Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Extraction
2.2. Descriptive Analysis
2.3. Disproportionality Analysis
- (i)
- Number of cases > 2;
- (ii)
- Chi-square (χ2) > 4;
- (iii)
- Proportional Reporting Ratio (PRR) > 2 [20].
2.4. Ethics
3. Results
3.1. Data Mining
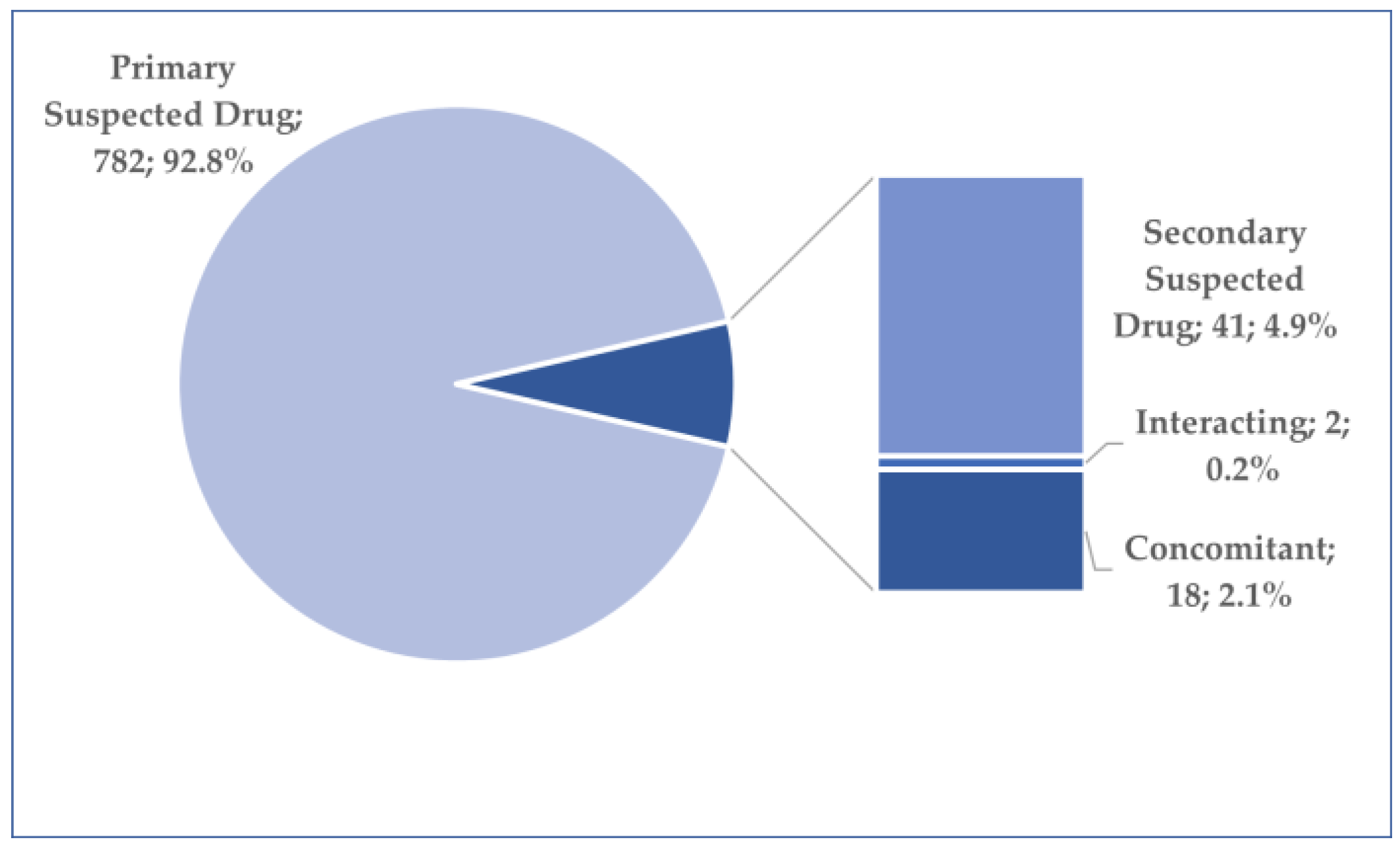
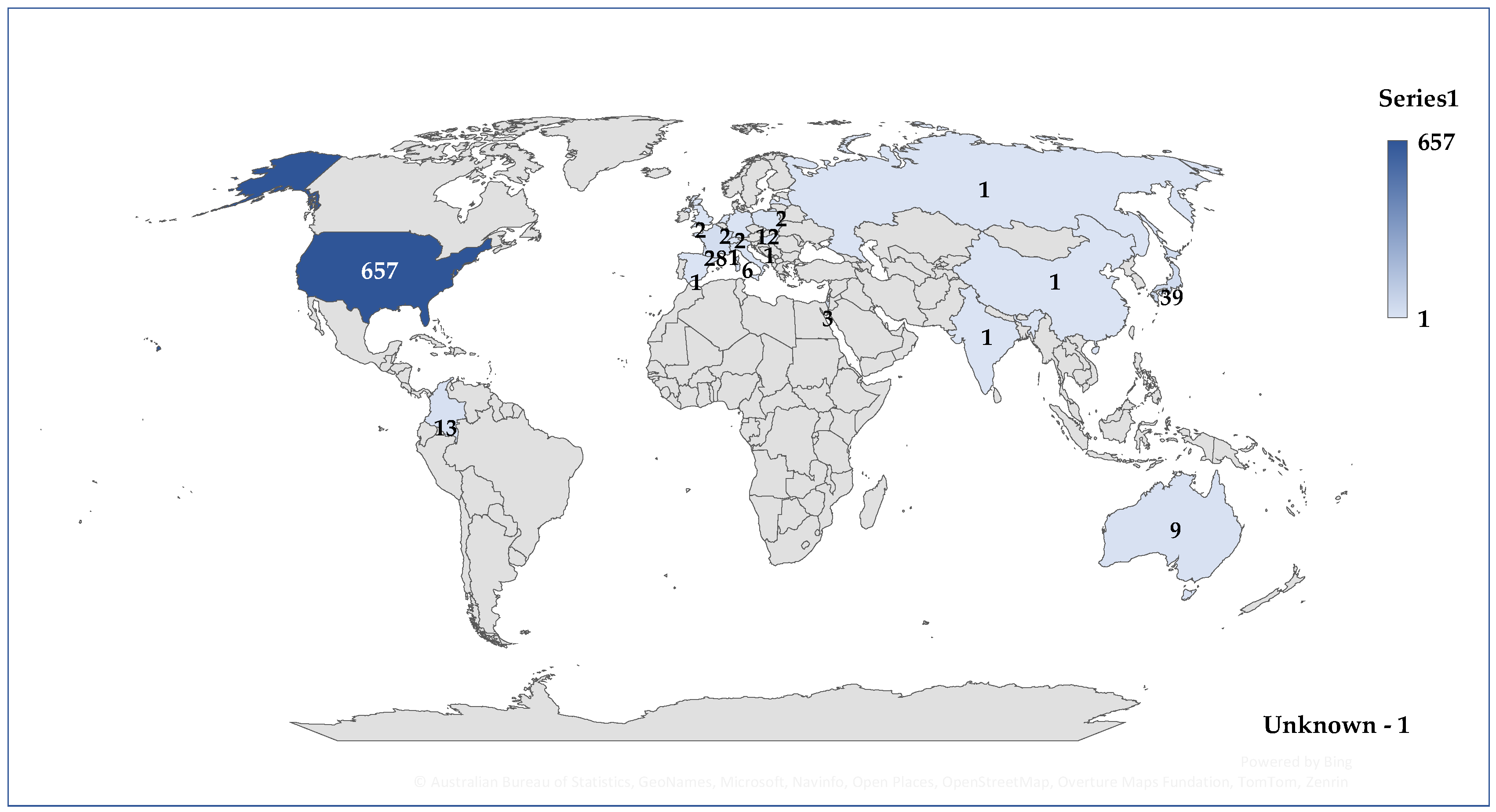
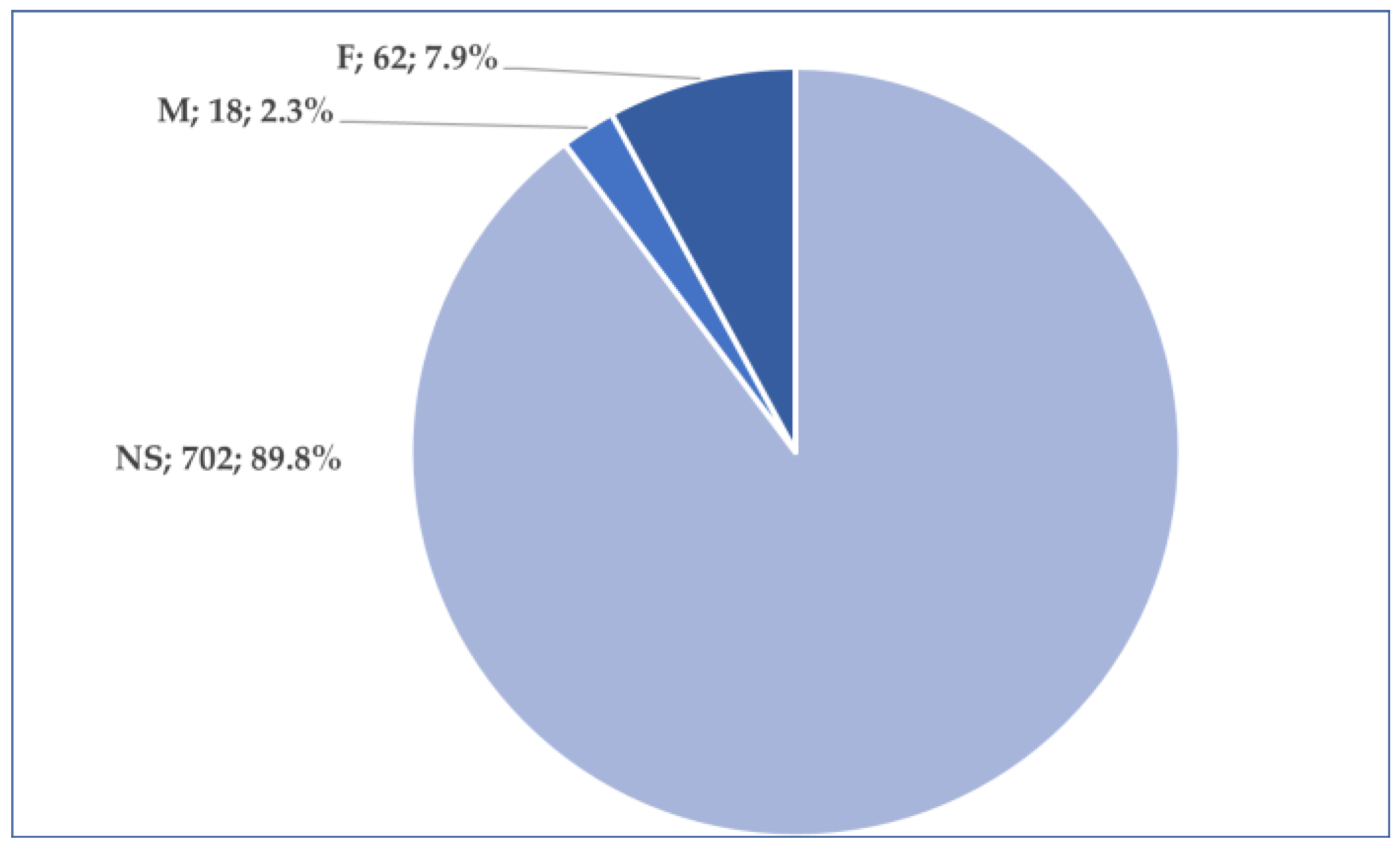
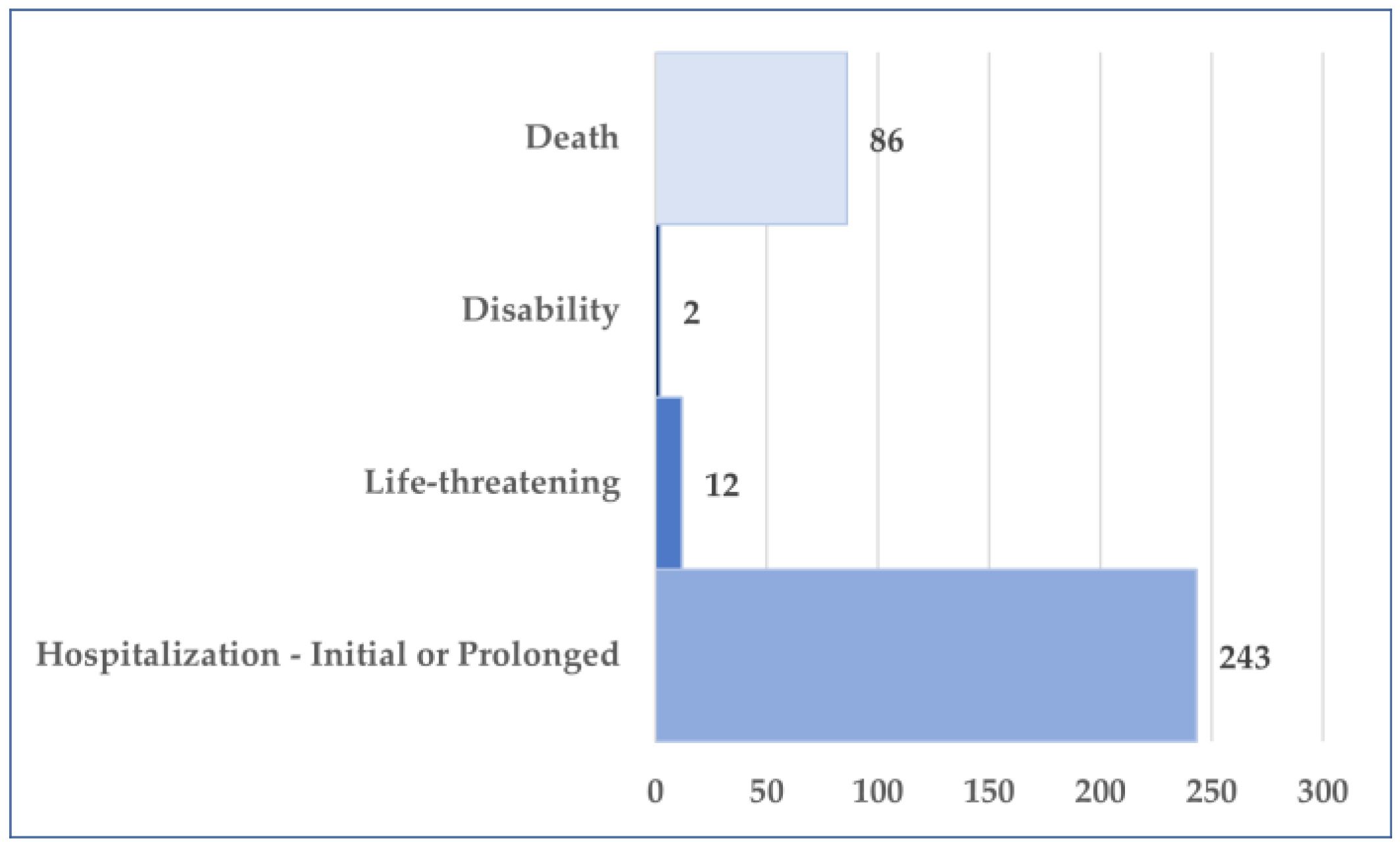
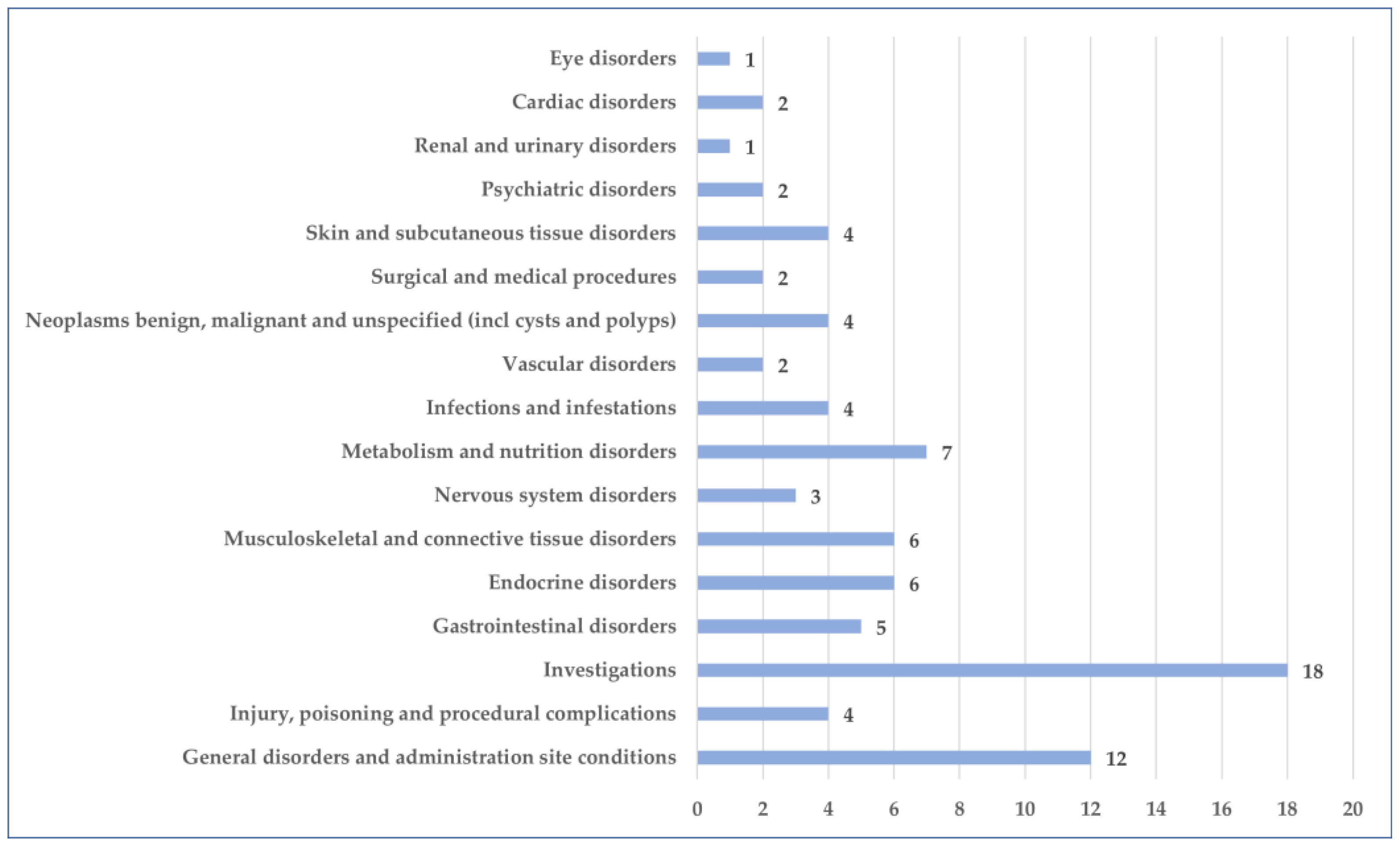
3.2. General Characteristics of Cases Related to Osilodrostat Use
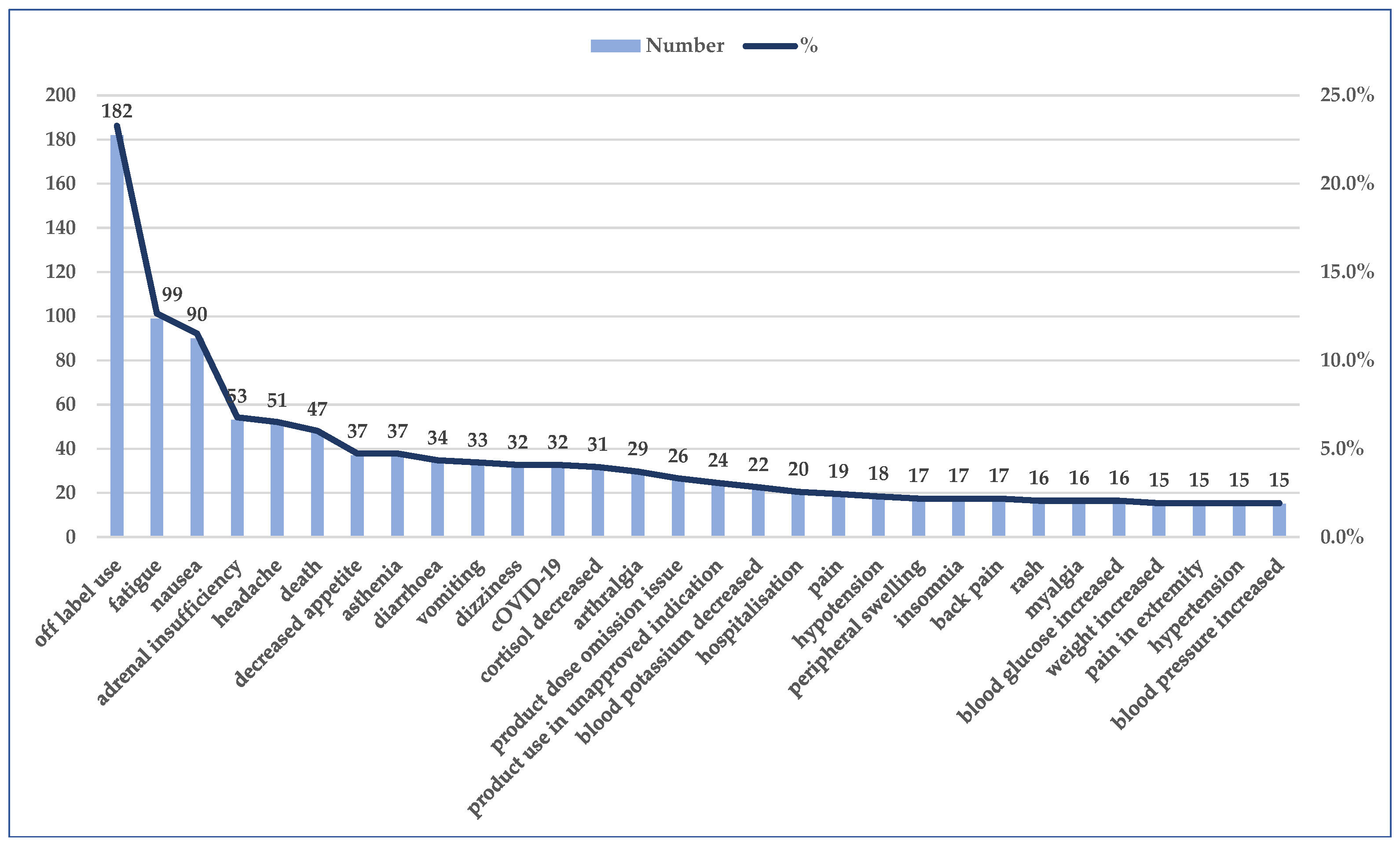
3.3. Analysis of Prefered Terms Distribution
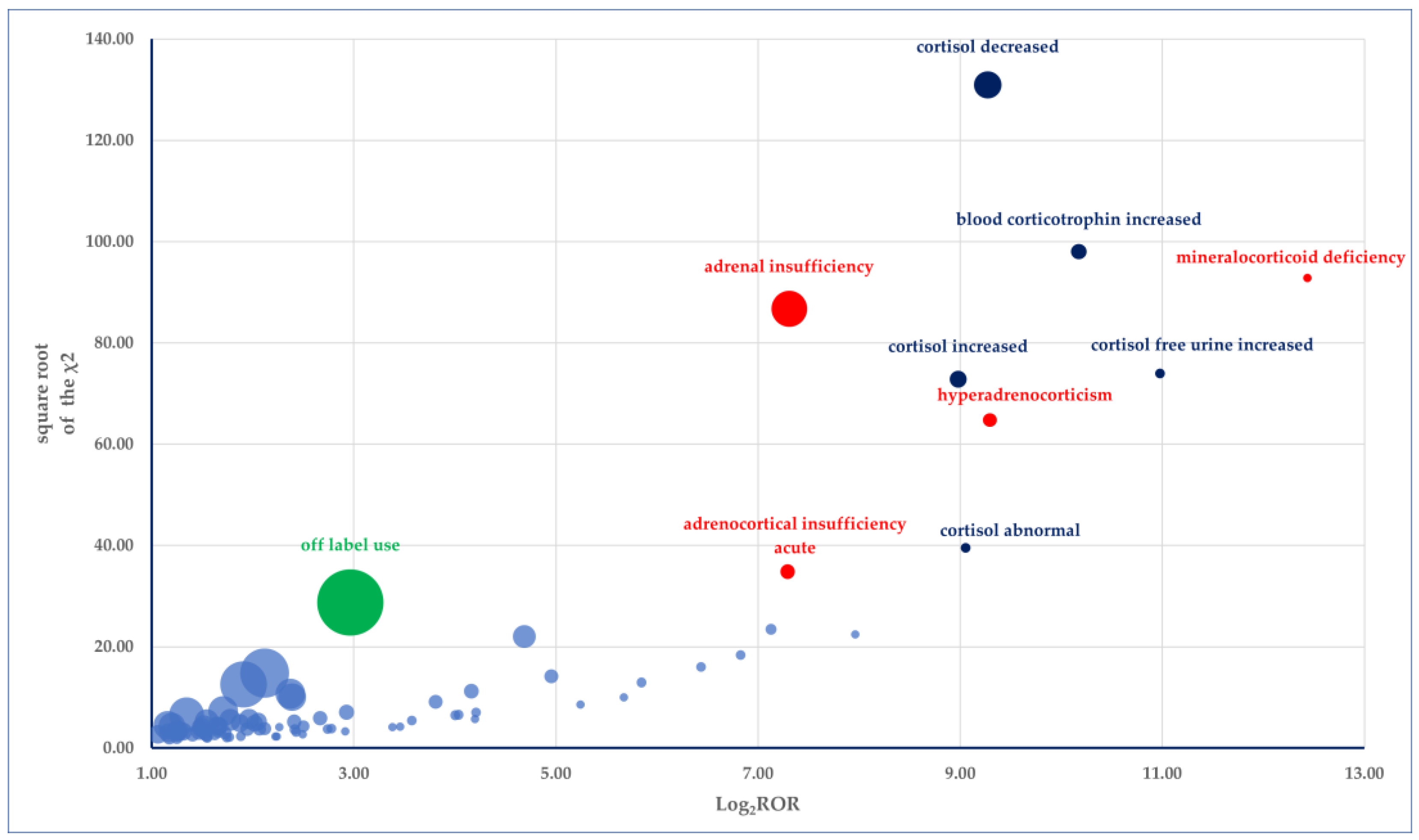
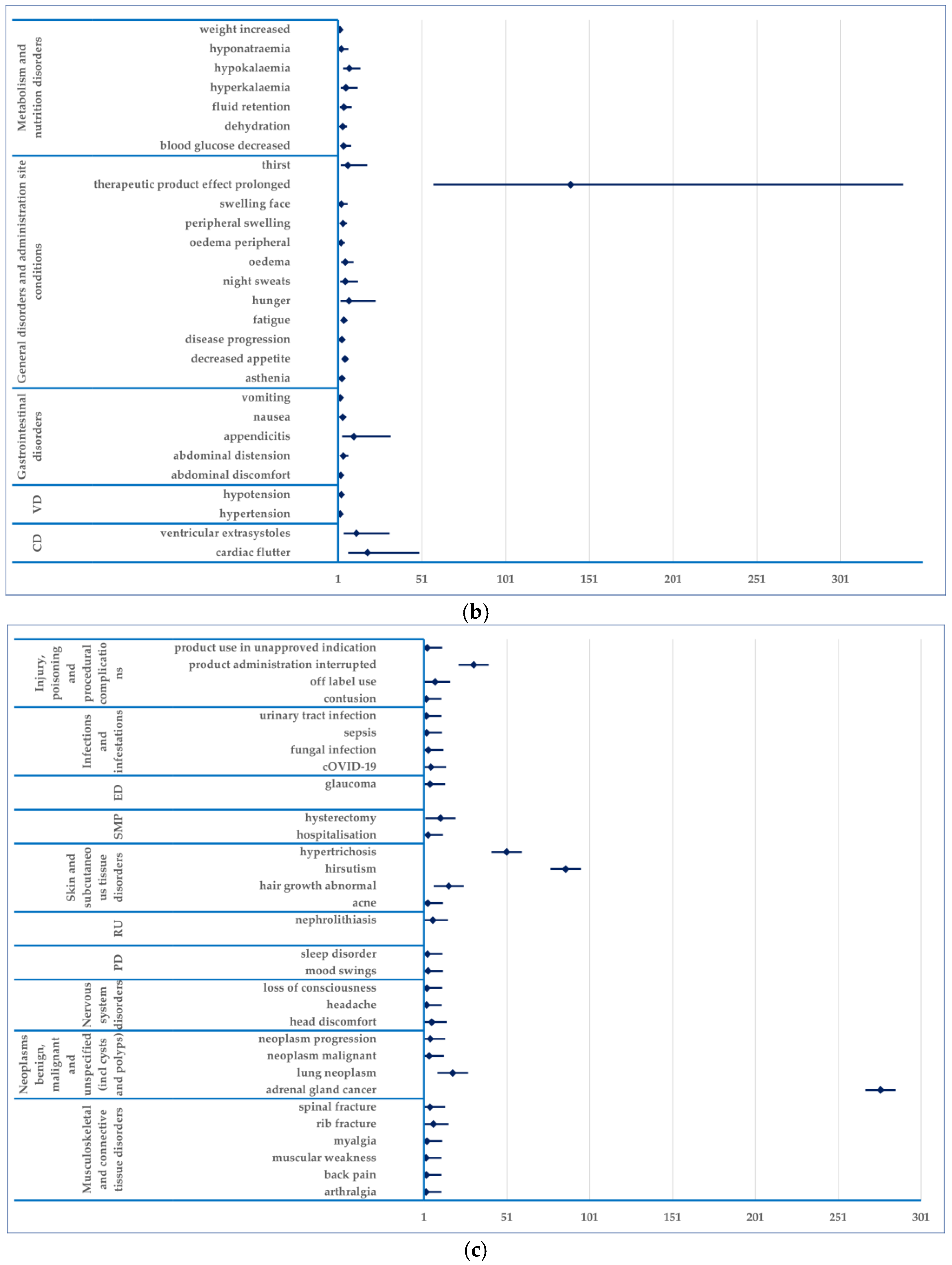
3.4. Disproportionality Analysis
- (i)
- Endocrine disorders: mineralocorticoid deficiency (PRR: 5513.88), hyperadrenocorticism (PRR: 621.28), glucocorticoid deficiency (PRR: 248.13); adrenocortical insufficiency acute (PRR: 154.92)
- (ii)
- Investigations: cortisol free urine increased (PRR: 2005.05), blood corticotrophin increased (PRR: 1140.80), cortisol decreased (PRR: 594.20), cortisol increased (PRR: 497.49), cortisol abnormal (PRR: 529.33)
- (iii)
- Neoplasms benign, malignant and unspecified (incl cysts and polyps): adrenal gland cancer (PRR: 274.78).
4. Discussions
Limitations of Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahakian, N.; Castinetti, F.; Brue, T.; Cuny, T. Current and Emerging Medical Therapies in Pituitary Tumors. J. Clin. Med. 2022, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Tritos, N.A. Adrenally Directed Medical Therapies for Cushing Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 16–25. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Desai, D.S.; Herrera, J.B. Osilodrostat: A Novel Steroidogenesis Inhibitor to Treat Cushing’s Disease. Ann. Pharmacother. 2021, 55, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Skinner, B.W. Osilodrostat for the Treatment of Cushing’s Disease. Expert Opin. Pharmacother. 2021, 22, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Popa Ilie, I.R.; Herdean, A.M.; Herdean, A.I.; Georgescu, C.E. Spontaneous Remission of Cushing’s Disease: A Systematic Review. Ann. Endocrinol. 2021, 82, 613–621. [Google Scholar] [CrossRef]
- Rubinstein, G.; Osswald, A.; Zopp, S.; Ritzel, K.; Theodoropoulou, M.; Beuschlein, F.; Reincke, M. Therapeutic Options after Surgical Failure in Cushing’s Disease: A Critical Review. Best Pr. Res. Clin. Endocrinol. Metab. 2019, 33, 101270. [Google Scholar] [CrossRef]
- Tritos, N.A.; Biller, B.M.K. Current Management of Cushing’s Disease. J. Intern. Med. 2019, 286, 526–541. [Google Scholar] [CrossRef]
- Pivonello, R.; De Leo, M.; Cozzolino, A.; Colao, A. The Treatment of Cushing’s Disease. Endocr. Rev. 2015, 36, 385–486. [Google Scholar] [CrossRef]
- Feelders, R.A.; Newell-Price, J.; Pivonello, R.; Nieman, L.K.; Hofland, L.J.; Lacroix, A. Advances in the Medical Treatment of Cushing’s Syndrome. Lancet Diabetes Endocrinol. 2019, 7, 300–312. [Google Scholar] [CrossRef]
- Fleseriu, M.; Findling, J.W.; Koch, C.A.; Schlaffer, S.-M.; Buchfelder, M.; Gross, C. Changes in Plasma ACTH Levels and Corticotroph Tumor Size in Patients with Cushing’s Disease During Long-Term Treatment with the Glucocorticoid Receptor Antagonist Mifepristone. J. Clin. Endocrinol. Metab. 2014, 99, 3718–3727. [Google Scholar] [CrossRef]
- Langlois, F.; Chu, J.; Fleseriu, M. Pituitary-Directed Therapies for Cushing’s Disease. Front. Endocrinol. 2018, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Perosevic, M.; Tritos, N.A. Clinical Utility of Osilodrostat in Cushing’s Disease: Review of Currently Available Literature. Drug Des. Dev. Ther. 2023, 17, 1303–1312. [Google Scholar] [CrossRef]
- Duggan, S. Osilodrostat: First Approval. Drugs 2020, 80, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Broder, M.S.; Neary, M.P.; Chang, E.; Cherepanov, D.; Ludlam, W.H. Incidence of Cushing’s Syndrome and Cushing’s Disease in Commercially-Insured Patients <65 Years Old in the United States. Pituitary 2015, 18, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Nellesen, D.; Truong, H.L.; Neary, M.P.; Ludlam, W.H. Economic Burden of Multiple Chronic Comorbidities Associated with Cushing’s Disease, a Rare Endocrine Disorder. Expert Opin. Orphan Drugs 2016, 4, 291–297. [Google Scholar] [CrossRef]
- Vogel, U.; van Stekelenborg, J.; Dreyfus, B.; Garg, A.; Habib, M.; Hosain, R.; Wisniewski, A. Investigating Overlap in Signals from EVDAS, FAERS, and VigiBase®. Drug Saf. 2020, 43, 351–362. [Google Scholar] [CrossRef]
- Veronin, M.A.; Schumaker, R.P.; Dixit, R. The Irony of MedWatch and the FAERS Database: An Assessment of Data Input Errors and Potential Consequences. J. Pharm. Technol. 2020, 36, 164–167. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Zheng, Y.; Chen, J.; Li, J. A Disproportionality Analysis of FDA Adverse Event Reporting System (FAERS) Events for Ticagrelor. Front. Pharmacol. 2024, 15, 1251961. [Google Scholar] [CrossRef]
- Council for International Organizations of Medical Sciences (CIOMS). Practical Approaches to Risk Minimisation for Medicinal Products: Report of CIOMS Working Group VIII; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Evans, S.J.W.; Waller, P.C.; Davis, S. Use of Proportional Reporting Ratios (PRRs) for Signal Generation from Spontaneous Adverse Drug Reaction Reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef]
- Pivonello, R.; Isidori, A.M.; De Martino, M.C.; Newell-Price, J.; Biller, B.M.K.; Colao, A. Complications of Cushing’s Syndrome: State of the Art. Lancet Diabetes Endocrinol. 2016, 4, 611–629. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on Diagnosis and Management of Cushing’s Disease: A Guideline Update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Ferriere, A.; Tabarin, A. Cushing’s Syndrome: Treatment and New Therapeutic Approaches. Best Pr. Res. Clin. Endocrinol. Metab. 2020, 34, 101381. [Google Scholar] [CrossRef] [PubMed]
- Dzialach, L.; Sobolewska, J.; Respondek, W.; Szamotulska, K.; Witek, P. Cushing’s Disease: Long-Term Effectiveness and Safety of Osilodrostat in a Polish Group of Patients with Persistent Hypercortisolemia in the Experience of a Single Center. Biomedicines 2023, 11, 3227. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212801s000lbl.pdf (accessed on 1 February 2025).
- Pivonello, R.; Fleseriu, M.; Newell-Price, J.; Bertagna, X.; Findling, J.; Shimatsu, A.; Gu, F.; Auchus, R.; Leelawattana, R.; Lee, E.J.; et al. Efficacy and Safety of Osilodrostat in Patients with Cushing’s Disease (LINC 3): A Multicentre Phase III Study with a Double-Blind, Randomised Withdrawal Phase. Lancet Diabetes Endocrinol. 2020, 8, 748–761. [Google Scholar] [CrossRef]
- Fleseriu, M.; Newell-Price, J.; Pivonello, R.; Shimatsu, A.; Auchus, R.J.; Scaroni, C.; Belaya, Z.; Feelders, R.A.; Vila, G.; Houde, G.; et al. Long-Term Outcomes of Osilodrostat in Cushing’s Disease: LINC 3 Study Extension. Eur. J. Endocrinol. 2022, 187, 531–541. [Google Scholar] [CrossRef]
- Lonser, R.R.; Nieman, L.; Oldfield, E.H. Cushing’s Disease: Pathobiology, Diagnosis, and Management. J. Neurosurg. 2017, 126, 404–417. [Google Scholar] [CrossRef]
- Fleseriu, M.; Pivonello, R.; Newell-Price, J.; Gadelha, M.R.; Biller, B.M.K.; Auchus, R.J.; Feelders, R.A.; Shimatsu, A.; Witek, P.; Bex, M.; et al. Osilodrostat Improves Blood Pressure and Glycemic Control in Patients with Cushing’s Disease: A Pooled Analysis of LINC 3 and LINC 4 Studies. Pituitary 2025, 28, 22. [Google Scholar] [CrossRef]
- Gadelha, M.; Snyder, P.J.; Witek, P.; Bex, M.; Belaya, Z.; Turcu, A.F.; Feelders, R.A.; Heaney, A.P.; Paul, M.; Pedroncelli, A.M.; et al. Long-Term Efficacy and Safety of Osilodrostat in Patients with Cushing’s Disease: Results from the LINC 4 Study Extension. Front. Endocrinol. 2023, 14, 1236465. [Google Scholar] [CrossRef]
- Gadelha, M.; Bex, M.; Feelders, R.A.; Heaney, A.P.; Auchus, R.J.; Gilis-Januszewska, A.; Witek, P.; Belaya, Z.; Yu, Y.; Liao, Z.; et al. Randomized Trial of Osilodrostat for the Treatment of Cushing Disease. J. Clin. Endocrinol. Metab. 2022, 107, e2882–e2895. [Google Scholar] [CrossRef]
- Hakami, O.A.; Ahmed, S.; Karavitaki, N. Epidemiology and Mortality of Cushing’s Syndrome. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101521. [Google Scholar] [CrossRef]
- Lambert, J.K.; Goldberg, L.; Fayngold, S.; Kostadinov, J.; Post, K.D.; Geer, E.B. Predictors of Mortality and Long-Term Outcomes in Treated Cushing’s Disease: A Study of 346 Patients. J. Clin. Endocrinol. Metab. 2013, 98, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, O.; Olsson, D.S.; Papakokkinou, E.; Chantzichristos, D.; Dahlqvist, P.; Segerstedt, E.; Olsson, T.; Petersson, M.; Berinder, K.; Bensing, S.; et al. Overall and Disease-Specific Mortality in Patients with Cushing Disease: A Swedish Nationwide Study. J. Clin. Endocrinol. Metab. 2019, 104, 2375–2384. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Auchus, R.J.; Bancos, I.; Biller, B.M.K. Osilodrostat Treatment for Adrenal and Ectopic Cushing Syndrome: Integration of Clinical Studies with Case Presentations. J. Endocr. Soc. 2025, 9, bvaf027. [Google Scholar] [CrossRef]
- Hána, V.; Brutvan, T.; Krausová, A.; Kršek, M.; Hána, V. Severe Cushing’s Syndrome from an Ectopic Adrenocorticotropic Hormone-Secreting Neuroendocrine Tumour Treated by Osilodrostat. Endocrinol. Diabetes Metab. Case Rep. 2023, 2023, 23-0076. [Google Scholar] [CrossRef]
- Dormoy, A.; Haissaguerre, M.; Vitellius, G.; Do Cao, C.; Geslot, A.; Drui, D.; Lasolle, H.; Vieira-Pinto, O.; Salenave, S.; François, M.; et al. Efficacy and Safety of Osilodrostat in Paraneoplastic Cushing Syndrome: A Real-World Multicenter Study in France. J. Clin. Endocrinol. Metab. 2023, 108, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Heleno, C.T.; Hong, S.P.D.; Cho, H.-G.; Kim, M.J.; Park, Y.; Chae, Y.K. Cushing’s Syndrome in Adenocarcinoma of Lung Responding to Osilodrostat. Case Rep. Oncol. 2023, 16, 130–134. [Google Scholar] [CrossRef]
- Tabarin, A.; Haissaguerre, M.; Lassole, H.; Jannin, A.; Paepegaey, A.-C.; Chabre, O.; Young, J. Efficacy and Tolerance of Osilodrostat in Patients with Cushing’s Syndrome Due to Adrenocortical Carcinomas. Eur. J. Endocrinol. 2022, 186, K1–K4. [Google Scholar] [CrossRef]
- Malik, R.B.; Ben-Shlomo, A. Adrenal Cushing’s Syndrome Treated with Preoperative Osilodrostat and Adrenalectomy. AACE Clin. Case. Rep. 2022, 8, 267–270. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Puerto, M.; Nunes, M.-L.; Tabarin, A. Efficacy and Tolerance of Osilodrostat in Patients with Severe Cushing’s Syndrome Due to Non-Pituitary Cancers. Eur. J. Endocrinol. 2020, 183, L7–L9. [Google Scholar] [CrossRef]
- Bessiène, L.; Bonnet, F.; Tenenbaum, F.; Jozwiak, M.; Corchia, A.; Bertherat, J.; Groussin, L. Rapid Control of Severe Ectopic Cushing’s Syndrome by Oral Osilodrostat Monotherapy. Eur. J. Endocrinol. 2021, 184, L13–L15. [Google Scholar] [CrossRef]
- Bancos, I.; Geer, E.B.; Castinetti, F.; Feelders, R.; Fleseriu, M.; Pivonello, R.; Reincke, M.; Tabarin, A.; Le Mouhaër, J.; Stermenska, J.; et al. 7790 A Non-Interventional, Multinational, Phase IV Study to Evaluate the Long-Term Safety and Efficacy of Osilodrostat in Patients with Endogenous Cushing’s Syndrome (LINC 6): 1-Year Real-World Interim Analysis. J. Endocr. Soc. 2024, 8, bvae163-1098. [Google Scholar] [CrossRef]
- Tanaka, T.; Satoh, F.; Ujihara, M.; Midorikawa, S.; Kaneko, T.; Takeda, T.; Suzuki, A.; Sato, M.; Shimatsu, A. A Multicenter, Phase 2 Study to Evaluate the Efficacy and Safety of Osilodrostat, a New 11β-Hydroxylase Inhibitor, in Japanese Patients with Endogenous Cushing’s Syndrome Other than Cushing’s Disease. Endocr. J. 2020, 67, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Castinetti, F. Updates on the Role of Adrenal Steroidogenesis Inhibitors in Cushing’s Syndrome: A Focus on Novel Therapies. Pituitary 2016, 19, 643–653. [Google Scholar] [CrossRef]
- Bancos, I.; Hahner, S.; Tomlinson, J.; Arlt, W. Diagnosis and Management of Adrenal Insufficiency. Lancet Diabetes Endocrinol. 2015, 3, 216–226. [Google Scholar] [CrossRef]
- Gadelha, M.; Gatto, F.; Wildemberg, L.E.; Fleseriu, M. Cushing’s Syndrome. Lancet 2023, 402, 2237–2252. [Google Scholar] [CrossRef]
- Fleseriu, M.; Biller, B.M.K. Treatment of Cushing’s Syndrome with Osilodrostat: Practical Applications of Recent Studies with Case Examples. Pituitary 2022, 25, 795–809. [Google Scholar] [CrossRef]
- Fleseriu, M.; Pivonello, R.; Young, J.; Hamrahian, A.H.; Molitch, M.E.; Shimizu, C.; Tanaka, T.; Shimatsu, A.; White, T.; Hilliard, A.; et al. Osilodrostat, a Potent Oral 11β-Hydroxylase Inhibitor: 22-Week, Prospective, Phase II Study in Cushing’s Disease. Pituitary 2016, 19, 138–148. [Google Scholar] [CrossRef]
- Bertagna, X.; Pivonello, R.; Fleseriu, M.; Zhang, Y.; Robinson, P.; Taylor, A.; Watson, C.E.; Maldonado, M.; Hamrahian, A.H.; Boscaro, M.; et al. LCI699, a Potent 11β-Hydroxylase Inhibitor, Normalizes Urinary Cortisol in Patients with Cushing’s Disease: Results From a Multicenter, Proof-of-Concept Study. J. Clin. Endocrinol. Metab. 2014, 99, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, E.V.; Han, A.J.; Fleseriu, M. Updates in Adrenal Steroidogenesis Inhibitors for Cushing’s Syndrome—A Practical Guide. Best Pr. Res. Clin. Endocrinol. Metab. 2021, 35, 101490. [Google Scholar] [CrossRef]
- Poirier, J.; Bonnet-Serrano, F.; Thomeret, L.; Bouys, L.; Bertherat, J. Prolonged Adrenocortical Blockade Following Discontinuation of Osilodrostat. Eur. J. Endocrinol. 2023, 188, K29–K32. [Google Scholar] [CrossRef]
- Ferriere, A.; Salenave, S.; Puerto, M.; Young, J.; Tabarin, A. Prolonged Adrenal Insufficiency Following Discontinuation of Osilodrostat Treatment for Intense Hypercortisolism. Eur. J. Endocrinol. 2024, 190, L1–L3. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Azizi, M.; Menard, J.; Peyrard, S.; Watson, C.; Plouin, P.-F. Aldosterone Synthase Inhibition with LCI699. Hypertension 2010, 56, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.A.; White, W.B.; Krum, H.; Guo, W.; Bermann, G.; Trapani, A.; Lefkowitz, M.P.; Ménard, J. Effects of a Novel Aldosterone Synthase Inhibitor for Treatment of Primary Hypertension. Circulation 2011, 124, 1945–1955. [Google Scholar] [CrossRef]
- Nagendra, L.; Dutta, D.; Raizada, N.; Surana, V.; Selvan, C.; Bhattacharya, S. Efficacy and Safety of Osilodrostat in Managing Cushing’s Syndrome: A Systematic Review and Meta-Analysis. Indian J. Endocrinol. Metab. 2024, 28, 232–238. [Google Scholar] [CrossRef]
- Pivonello, R.; Simeoli, C.; Di Paola, N.; Colao, A. Cushing’s Disease: Adrenal Steroidogenesis Inhibitors. Pituitary 2022, 25, 726–732. [Google Scholar] [CrossRef]
- Fleseriu, M.; Biller, B.M.K.; Bertherat, J.; Young, J.; Hatipoglu, B.; Arnaldi, G.; O’Connell, P.; Izquierdo, M.; Pedroncelli, A.M.; Pivonello, R. Long-Term Efficacy and Safety of Osilodrostat in Cushing’s Disease: Final Results from a Phase II Study with an Optional Extension Phase (LINC 2). Pituitary 2022, 25, 959–970. [Google Scholar] [CrossRef]
- Detomas, M.; Altieri, B.; Deutschbein, T.; Fassnacht, M.; Dischinger, U. Metyrapone Versus Osilodrostat in the Short-Term Therapy of Endogenous Cushing’s Syndrome: Results From a Single Center Cohort Study. Front. Endocrinol. 2022, 13, 903545. [Google Scholar] [CrossRef] [PubMed]
- Trojak, B.; Astruc, K.; Pinoit, J.-M.; Chauvet-Gelinier, J.-C.; Ponavoy, E.; Bonin, B.; Gisselmann, A. Hypokalemia Is Associated with Lengthening of QT Interval in Psychiatric Patients on Admission. Psychiatry Res. 2009, 169, 257–260. [Google Scholar] [CrossRef]
- Thu Kyaw, M.; Maung, Z.M. Hypokalemia-Induced Arrhythmia: A Case Series and Literature Review. Cureus 2022, 14, e22940. [Google Scholar] [CrossRef]
- Hunter, R.W.; Bailey, M.A. Hyperkalemia: Pathophysiology, Risk Factors and Consequences. Nephrol. Dial. Transplant. 2019, 34, iii2–iii11. [Google Scholar] [CrossRef]
- Kallergis, E.M.; Goudis, C.A.; Simantirakis, E.N.; Kochiadakis, G.E.; Vardas, P.E. Mechanisms, Risk Factors, and Management of Acquired Long QT Syndrome: A Comprehensive Review. Sci. World J. 2012, 2012, 212178. [Google Scholar] [CrossRef]
- Mandyam, M.C.; Soliman, E.Z.; Alonso, A.; Dewland, T.A.; Heckbert, S.R.; Vittinghoff, E.; Cummings, S.R.; Ellinor, P.T.; Chaitman, B.R.; Stocke, K.; et al. The QT Interval and Risk of Incident Atrial Fibrillation. Heart Rhythm. 2013, 10, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chun, K.-H.; Hwang, I.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Joung, B.; Lee, M.-H.; Pak, H.-N. Clinical and Genetic Relationships between the QTc Interval and Risk of a Stroke among Atrial Fibrillation Patients Undergoing Catheter Ablation. Int. J. Arrhythmia 2020, 21, 9. [Google Scholar] [CrossRef]
- De Leo, M.; Pivonello, R.; Auriemma, R.S.; Cozzolino, A.; Vitale, P.; Simeoli, C.; De Martino, M.C.; Lombardi, G.; Colao, A. Cardiovascular Disease in Cushing’s Syndrome: Heart versus Vasculature. Neuroendocrinology 2010, 92, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kamenický, P.; Redheuil, A.; Roux, C.; Salenave, S.; Kachenoura, N.; Raissouni, Z.; Macron, L.; Guignat, L.; Jublanc, C.; Azarine, A.; et al. Cardiac Structure and Function in Cushing’s Syndrome: A Cardiac Magnetic Resonance Imaging Study. J. Clin. Endocrinol. Metab. 2014, 99, E2144–E2153. [Google Scholar] [CrossRef]
- Rotenberg, S.; McGrath, J.J. Inter-Relation between Autonomic and HPA Axis Activity in Children and Adolescents. Biol. Psychol. 2016, 117, 16–25. [Google Scholar] [CrossRef]
- Hasenmajer, V.; Sbardella, E.; Sciarra, F.; Minnetti, M.; Isidori, A.M.; Venneri, M.A. The Immune System in Cushing’s Syndrome. Trends Endocrinol. Metab. 2020, 31, 655–669. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S. Mechanics Insights of Alpha-Lipoic Acid against Cardiovascular Diseases during COVID-19 Infection. Int. J. Mol. Sci. 2021, 22, 7979. [Google Scholar] [CrossRef]
- Isidori, A.M.; Pofi, R.; Hasenmajer, V.; Lenzi, A.; Pivonello, R. Use of Glucocorticoids in Patients with Adrenal Insufficiency and COVID-19 Infection. Lancet Diabetes Endocrinol. 2020, 8, 472–473. [Google Scholar] [CrossRef]
- Isidori, A.M.; Venneri, M.A.; Graziadio, C.; Simeoli, C.; Fiore, D.; Hasenmajer, V.; Sbardella, E.; Gianfrilli, D.; Pozza, C.; Pasqualetti, P.; et al. Effect of Once-Daily, Modified-Release Hydrocortisone versus Standard Glucocorticoid Therapy on Metabolism and Innate Immunity in Patients with Adrenal Insufficiency (DREAM): A Single-Blind, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2018, 6, 173–185. [Google Scholar] [CrossRef]
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real Estimates of Mortality Following COVID-19 Infection. Lancet Infect. Dis. 2020, 20, 773. [Google Scholar] [CrossRef]
- Witek, P.; Mehlich, A.; Stasiewicz, A.; Jawiarczyk-Przybyłowska, A.; Bolanowski, M. Osilodrostat—An Emerging Drug for the Medical Management of Cushing’s Disease. Endokrynol. Pol. 2022, 73, 371–374. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Szatko, A.; Papierska, L.; Zgliczyński, W.; Glinicki, P. Musculoskeletal Complications of Cushing Syndrome. Rheumatology 2023, 61, 271–282. [Google Scholar] [CrossRef]
- Sun, H.; Wu, C.; Hu, B.; Xie, G. High Prevalence of Vertebral Fractures Associated with Preoperative Cortisol Levels in Patients with Recent Diagnosis of Cushing Disease. Ann. Med. 2023, 55, 2282183. [Google Scholar] [CrossRef]
- Vogel, F.; Braun, L.T.; Rubinstein, G.; Zopp, S.; Künzel, H.; Strasding, F.; Albani, A.; Riester, A.; Schmidmaier, R.; Bidlingmaier, M.; et al. Persisting Muscle Dysfunction in Cushing’s Syndrome Despite Biochemical Remission. J. Clin. Endocrinol. Metab. 2020, 105, e4490–e4498. [Google Scholar] [CrossRef]
- Zdrojowy-Wełna, A.; Stachowska, B.; Bolanowski, M. Cushing’s Disease and Bone. Pituitary 2024, 27, 837–846. [Google Scholar] [CrossRef]
- Sher, L.B.; Woitge, H.W.; Adams, D.J.; Gronowicz, G.A.; Krozowski, Z.; Harrison, J.R.; Kream, B.E. Transgenic Expression of 11β-Hydroxysteroid Dehydrogenase Type 2 in Osteoblasts Reveals an Anabolic Role for Endogenous Glucocorticoids in Bone. Endocrinology 2004, 145, 922–929. [Google Scholar] [CrossRef]
- Zhou, H.; Cooper, M.S.; Seibel, M.J. Endogenous Glucocorticoids and Bone. Bone Res. 2013, 1, 107–119. [Google Scholar] [CrossRef]
- Björnsdottir, S.; Sääf, M.; Bensing, S.; Kämpe, O.; Michaëlsson, K.; Ludvigsson, J.F. Risk of Hip Fracture in Addison’s Disease: A Population-Based Cohort Study. J. Intern. Med. 2011, 270, 187–195. [Google Scholar] [CrossRef]
- Ragnarsson, O.; Juhlin, C.C.; Torpy, D.J.; Falhammar, H. A Clinical Perspective on Ectopic Cushing’s Syndrome. Trends Endocrinol. Metab. 2024, 35, 347–360. [Google Scholar] [CrossRef]
- Tauchmanovà, L.; Pivonello, R.; Di Somma, C.; Rossi, R.; De Martino, M.C.; Camera, L.; Klain, M.; Salvatore, M.; Lombardi, G.; Colao, A. Bone Demineralization and Vertebral Fractures in Endogenous Cortisol Excess: Role of Disease Etiology and Gonadal Status. J. Clin. Endocrinol. Metab. 2006, 91, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Cosentini, D.; Grisanti, S.; Hadoux, J.; Libè, R.; Frigerio, M.; Laganà, M.; Deschamps, F.; Zamparini, M.; Lamartina, L.; Pedersini, R.; et al. Progression of Vertebral Fractures in Patients with Adrenocortical Carcinoma Undergoing Mitotane Therapy. J. Clin. Endocrinol. Metab. 2022, 107, e2167–e2176. [Google Scholar] [CrossRef]
- Tang, A.; O’Sullivan, A.J.; Diamond, T.; Gerard, A.; Campbell, P. Psychiatric Symptoms as a Clinical Presentation of Cushing’s Syndrome. Ann. Gen. Psychiatry. 2013, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Simeoli, C.; De Martino, M.C.; Cozzolino, A.; De Leo, M.; Iacuaniello, D.; Pivonello, C.; Negri, M.; Pellecchia, M.T.; Iasevoli, F.; et al. Neuropsychiatric Disorders in Cushing’s Syndrome. Front. Neurosci. 2015, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Starkman, M.N. Neuropsychiatric Findings in Cushing Syndrome and Exogenous Glucocorticoid Administration. Endocrinol. Metab. Clin. North Am. 2013, 42, 477–488. [Google Scholar] [CrossRef]
- Starkman, M.N.; Schteingart, D.E.; Schork, A.M. Depressed Mood and Other Psychiatric Manifestations of Cushingʼs Syndrome: Relationship to Hormone Levels. Psychosom. Med. 1981, 43, 3–18. [Google Scholar] [CrossRef]
- Zhang, C.D.; Li, D.; Singh, S.; Suresh, M.; Thangamuthu, K.; Nathani, R.; Achenbach, S.J.; Atkinson, E.J.; Van Gompel, J.J.; Young, W.F.; et al. Glucocorticoid Withdrawal Syndrome Following Surgical Remission of Endogenous Hypercortisolism: A Longitudinal Observational Study. Eur. J. Endocrinol. 2023, 189, 29–39. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa Ilie, I.R.; Butuca, A.; Homorodean, C.; Dobrea, C.M.; Morgovan, C.; Frum, A.; Ghibu, S. Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database. J. Clin. Med. 2025, 14, 3518. https://doi.org/10.3390/jcm14103518
Popa Ilie IR, Butuca A, Homorodean C, Dobrea CM, Morgovan C, Frum A, Ghibu S. Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database. Journal of Clinical Medicine. 2025; 14(10):3518. https://doi.org/10.3390/jcm14103518
Chicago/Turabian StylePopa Ilie, Ioana Rada, Anca Butuca, Calin Homorodean, Carmen Maximiliana Dobrea, Claudiu Morgovan, Adina Frum, and Steliana Ghibu. 2025. "Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database" Journal of Clinical Medicine 14, no. 10: 3518. https://doi.org/10.3390/jcm14103518
APA StylePopa Ilie, I. R., Butuca, A., Homorodean, C., Dobrea, C. M., Morgovan, C., Frum, A., & Ghibu, S. (2025). Osilodrostat Safety Profile: Findings from Real-World Data in the FAERS Database. Journal of Clinical Medicine, 14(10), 3518. https://doi.org/10.3390/jcm14103518








