients. Int. Dent. J. 2023, 73, 403–409. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.J.; Yoon, A. Craniofacial anatomical determinants of pediatric sleep-disordered breathing: A comprehensive review. J. Prosthodont. 2024, 34, 26–34. [Google Scholar] [CrossRef]
- Souki, B.Q.; Lopes, P.B.; Veloso, N.C.; Avelino, R.A.; Pereira, T.B.; Souza, P.E.; Franco, L.P.; Becker, H.M. Facial soft tissues of mouth-breathing children: Do expectations meet reality? Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Man, P.P.; Jang, S.; Yi, H.K. Nasal obstruction promotes alveolar bone destruction in the juvenile rat model. J. Dent. Sci. 2022, 17, 176–183. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, L.; Huang, X.; Li, C.; Liu, J.; Hu, Y. Effects of mouth breathing on facial skeletal development in children: A systematic review and meta-analysis. BMC Oral Health 2021, 21, 108. [Google Scholar] [CrossRef]
- Fraga, W.S.; Seixas, V.M.; Santos, J.C.; Paranhos, L.R.; César, C.P. Mouth breathing in children and its impact in dental malocclusion: A systematic review of observational studies. Minerva Stomatol. 2018, 67, 129–138. [Google Scholar] [CrossRef]
- Frankel, A.; Mehta, U. Nasal Analysis. In Master Techniques in Rhinoplasty; Elsevier: Amsterdam, The Netherlands, 2011; pp. 31–42. [Google Scholar]
- Li, J.; Zhao, Z.; Zheng, L.; Daraqel, B.; Liu, J.; Hu, Y. Effects of mouth breathing on maxillofacial and airway development in children and adolescents with different cervical vertebral maturation stages: A cross-sectional study. BMC Oral Health 2022, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Hodges-Simeon, C.R.; Albert, G.; Richardson, G.B.; McHale, T.S.; Weinberg, S.M.; Gurven, M.; Gaulin, S.J.C. Was facial width-to-height ratio subject to sexual selection pressures? A life course approach. PLoS ONE 2021, 16, e0240284. [Google Scholar] [CrossRef] [PubMed]
- Inada, E.; Saitoh, I.; Kaihara, Y.; Murakami, D.; Nogami, Y.; Kiyokawa, Y.; Tanaka, R.; Sakata, K.; Yamasaki, Y. Factors related to mouth breathing syndrome in preschool children and the effects of incompetent lip seal: An exploratory study. Clin. Exp. Dent. Res. 2022, 8, 1555–1560. [Google Scholar] [CrossRef]
- Basheer, B.; Hegde, K.S.; Bhat, S.; Umar, D.; Baroudi, K. Influence of Mouth Breathing on the Dentofacial Growth of Children: A Cephalometric Study. J. Int. Oral Health 2014, 6, 50–55. [Google Scholar]
- De Menezes, V.A.; Leal, R.B.; Pessoa, R.S.; Pontes, R.M. Prevalence and factors related to mouth breathing in school children at the Santo Amaro project-Recife, 2005. Braz. J. Otorhinolaryngol. 2006, 72, 394–399. [Google Scholar] [CrossRef]
- Saitoh, I.; Inada, E.; Kaihara, Y.; Nogami, Y.; Murakami, D.; Kubota, N.; Sakurai, K.; Shirazawa, Y.; Sawami, T.; Goto, M.; et al. An exploratory study of the factors related to mouth breathing syndrome in primary school children. Arch. Oral Biol. 2018, 92, 57–61. [Google Scholar] [CrossRef]
- Grippaudo, C.; Paolantonio, E.G.; Antonini, G.; Saulle, R.; La Torre, G.; Deli, R. Association between oral habits, mouth breathing and malocclusion. Acta Otorhinolaryngol. Ital. 2016, 36, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, E.G.; Ludovici, N.; Saccomanno, S.; La Torre, G.; Grippaudo, C. Association between oral habits, mouth breathing and malocclusion in Italian preschoolers. Eur. J. Paediatr. Dent. 2019, 20, 204–208. [Google Scholar] [CrossRef]
- Harari, D.; Redlich, M.; Miri, S.; Hamud, T.; Gross, M. The effect of mouth breathing versus nasal breathing on dentofacial and craniofacial development in orthodontic patients. Laryngoscope 2010, 120, 2089–2093. [Google Scholar] [CrossRef] [PubMed]
- Festa, P.; Mansi, N.; Varricchio, A.M.; Savoia, F.; Calì, C.; Marraudino, C.; De Vincentiis, G.C.; Galeotti, A. Association between upper airway obstruction and malocclusion in mouth-breathing children. Acta Otorhinolaryngol. Ital. 2021, 41, 436–442. [Google Scholar] [CrossRef]
- Lanzer, B.; Bantleon, H.P. Äquilibrium von Zunge, Lippen und Wangen. Informationen aus Orthod. Kieferorthopädie 2016, 48, 29–34. [Google Scholar] [CrossRef]
- Lione, R.; Franchi, L.; Huanca Ghislanzoni, L.T.; Primozic, J.; Buongiorno, M.; Cozza, P. Palatal surface and volume in mouth-breathing subjects evaluated with three-dimensional analysis of digital dental casts-a controlled study. Eur. J. Orthod. 2015, 37, 101–104. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Kaihara, Y.; Yamasaki, Y. Factors related to mouth-breathing syndrome and the influence of an incompetent lip seal on facial soft tissue form in children. Pediatr. Dent. J. 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Mancini, F.; Sousa, F.S.; Hummel, A.D.; Falcao, A.E.; Yi, L.C.; Ortolani, C.F.; Sigulem, D.; Pisa, I.T. Classification of postural profiles among mouth-breathing children by learning vector quantization. Methods Inf. Med. 2011, 50, 349–357. [Google Scholar] [CrossRef]
- Neiva, P.D.; Kirkwood, R.N.; Mendes, P.L.; Zabjek, K.; Becker, H.G.; Mathur, S. Postural disorders in mouth breathing children: A systematic review. Braz. J. Phys. Ther. 2018, 22, 7–19. [Google Scholar] [CrossRef]
- Sabatucci, A.; Raffaeli, F.; Mastrovincenzo, M.; Luchetta, A.; Giannone, A.; Ciavarella, D. Breathing pattern and head posture: Changes in craniocervical angles. Minerva Stomatol. 2015, 64, 59–74. [Google Scholar]
- Cerruto, C.; Di Vece, L.; Doldo, T.; Giovannetti, A.; Polimeni, A.; Goracci, C. A computerized photographic method to evaluate changes in head posture and scapular position following rapid palatal expansion: A pilot study. J. Clin. Pediatr. Dent. 2012, 37, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Gomes Lde, C.; Horta, K.O.; Goncalves, J.R.; Santos-Pinto, A.D. Systematic review: Craniocervical posture and craniofacial morphology. Eur. J. Orthod. 2014, 36, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Ungkanont, K.; Areyasathidmon, S. Factors affecting quality of life of pediatric outpatients with symptoms suggestive of sleep-disordered breathing. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1945–1948. [Google Scholar] [CrossRef]
- Bonuck, K.; Parikh, S.; Bassila, M. Growth failure and sleep disordered breathing: A review of the literature. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Leal, R.B.; Gomes, M.C.; Granville-Garcia, A.F.; Goes, P.S.; de Menezes, V.A. Impact of breathing patterns on the quality of life of 9- to 10-year-old schoolchildren. Am. J. Rhinol. Allergy 2016, 30, 147–152. [Google Scholar] [CrossRef]
- Uhlig, S.E.; Marchesi, L.M.; Duarte, H.; Araújo, M.T. Association between respiratory and postural adaptations and self-perception of school-aged children with mouth breathing in relation to their quality of life. Braz. J. Phys. Ther. 2015, 19, 201–210. [Google Scholar] [CrossRef][Green Version]
- Keeler, J.; Most, S.P. Measuring Nasal Obstruction. Facial Plast. Surg. Clin. N. Am. 2016, 24, 315–322. [Google Scholar] [CrossRef]
- Kalaskar, R.; Bhaje, P.; Kalaskar, A.; Faye, A. Sleep Difficulties and Symptoms of Attention-deficit Hyperactivity Disorder in Children with Mouth Breathing. Int. J. Clin. Pediatr. Dent. 2021, 14, 604–609. [Google Scholar] [CrossRef]
- DeLong, G.F. Habitual Mouth-Breathing and Consequent Malocclusion of the teeth. In The Dental Cosmos, 1887, Vol. 29: A Monthly Record of Dental Science; Forgotten Books: London, UK, 1909; Volume 51, pp. 200–204. [Google Scholar]
- Moss, M.L.; Salentijn, L. The primary role of functional matrices in facial growth. Am. J. Orthod. 1969, 55, 566–577. [Google Scholar] [CrossRef]
- Costa, J.G.; Costa, G.S.; Costa, C.; Vilella, O.V.; Mattos, C.T.; Cury-Saramago, A.A. Clinical recognition of mouth breathers by orthodontists: A preliminary study. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 646–653. [Google Scholar] [CrossRef]
- Wasnik, M.; Kulkarni, S.; Gahlod, N.; Khekade, S.; Bhattad, D.; Shukla, H. Mouth breathing habit: A review. Int. J. Community Med. Public Health 2020, 8, 495–501. [Google Scholar] [CrossRef]


| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keleş, C.-F.; Morais, D.; Marya, A.; Chawshli, O.F.; Venugopal, A.; Botzenhart, U.U. Open Mouth Posture Syndrome (OMPS): Classification. J. Clin. Med. 2025, 14, 3586. https://doi.org/10.3390/jcm14103586
Keleş C-F, Morais D, Marya A, Chawshli OF, Venugopal A, Botzenhart UU. Open Mouth Posture Syndrome (OMPS): Classification. Journal of Clinical Medicine. 2025; 14(10):3586. https://doi.org/10.3390/jcm14103586
Chicago/Turabian StyleKeleş, Can-Florian, David Morais, Anand Marya, Omar Fawzi Chawshli, Adith Venugopal, and Ute Ulrike Botzenhart. 2025. "Open Mouth Posture Syndrome (OMPS): Classification" Journal of Clinical Medicine 14, no. 10: 3586. https://doi.org/10.3390/jcm14103586
APA StyleKeleş, C.-F., Morais, D., Marya, A., Chawshli, O. F., Venugopal, A., & Botzenhart, U. U. (2025). Open Mouth Posture Syndrome (OMPS): Classification. Journal of Clinical Medicine, 14(10), 3586. https://doi.org/10.3390/jcm14103586









