Advances in Intraperitoneal Chemotherapy for Gastric Cancer Patients with Peritoneal Metastases: Current Status of Treatment and Institutional Insights
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Treatment
2.2. Assessment of Response and Toxicity
2.3. Statistical Analysis
3. Results
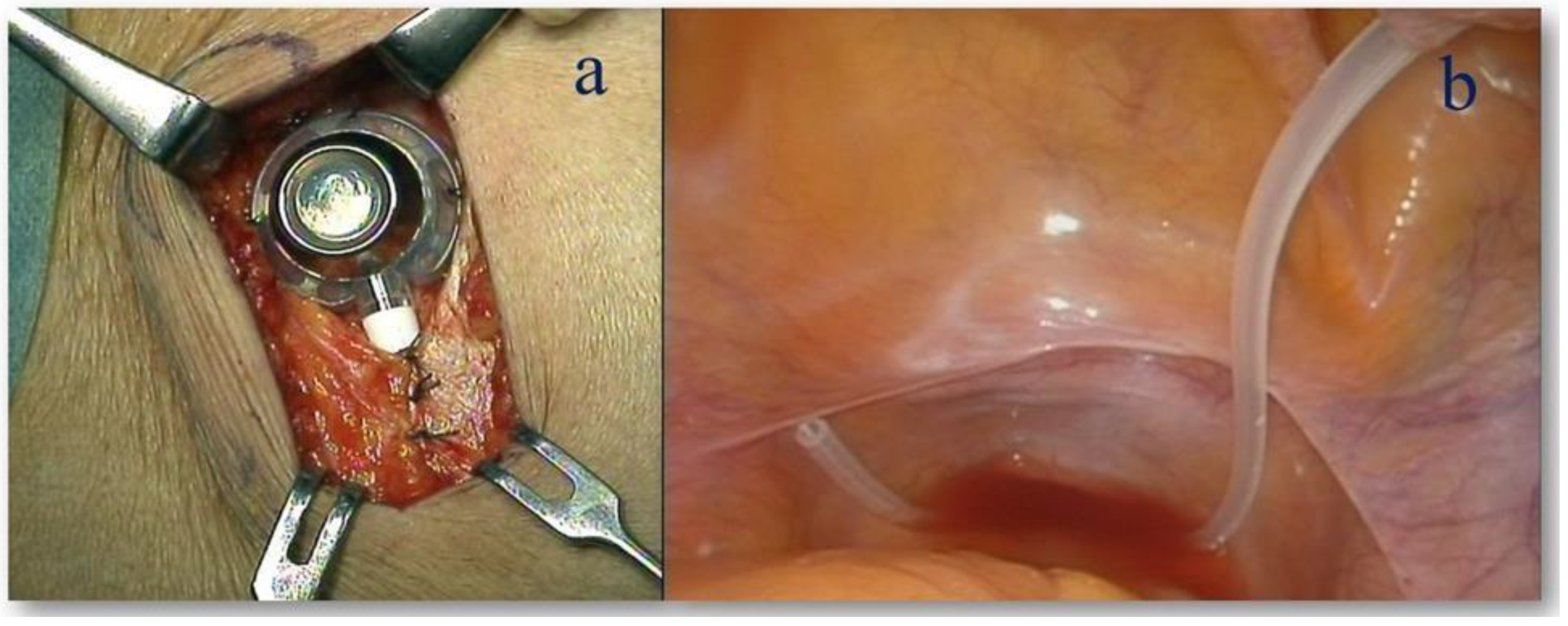
3.1. Intraperitoneal Administration of Paclitaxel (IP-PTX) Combined with S-1 Plus Oxaliplatin (SOX) for GC with PM
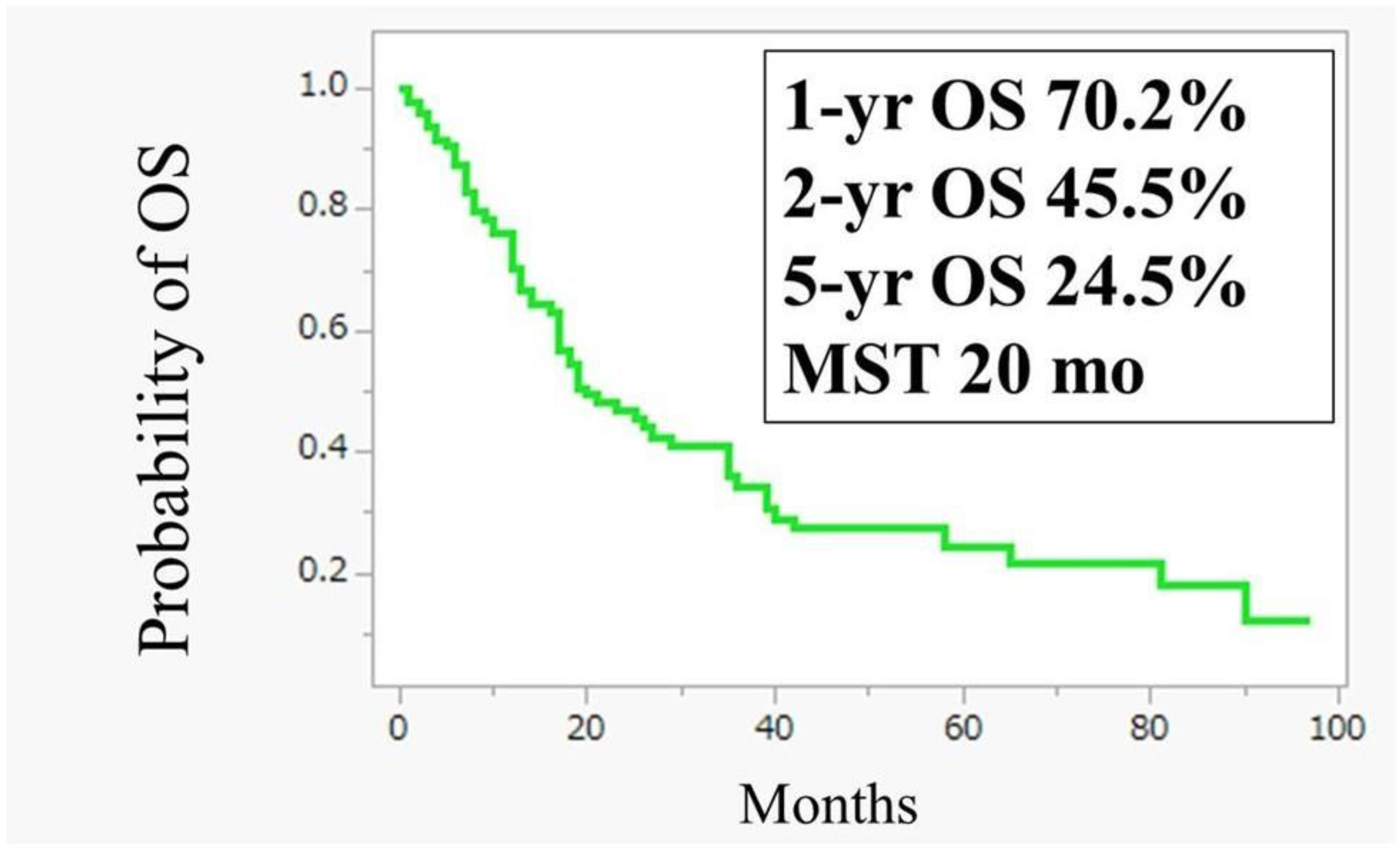
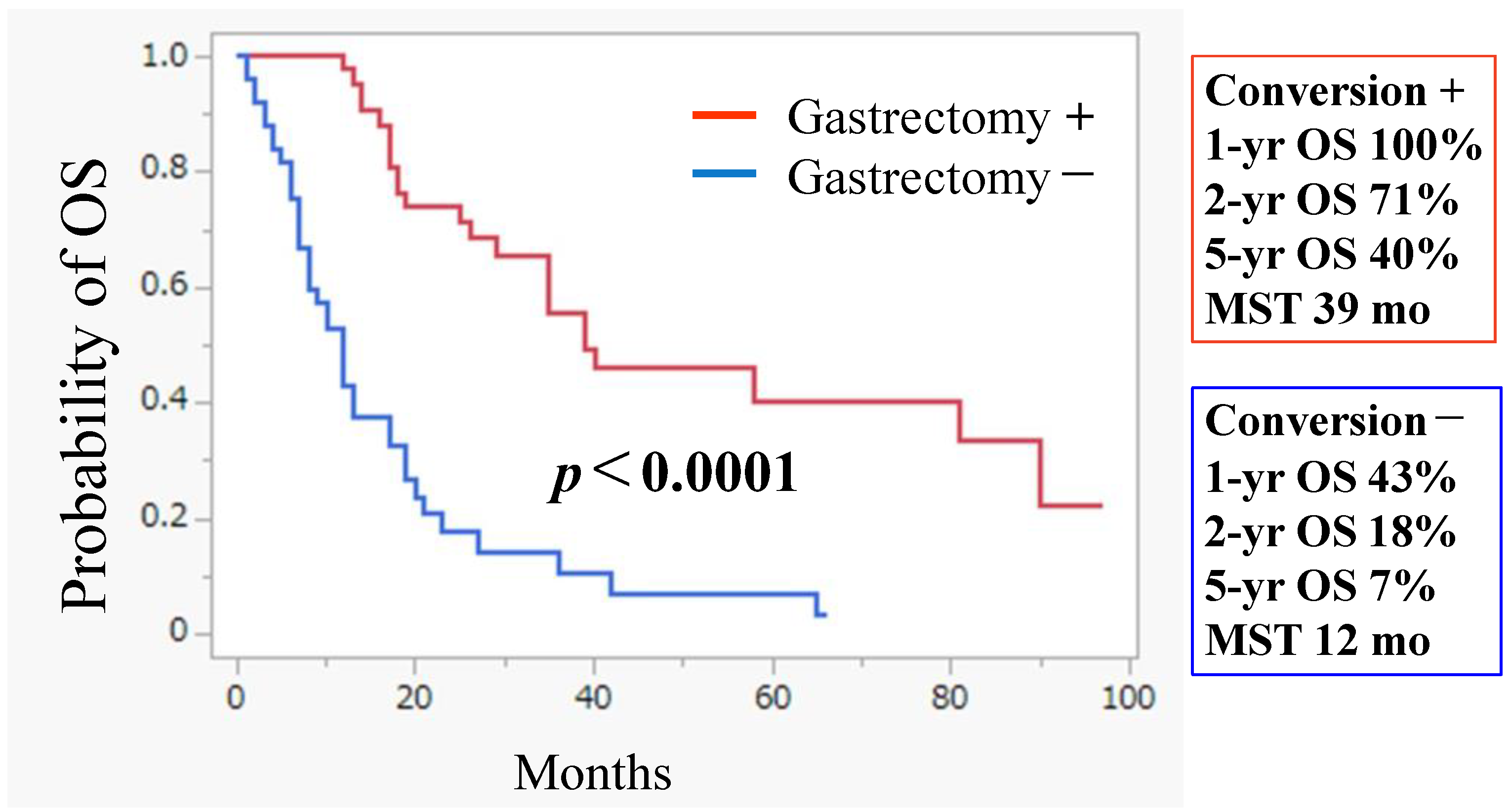
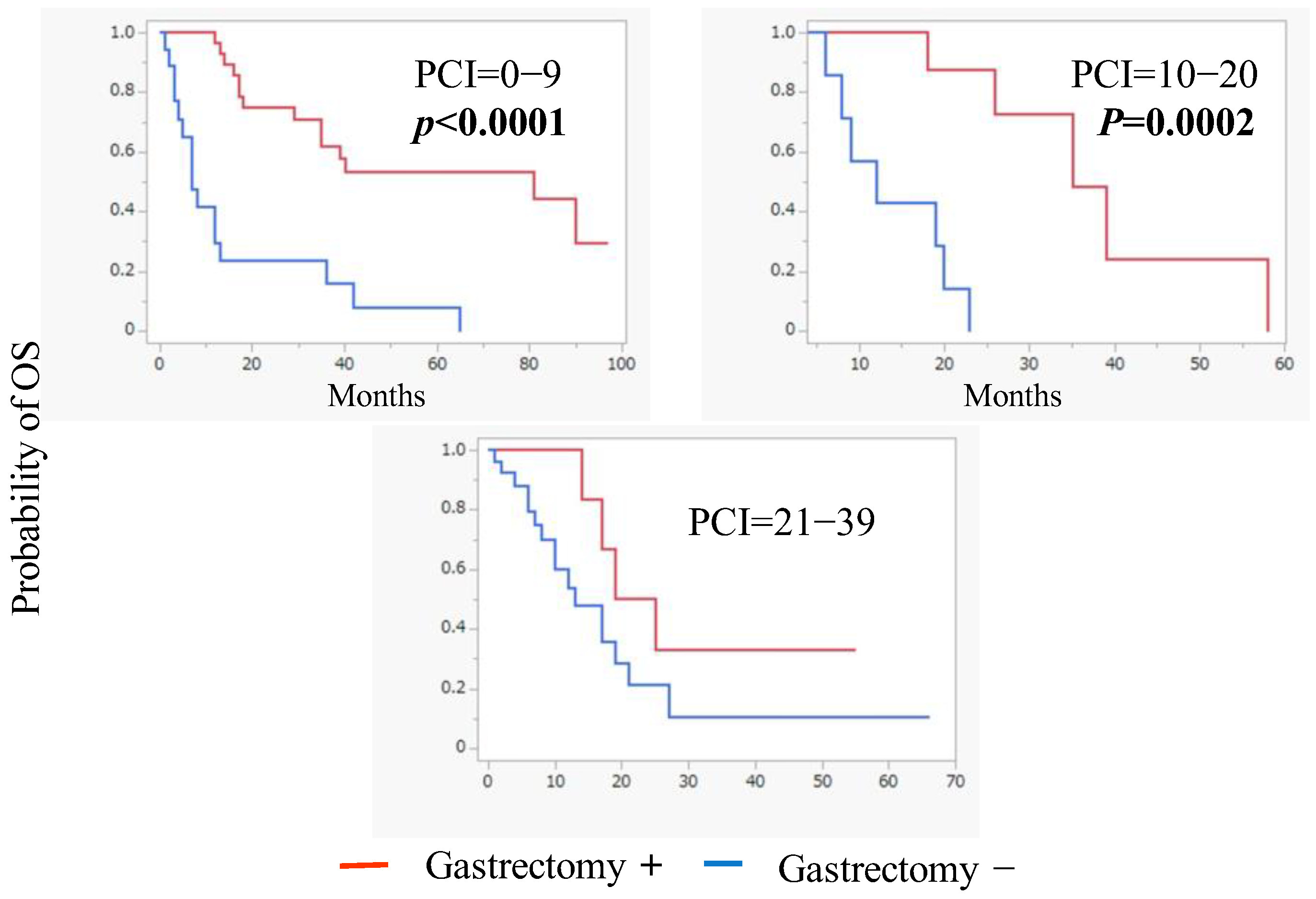
3.2. Conversion Surgery
4. Discussion
4.1. Promising Strategy Against Peritoneal Disease
4.2. Future Perspectives on Treatment for Peritoneal Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric Cancer |
| PM | Peritoneal Metastasis |
| OS | Overall Survival |
| PFS | Progression Free Survival |
| HIPEC | Hyperthermic Intraperitoneal Chemotherapy |
| CLDN18.2 | Claudin 18 isoform 2 |
| MST | Mean Survival Time |
| IP-PTX | Intraperitoneal–Paclitaxel |
| miR | microRNA |
| MMT | Mesothelial Mesenchymal Transition |
| AAV | Adeno-Associated Virus |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I. Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Johnston, F.M. Current role for cytoreduction and HIPEC for gastric cancer with peritoneal disease. J. Surg. Oncol. 2022, 125, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.N.; Wen, F.; Xu, P.; Zhang, S. Prognostic significance of malignant ascites in gastric cancer patients with peritoneal metastasis: A systemic review and meta-analysis. World J. Clin. Cases 2019, 7, 3247–3258. [Google Scholar] [CrossRef]
- Yarema, R.; Ohorchak, M.; Hyrya, P.; Kovalchuk, Y.; Safiyan, V.; Karelin, I.; Ferneza, S.; Fetsych, M.; Matusyak, M.; Oliynyk, Y.; et al. Gastric cancer with peritoneal metastases: Efficiency of standard treatment methods. World J. Gastrointest. Oncol. 2020, 12, 569–581. [Google Scholar] [CrossRef]
- Coccolini, F.; Cotte, E.; Glehen, O.; Lotti, M.; Poiasina, E.; Catena, F.; Yonemura, Y.; Ansaloni, L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur. J. Surg. Oncol. 2014, 40, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wu, N.D.; Liu, B.R. Regional but fatal: Intraperitoneal metastasis in gastric cancer. World J. Gastroenterol. 2016, 22, 7478–7485. [Google Scholar] [CrossRef]
- Kinoshita, J.; Yamaguchi, T.; Moriyama, H.; Fushida, S. Current status of conversion surgery for stage IV gastric cancer. Surg. Today 2021, 51, 1736–1754. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Yoo, C.H.; Noh, S.H.; Shin, D.W.; Choi, S.H.; Min, J.S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 2000, 87, 236–242. [Google Scholar] [CrossRef]
- Chan, D.Y.; Syn, N.L.; Yap, R.; Phua, J.N.; Soh, T.I.; Chee, C.E.; Nga, M.E.; Shabbir, A.; So, J.B.; Yong, W.P. Conversion Surgery Post-Intraperitoneal Paclitaxel and Systemic Chemotherapy for Gastric Cancer Carcinomatosis Peritonei. Are We Ready? J. Gastrointest. Surg. 2017, 21, 425–433. [Google Scholar] [CrossRef]
- Gwee, Y.X.; Chia, D.K.A.; So, J.; Ceelen, W.; Yong, W.P.; Tan, P.; Ong, C.J.; Sundar, R. Integration of Genomic Biology into Therapeutic Strategies of Gastric Cancer Peritoneal Metastasis. J. Clin. Oncol. 2022, 40, 2830. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Y.; Zhuang, W.; Fang, S.; Chen, Q.; Lian, M.; Lv, C.; Weng, J.; Wei, R.; Lin, Y.; et al. Gastric cancer peritoneal metastasis related signature predicts prognosis and sensitivity to immunotherapy in gastric cancer. J. Cell. Mol. Med. 2023, 27, 3578–3590. [Google Scholar] [CrossRef]
- Allen, C.J.; Blumenthaler, A.N.; Das, P.; Minsky, B.D.; Blum, M.; Roy-Chowdhuri, S.; Ajani, J.A.; Ikoma, N.; Mansfield, P.F.; Badgwell, B.D. Staging laparoscopy and peritoneal cytology in patients with early stage gastric adenocarcinoma. World J. Surg. Oncol. 2020, 18, 39. [Google Scholar] [CrossRef]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Saito, T.; Nakanishi, H.; Mochizuki, Y.; Ito, S.; Ito, Y.; Misawa, K.; Yatabe, Y.; Yamamichi, K.; Kondo, E. Preferential HER2 expression in liver metastases and EGFR expression in peritoneal metastases in patients with advanced gastric cancer. Gastric Cancer 2015, 18, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Boku, N.; Yamaguchi, T.; Ohtsu, A.; Mizusawa, J.; Nakamura, K.; Fukuda, H. Inter-institutional heterogeneity in outcomes of chemotherapy for metastatic gastric cancer: Correlative study in the JCOG9912 phase III trial. ESMO Open 2016, 1, e000031. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli Liu, T.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef]
- Ishigami, H.; Kitayama, J.; Kaisaki, S.; Hidemura, A.; Kato, M.; Otani, K.; Kamei, T.; Soma, D.; Miyato, H.; Yamashita, H.; et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann. Oncol. 2010, 21, 67–70. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Yamaguchi, K.; Boku, N.; Hyodo, I.; Mizusawa, J.; Hara, H.; Nishina, T.; Sakamoto, T.; Shitara, K.; Shinozaki, K.; et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer 2020, 23, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: The randomized, phase 3 GLOW trial. Nat. Med. 2023, 29, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Ohzawa, H.; Kawashima, R.; Matsumoto, S.; Kurashina, K.; Saito, S.; Miyato, H.; Hosoya, Y.; Sata, N.; Kitayama, J.; et al. Expression of Claudin18.2 in metastatic lesions in peritoneum of gastric cancer. J. Gastrointest. Oncol. 2025, 16, 67–76. [Google Scholar] [CrossRef]
- Boku, N.; Omori, T.; Shitara, K.; Sakuramoto, S.; Yamaguchi, K.; Kato, K.; Kadowaki, S.; Tsuji, K.; Ryu, M.H.; Oh, D.Y.; et al. Nivolumab plus chemotherapy in patients with HER2-negative, previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: 3-year follow-up of the ATTRACTION-4 randomized, double-blind, placebo-controlled, phase 3 trial. Gastric Cancer 2024, 27, 1287–1301. [Google Scholar] [CrossRef]
- Saito, S.; Yamaguchi, H.; Ohzawa, H.; Miyato, H.; Kanamaru, R.; Kurashina, K.; Hosoya, Y.; Lefor, A.K.; Sata, N.; Kitayama, J. Intraperitoneal Administration of Paclitaxel Combined with S-1 Plus Oxaliplatin as Induction Therapy for Patients with Advanced Gastric Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3863–3870. [Google Scholar] [CrossRef]
- Ishigami, H.; Fujiwara, Y.; Fukushima, R.; Nashimoto, A.; Yabusaki, H.; Imano, M.; Imamoto, H.; Kodera, Y.; Uenosono, Y.; Amagai, K.; et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOE-NIX-GC Trial. J. Clin. Oncol. 2018, 36, 1922–1929. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Soma, D.; Kitayama, J.; Konno, T.; Ishihara, K.; Yamada, J.; Kamei, T.; Ishigami, H.; Kaisaki, S.; Nagawa, H. Intraperitoneal administration of paclitaxel solubilized with poly(2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) for peritoneal dissemination of gastric cancer. Cancer Sci. 2009, 100, 1979–1985. [Google Scholar] [CrossRef]
- Ye, Z.; Yu, P.; Cao, Y.; Chai, T.; Huang, S.; Cheng, X.; Du, Y. Prediction of Peritoneal Cancer Index and Prognosis in Peritoneal Metastasis of Gastric Cancer Using NLR-PLR-DDI Score: A Retrospective Study. Cancer Manag. Res. 2022, 14, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Steuperaert, M.; Debbaut, C.; Segers, P.; Ceelen, W. Modelling drug transport during intraperitoneal chemotherapy. Pleura Peritoneum 2017, 2, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Peritoneal-plasma barrier. Cancer Treat. Res. 1996, 82, 53–63. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Li, Y.; Lin, S.; Tomuleasa, C. microRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J. BUON 2014, 19, 228–236. [Google Scholar] [PubMed]
- Li, X.; Zhang, Y.; Zhang, H.; Liu, X.; Gong, T.; Li, M.; Sun, L.; Ji, G.; Shi, Y.; Han, Z.; et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol. Cancer Res. 2011, 9, 824–833. [Google Scholar] [CrossRef]
- Ohzawa, H.; Kimura, Y.; Saito, A.; Yamaguchi, H.; Miyato, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Lefor, A.K.; Sata, N.; et al. Ratios of miRNAs in Peritoneal Exosomes are Useful Biomarkers to Predict Tumor Response to Intraperitoneal Chemotherapy in Patients with Peritoneal Metastases from Gastric Cancer. Ann. Surg. Oncol. 2020, 27, 5057–5064. [Google Scholar] [CrossRef]
- Yan, B.; Guo, Q.; Fu, F.J.; Wang, Z.; Yin, Z.; Wei, Y.B.; Yang, J.R. The role of miR-29b in cancer: Regulation, function, and signaling. Onco Targets Ther. 2015, 8, 539–548. [Google Scholar] [CrossRef]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A systematic review of miR-29 in cancer. Mol. Ther. Oncolytics 2019, 12, 173–194. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ohzawa, H.; Kimura, Y.; Takahashi, R.; Matsumiya, M.; Tamura, K.; Futoh, Y.; Miyato, H.; Saito, S.; Yamaguchi, H.; et al. Kitayama: Intraperitoneal administration of adeno-associated virus encoding microRNA-29b for the treatment of peritoneal metastasis. Cancer Gene Ther. 2024, 31, 1818–1830. [Google Scholar] [CrossRef]
- Saito, H.; Fushida, S.; Harada, S.; Miyashita, T.; Oyama, K.; Yamaguchi, T.; Tsukada, T.; Kinoshita, J.; Tajima, H.; Ninomiya, I.; et al. Importance of human peritoneal mesothelial cells in the progression, fibrosis, and control of gastric cancer: Inhibition of growth and fibrosis by tranilast. Gastric Cancer 2018, 21, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, P.; Jiménez-Heffernan, J.A.; Guerra-Azcona, G.; Pérez-Lozano, M.L.; Rynne-Vidal, Á.; Albar-Vizcaíno, P.; Gil-Vera, F.; Martín, P.; Coronado, M.J.; Barcena, C.; et al. Mesothelial-to-mesenchymal transition in the pathogenesis of post-surgical peritoneal adhesions. J. Pathol. 2016, 239, 48–59. [Google Scholar] [CrossRef]
- Kojima, M.; Higuchi, Y.; Yokota, M.; Ishii, G.; Saito, N.; Aoyagi, K.; Sasaki, H.; Ochiai, A. Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS ONE 2014, 9, e88018. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Feng, M.; Guan, W. Mechanisms of peritoneal dissemination in gastric cancer. Oncol. Lett. 2017, 14, 6991–6998. [Google Scholar] [CrossRef]
- Yoshihara, M.; Kajiyama, H.; Yokoi, A.; Sugiyama, M.; Koya, Y.; Yamakita, Y.; Liu, W.; Nakamura, K.; Moriyama, Y.; Yasui, H.; et al. Ovarian cancer-associated mesothelial cells induce acquired platinum-resistance in peritoneal metastasis via the FN1/Akt signaling pathway. Int. J. Cancer 2020, 146, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Safarzadeh, A.; Beyranvand, F.; Ahmadpour, F.; Hajiasgharzadeh, K.; Baghbanzadeh, A.; Baradaran, B. The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J. Cell. Physiol. 2019, 234, 19280–19297. [Google Scholar] [CrossRef]
- Kimura, Y.; Ohzawa, H.; Miyato, H.; Kaneko, Y.; Kuchimaru, T.; Takahashi, R.; Yamaguchi, H.; Kurashina, K.; Saito, S.; Hosoya, Y.; et al. Intraperitoneal transfer of microRNA-29b-containing small extracellular vesicles can suppress peritoneal metastases of gastric cancer. Cancer Sci. 2023, 114, 2939–2950. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Yamaguchi, H.; Saito, A.; Kaneko, Y.; Ohzawa, H.; Yokota, S.; Kitayama, J. Advances in Intraperitoneal Chemotherapy for Gastric Cancer Patients with Peritoneal Metastases: Current Status of Treatment and Institutional Insights. J. Clin. Med. 2025, 14, 3521. https://doi.org/10.3390/jcm14103521
Saito S, Yamaguchi H, Saito A, Kaneko Y, Ohzawa H, Yokota S, Kitayama J. Advances in Intraperitoneal Chemotherapy for Gastric Cancer Patients with Peritoneal Metastases: Current Status of Treatment and Institutional Insights. Journal of Clinical Medicine. 2025; 14(10):3521. https://doi.org/10.3390/jcm14103521
Chicago/Turabian StyleSaito, Shin, Hironori Yamaguchi, Akira Saito, Yuki Kaneko, Hideyuki Ohzawa, Shinichiro Yokota, and Joji Kitayama. 2025. "Advances in Intraperitoneal Chemotherapy for Gastric Cancer Patients with Peritoneal Metastases: Current Status of Treatment and Institutional Insights" Journal of Clinical Medicine 14, no. 10: 3521. https://doi.org/10.3390/jcm14103521
APA StyleSaito, S., Yamaguchi, H., Saito, A., Kaneko, Y., Ohzawa, H., Yokota, S., & Kitayama, J. (2025). Advances in Intraperitoneal Chemotherapy for Gastric Cancer Patients with Peritoneal Metastases: Current Status of Treatment and Institutional Insights. Journal of Clinical Medicine, 14(10), 3521. https://doi.org/10.3390/jcm14103521




