Clinical Efficacy of Platelet-Rich Plasma and Hyaluronic Acid Versus Hyaluronic Acid for Knee Osteoarthritis with MRI Analysis: A Randomized Controlled Trial
Abstract
1. Introduction
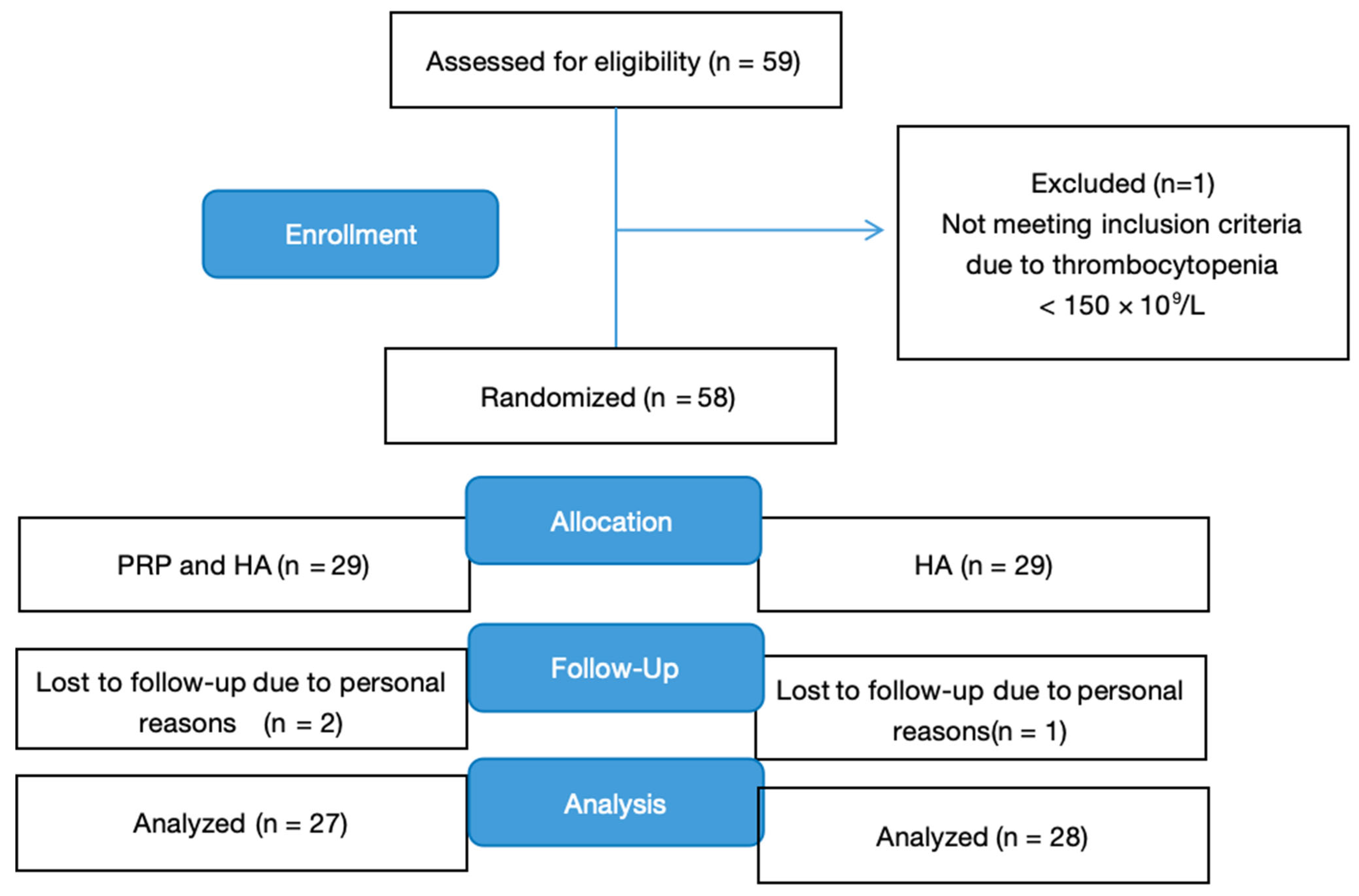
2. Materials and Methods
2.1. Study Design
2.2. Randomization and Masking
2.3. Participants
2.4. Intervention
2.4.1. The HA+PRP Group
2.4.2. The HA Group
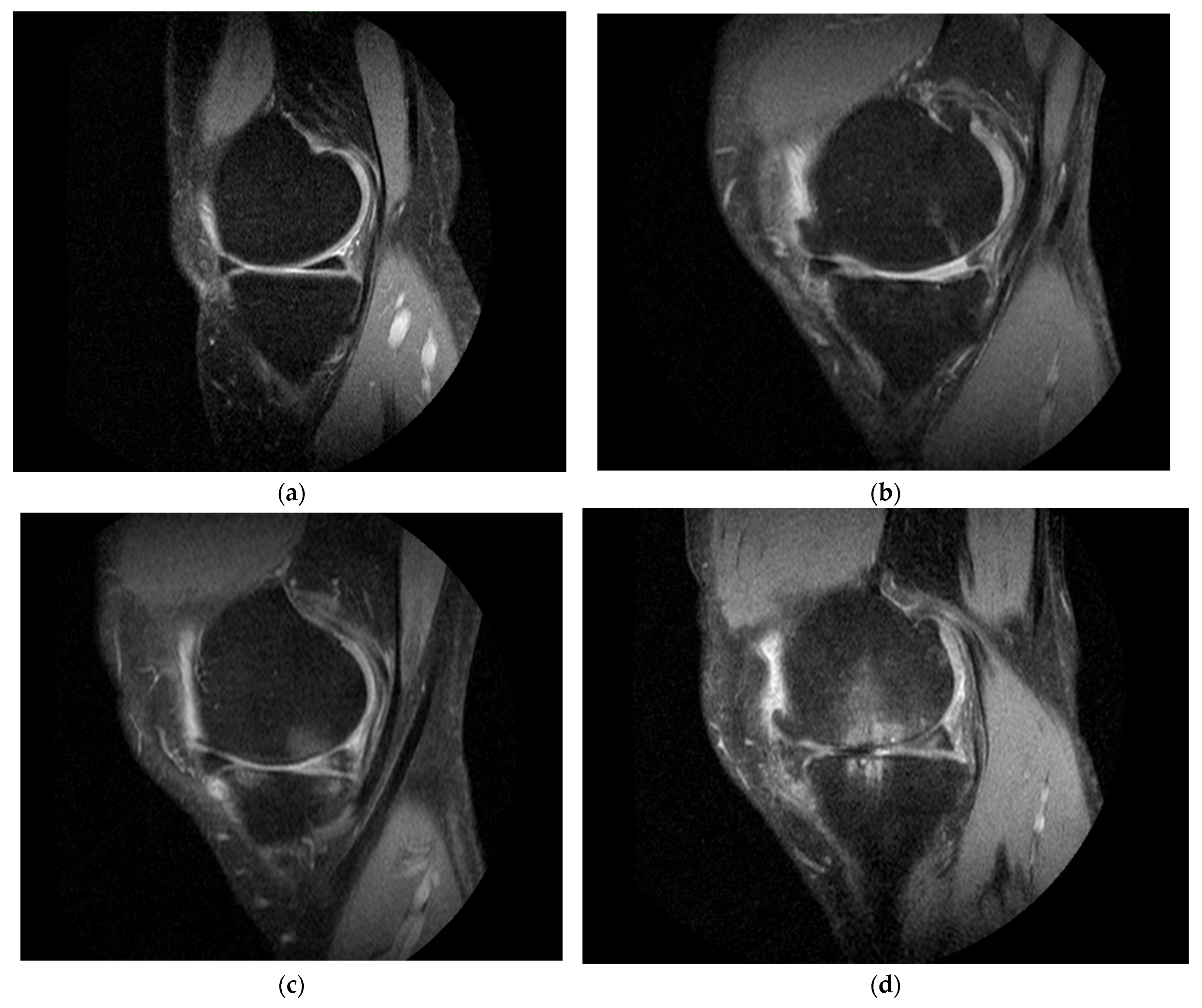
2.5. MRI Outcomes
3. Statistical Analysis
Sample Size Calculation
4. Results
4.1. Demographic Profile and Baseline Characteristics
4.2. Primary Outcome
VAS
4.3. Secondary Outcomes
4.3.1. WOMAC and EQ-5D-5L
4.3.2. MRI Outcome Measures
5. Discussion
Limitations and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fusco, M.; Skaper, S.D.; Coaccioli, S.; Varrassi, G.; Paladini, A. Degenerative joint diseases and neuroinflammation. Pain Pract. 2017, 17, 522–532. [Google Scholar] [CrossRef]
- Paterson, K.L.; Nicholls, M.; Bennell, K.L.; Bates, D. Intra-articular injection of photo-activated platelet-rich plasma in patients with knee osteoarthritis: A double-blind randomized controlled pilot study. BMC Musculoskelet. Disord. 2016, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Solomon, C.G. Osteoarthritis of the knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Ip, H.L.; Nath, D.K.; Sawleh, S.H.; Kabir, M.H.; Jahan, N. Regenerative medicine for knee osteoarthritis—The efficacy and safety of intra-articular platelet-rich plasma and mesenchymal stem cells injections: A literature review. Cureus 2020, 12, e10575. [Google Scholar] [CrossRef]
- Curran, M.P. Hyaluronic acid (Supartz®): A review of its use in osteoarthritis of the knee. Drugs Aging 2010, 27, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Y. Intra-articular hyaluronic acid injections for knee osteoarthritis. Am. Fam. Physician 2000, 62, 565–570. [Google Scholar]
- Iaconisi, G.N.; Gallo, N.; Caforio, L.; Ricci, V.; Fiermonte, G.; Della Tommasa, S.; Bernetti, A.; Dolce, V.; Farì, G.; Capobianco, L. Clinical and Biochemical Implications of Hyaluronic Acid in Musculoskeletal Rehabilitation: A Comprehensive Review. J. Pers. Med. 2023, 13, 1647. [Google Scholar] [CrossRef]
- Laudy, A.B.; Bakker, E.W.; Rekers, M.; Moen, M.H. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 657–672. [Google Scholar] [CrossRef]
- Renevier, J.L.; Marc, J.F.; Adam, P.; Sans, N.; Le Coz, J.; Prothoy, I. Cellular matrix™ PRP-HA: A new treatment option with platelet-rich plasma and hyaluronic acid for patients with osteoarthritis having had an unsatisfactory clinical response to hyaluronic acid alone: Results of a pilot multicenter French study with long-term follow-up. Int. J. Clin. Rheumatol. 2018, 13, 230–238. [Google Scholar]
- Gupta, A.; Jeyaraman, M.; Potty, A.G. Leukocyte-rich vs leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis. Biomedicines 2023, 11, 141. [Google Scholar] [CrossRef]
- LaBaze, D.; Li, H. Platelet rich plasma: Biology and clinical usage in orthopedics. In Orthopedic Biomaterials: Progress in Biology, Manufacturing, and Industry Perspectives; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 243–286. [Google Scholar]
- Abbas, A.; Du, J.T.; Dhotar, H.S. The Effect of Leukocyte Concentration on Platelet-Rich Plasma Injections for Knee Osteoarthritis: A Network Meta-Analysis. J. Bone Jt. Surg. 2022, 104, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Saturveithan, C.; Premganesh, G.; Fakhrizzaki, S.; Mahathir, M.; Karuna, K.; Rauf, K.; William, H.; Akmal, H.; Sivapathasundaram, N.; Jaspreet, K. Intra-articular hyaluronic acid (HA) and platelet-rich plasma (PRP) injection versus hyaluronic acid (HA) injection alone in patients with Grade III and IV knee osteoarthritis (OA): A retrospective study on functional outcome. Malays. Orthop. J. 2016, 10, 35–40. [Google Scholar]
- Raeissadat, S.A.; Rayegani, S.M.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee osteoarthritis injection choices: Platelet-rich plasma (PRP) versus hyaluronic acid (A one-year randomized clinical trial). Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peterfy, C.G.; Guermazi, A.; Zaim, S.; Tirman, P.F.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Tubach, F.; Ravaud, P.; Baron, G.; Falissard, B.; Logeart, I.; Bellamy, N.; Bombardier, C.; Felson, D.T.; Dougados, M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: The minimal clinically important improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Karasavvidis, T.; Totlis, T.; Gilat, R.; Cole, B.J. Platelet-rich plasma combined with hyaluronic acid improves pain and function compared with hyaluronic acid alone in knee osteoarthritis: A systematic review and meta-analysis. Arthroscopy 2021, 37, 1277–1287. [Google Scholar] [CrossRef]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tenton, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: Results at 5 years of a double-blind, randomized controlled trial. Am. J. Sports Med. 2019, 47, 347–354. [Google Scholar] [CrossRef]
- Xu, Z.; He, Z.; Shu, L.; Li, X.; Ma, M.; Ye, C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthroscopy 2021, 37, 903–915. [Google Scholar] [CrossRef]
- Marmotti, A.; Bruzzone, M.; Bonasia, D.E.; Castoldi, F.; Rossi, R.; Piras, L.; Maiello, A.; Realmuto, C.; Peretti, G.M. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Hummer, C.D.; Angst, F.; Ngai, W.; Whittington, C.; Yoon, S.S.; Duarte, L.; Manitt, C.; Schemitsch, E. High molecular weight intraarticular hyaluronic acid for the treatment of knee osteoarthritis: A network meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 702. [Google Scholar] [CrossRef]
- Trigkilidas, D.; Anand, A. The effectiveness of hyaluronic acid intra-articular injections in managing osteoarthritic knee pain. Ann. R. Coll. Surg. Engl. 2013, 95, 545–551. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, J.; Han, S.; Li, Z.; Fan, T.; Zeng, M.; Wen, X.; Peng, Y.; Jiang, L.; Han, W.; et al. A model-based quantitative analysis of efficacy and associated factors of platelet-rich plasma treatment for osteoarthritis. Int. J. Surg. 2023, 109, 1742–1752. [Google Scholar] [CrossRef]
- Duif, C.; Vogel, T.; Topcuoglu, F.; Spyrou, G.; von Schulze Pellengahr, C.; Lahner, M. Does intraoperative application of leukocyte-poor platelet-rich plasma during arthroscopy for knee degeneration affect postoperative pain, function and quality of life? A 12-month randomized controlled double-blind trial. Arch. Orthop. Trauma. Surg. 2015, 135, 971–977. [Google Scholar] [CrossRef]
- Guney, A.; Akar, M.; Karaman, I.; Oner, M.; Guney, B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Berrigan, W.A.; Bailowitz, Z.; Park, A.; Reddy, A.; Liu, R.; Lansdown, D. A greater platelet dose may yield better clinical outcomes for platelet-rich plasma in the treatment of knee osteoarthritis: A systematic review. Arthroscopy 2025, 41, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; De Marziani, L.; Andriolo, L.; Di Martino, A.; Romandini, I.; Zaffagnini, S.; Filardo, G. Influence of platelet concentration on the clinical outcome of platelet-rich plasma injections in knee osteoarthritis. Am. J. Sports Med. 2024, 52, 3223–3231. [Google Scholar] [CrossRef]
- Bensa, A.; Previtali, D.; Sangiorgio, A.; Boffa, A.; Salerno, M.; Filardo, G. PRP injections for the treatment of knee osteoarthritis: The improvement is clinically significant and influenced by platelet concentration: A meta-analysis of randomized controlled trials. Am. J. Sports Med. 2025, 53, 745–754. [Google Scholar] [CrossRef]
- Ciapini, G.; Simonetti, M.; Giuntoli, M.; Varchetta, G.; De Franco, S.; Ipponi, E.; Scaglione, M.; Parchi, P.D. Is the combination of platelet-rich plasma and hyaluronic acid the best injective treatment for Grade II–III knee osteoarthritis? A prospective study. Adv. Orthop. 2023, 2023, 1868943. [Google Scholar] [CrossRef]
- Guillibert, C.; Charpin, C.; Raffray, M.; Benmenni, A.; Dehaut, F.X.; El Ghobeira, G.; Giorgi, R.; Magalon, J.; Arniaud, D. Single injection of high volume of autologous pure PRP provides a significant improvement in knee osteoarthritis: A prospective routine care study. Int. J. Mol. Sci. 2019, 20, 1327. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.A.; Ganai, A.A.; Jehangir, M.; Parry, A.H.; Sath, S.; Qayoom, S. MRI-based cartilage changes and clinical effectiveness of autologous intra-articular platelet-rich plasma injections in symptomatic patients with moderate osteoarthritis of the knee. Egypt. J. Radiol. Nucl. Med. 2014, 55, 30–39. [Google Scholar] [CrossRef]
- Wang, Y.; Hall, S.; Hanna, F.; Wluka, A.E.; Grant, G.; Marks, P.; Feletar, M.; Cicuttini, F.M. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: A two-year single-blind clinical trial. BMC Musculoskelet. Disord. 2011, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Alliston, T.; Hernandez, C.J.; Findlay, D.M.; Felson, D.T.; Kennedy, O.D. Bone marrow lesions in osteoarthritis: What lies beneath? J. Orthop. Res. 2018, 36, 1818–1825. [Google Scholar] [CrossRef]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef]
- Wu, P.I.; Diaz, R.; Borg-Stein, J. Platelet-rich plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef]


| Characteristic | Treatment | |
|---|---|---|
| HA+PRP | HA | |
| n = 29 | n = 29 | |
| Sex, n (%) | ||
| Female | 13 (44.8%) | 13 (44.8%) |
| Male | 16 (55.2%) | 16 (55.2%) |
| Ethnicity n (%) | ||
| Chinese | 25 (86.2%) | 23 (79.3%) |
| Malay | 1 (3.5%) | 1 (3.5%) |
| Indian | 1 (3.5%) | 4 (13.8%) |
| Others | 2 (6.9%) | 1 (3.5%) |
| Age | ||
| Mean (SD) | 57.7 (8.0) | 56.7 (8.1) |
| Min–max | 42–77 | 43–73 |
| BMI, mean (SD) | 27.0 (6.1) | 26.9 (3.1) |
| Baseline measures | ||
| VAS, mean (SD) | 52.9 (12.5) | 57.5 (13.8) |
| WOMAC, mean (SD) | ||
| Total | 54.4 (17.1) | 52.0 (19.0) |
| Pain | 10.5 (3.3) | 10.9 (3.9) |
| Stiffness | 5.2 (1.6) | 4.7 (1.9) |
| Difficulty | 38.7 (13.1) | 36.4 (14.1) |
| EQ-5D-5L, mean (SD) | 3.9 (3.2) | 3.3 (2.8) |
| WORMS score (SD) | ||
| Total score | 77.7 (44.8) | 63.6 (43.2) |
| Bone marrow edema | 5.5 (4.7) | 5.7 (4.4) |
| Synovitis | 1.6 (0.7) | 1.6 (0.7) |
| Predictor | Treatment | ||
|---|---|---|---|
| HA+PRP (n = 29) | HA (n = 29) | p-Value | |
| WOMAC total score (95% CI) | |||
| At baseline | 54.4 | 52 | |
| Month 1 | (−)7.1 (−13.4 to −0.86) | (−)9.8 (−16.0 to −3.5) | 0.563 |
| Month 2 | (−)11.3 (−17.6 to −5.1) | (−)10.7 (−16.9 to −4.4) | 0.885 |
| Month 6 | (−)10.8 (−17.0 to −4.5) | (−)12.9 (−19.1 to −6.6) | 0.642 |
| Month 12 | (−)13.4 (−19.7 to −7.2) | (−)13.2 (−19.5 to −6.9) | 0.957 |
| WOMAC pain score (95% CI) | |||
| At baseline | 10.5 | 10.9 | |
| Month 1 | (−)1.1 (−2.5 to 0.2) | (−)2.2 (−3.5 to −0.8) | 0.267 |
| Month 2 | (−)2.1 (−3.4 to −0.8) | (−)2.5 (−3.9 to −1.2) | 0.667 |
| Month 6 | (−)1.8 (−3.2 to −0.5) | (−)2.7 (−3.9 to −1.3) | 0.390 |
| Month 12 | (−)2.2 (−3.6 to −0.9) | (−)2.7 (−3.9 to −1.3) | 0.667 |
| WOMAC stiffness score (95% CI) | |||
| At baseline | 5.2 | 4.7 | |
| Month 1 | (−)0.9 (−1.6 to −0.3) | (−)0.9 (−1.5 to −0.2) | 0.826 |
| Month 2 | (−)1.3 (−1.9 to −0.7) | (−)1.1 (−1.7 to −0.4) | 0.608 |
| Month 6 | (−)0.9 (−1.6 to −0.3) | (−)1.3 (−1.9 to −0.7) | 0.420 |
| Month 12 | (−)1.4 (−2.1 to −0.8) | (−)1.6 (−2.3 to −0.9) | 0.660 |
| WOMAC difficulty score (95% CI) | |||
| At baseline | 38.7 | 36.4 | |
| Month 1 | (−)5.0 (−9.6 to −0.4) | (−)6.7 (−11.3 to −2.1) | 0.618 |
| Month 2 | (−)7.9 (−12.5 to −3.3) | (−)7.1 (−11.7 to −2.5) | 0.803 |
| Month 6 | (−)8.0 (−12.6 to −3.4) | (−)8.9 (−13.5 to −4.3) | 0.787 |
| Month 12 | (−)9.8 (−14.4 to −5.2) | (−)8.9 (−13.5 to −4.3) | 0.795 |
| EQ-5D-5L score (95% CI) | |||
| At baseline | 3.9 | 3.3 | |
| Month 1 | 0.1 (−0.002 to 0.2) | 0.1 (0.01 to 0.2) | 0.835 |
| Month 2 | 0.1 (0.03 to 0.2) | 0.2 (0.1 to 0.3) | 0.667 |
| Month 6 | 0.1 (−0.01 to 0.2) | 0.1 (0.03 to 0.2) | 0.600 |
| Month 12 | 0.1 (−0.001 to 0.2) | 0.2 (0.1 to 0.3) | 0.395 |
| Total WORMS score (95% CI) | |||
| At baseline | 77.7 | 63.6 | |
| Month 12 | 1.2 (−3.6 to 5.9) | 1.3 (−3.5 to 6.1) | 0.962 |
| Bone marrow edema (95% CI) | |||
| At baseline | 5.5 | 5.7 | |
| Month 12 | (−)0.7 (−1.6 to 0.2) | 0.7 (−0.2 to 1.6) | 0.030 |
| Synovitis | |||
| At baseline | 1.6 | 1.6 | |
| Month 12 | (−)0.04 (−0.1 to 0.1) | (−)0.03 (−0.1 to 0.1) | 0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chew, K.; Goh, P.; Tun, M.H.; Sheah, K.; Tan, V.; Lim, B.; Ng, C.S.; Tan, B. Clinical Efficacy of Platelet-Rich Plasma and Hyaluronic Acid Versus Hyaluronic Acid for Knee Osteoarthritis with MRI Analysis: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 3553. https://doi.org/10.3390/jcm14103553
Zhang M, Chew K, Goh P, Tun MH, Sheah K, Tan V, Lim B, Ng CS, Tan B. Clinical Efficacy of Platelet-Rich Plasma and Hyaluronic Acid Versus Hyaluronic Acid for Knee Osteoarthritis with MRI Analysis: A Randomized Controlled Trial. Journal of Clinical Medicine. 2025; 14(10):3553. https://doi.org/10.3390/jcm14103553
Chicago/Turabian StyleZhang, Mandy, Kelvin Chew, Patrick Goh, Mon Hnin Tun, Kenneth Sheah, Victor Tan, Baoying Lim, Chung Sien Ng, and Benedict Tan. 2025. "Clinical Efficacy of Platelet-Rich Plasma and Hyaluronic Acid Versus Hyaluronic Acid for Knee Osteoarthritis with MRI Analysis: A Randomized Controlled Trial" Journal of Clinical Medicine 14, no. 10: 3553. https://doi.org/10.3390/jcm14103553
APA StyleZhang, M., Chew, K., Goh, P., Tun, M. H., Sheah, K., Tan, V., Lim, B., Ng, C. S., & Tan, B. (2025). Clinical Efficacy of Platelet-Rich Plasma and Hyaluronic Acid Versus Hyaluronic Acid for Knee Osteoarthritis with MRI Analysis: A Randomized Controlled Trial. Journal of Clinical Medicine, 14(10), 3553. https://doi.org/10.3390/jcm14103553







