1. Introduction
Uveitis is an intraocular inflammatory disease that affects the uveal tract (iris, choroid, and ciliary body) and adjacent structures, including the sclera, cornea, vitreous humor, retina, and optic nerve head. It is the most common form of ocular inflammation, with an annual incidence of 52 cases per 100,000 individuals and a global prevalence of approximately 0.1% [
1]. Uveitis predominantly affects individuals between 20 and 50 years of age (60–80%), with lower prevalence in children under 10 (7%) and adults over 70 (7.3%) [
2]. This condition accounts for 10% of vision loss and contributes to 5–20% of legal blindness in developed countries [
3]. Although the underlying pathogenesis remains poorly understood, uveitis is recognized as an immune-mediated disorder with contributions from endogenous and possibly environmental factors [
4].
Classification systems now emphasize anatomical and etiological criteria [
5]. Based on etiology, uveitis is grouped into infectious, systemic immune-mediated, ocular-specific syndromes, and masquerade syndromes (e.g., lymphoma, leukemia). Despite thorough diagnostic workups, 30–60% of cases remain idiopathic [
6]. The term “idiopathic immune-mediated uveitis” (IIMU) designates non-infectious, non-neoplastic, non-traumatic, and non-systemic autoimmune uveitis that responds favorably to immunomodulatory therapy, including corticosteroids, immunosuppressants, and biologics. Typically, bilateral, chronic, or recurrent IIMU significantly impairs quality of life.
Traditionally regarded as an isolated ocular disorder, IIMU may also involve systemic immune-mediated manifestations, including audiovestibular dysfunction. Inner-ear disorders can produce a spectrum of auditory and vestibular symptoms, such as sensorineural hearing loss (SNHL), tinnitus, vertigo, imbalance, and aural fullness. These symptoms, although common, are often underdiagnosed in ophthalmology settings or misattributed to unrelated causes. Mismanagement may result in poor quality of life and secondary psychiatric symptoms [
7]. The anatomical, embryological, and immunological similarities between ocular and inner-ear tissues suggest a potential shared immune-mediated mechanism. In recent years, interest in the immunopathogenesis of cochleovestibular disorders has grown [
8].
Diagnosing immune-mediated inner-ear dysfunction remains challenging due to the absence of specific biomarkers [
9]. Clinical suspicion, multidisciplinary collaboration, and early intervention are critical, as prompt treatment may prevent irreversible cochleovestibular damage [
10].
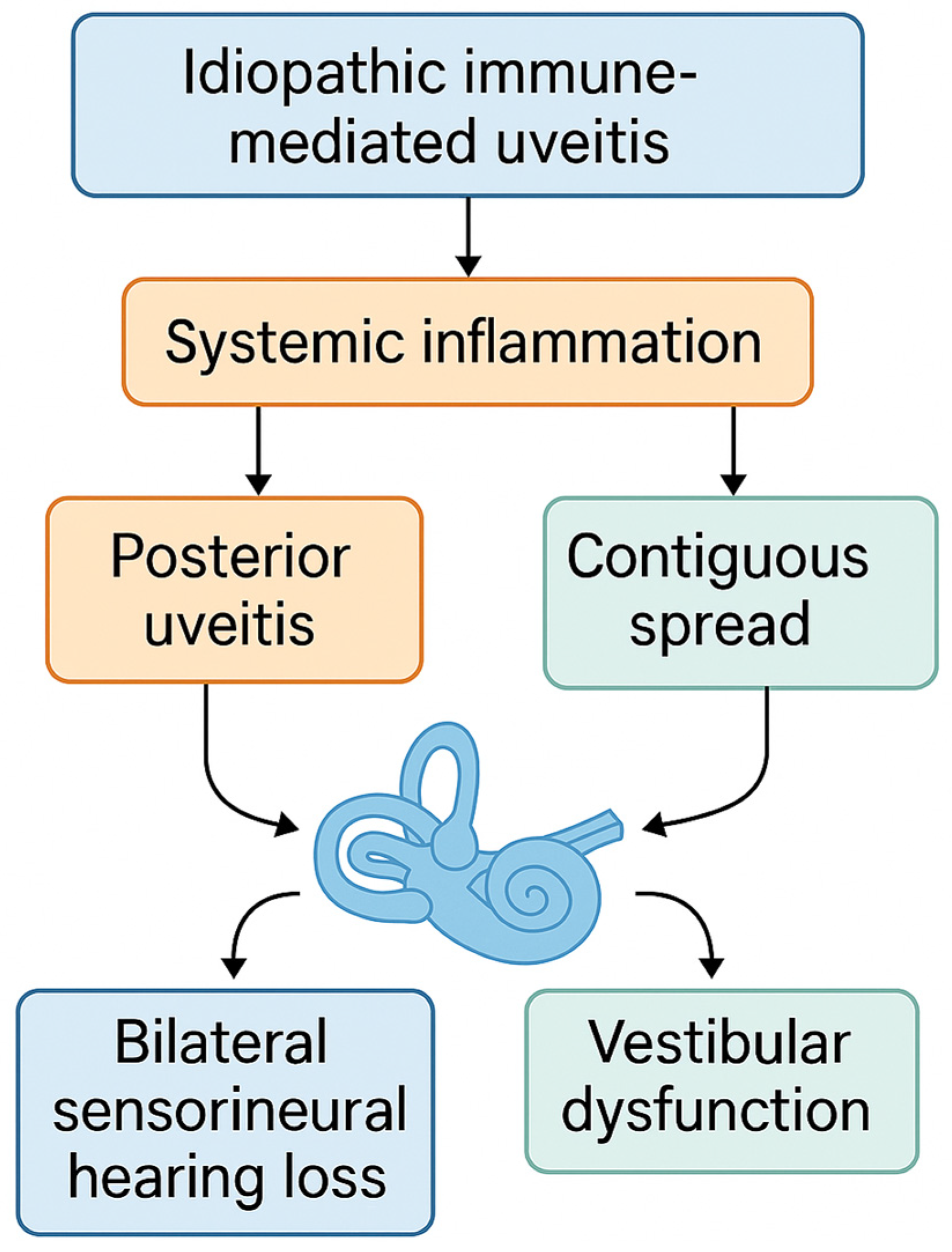
Despite increasing recognition of immune-mediated cochleovestibular conditions, little is known about the prevalence and characteristics of audiovestibular involvement in IIMU. This cross-sectional observational study aims to investigate the prevalence of SNHL and vestibular dysfunction in patients with IIMU. We hypothesize that audiovestibular symptoms represent an extrapolation of the immune-mediated process beyond the uveal tract and that their presence is associated with delayed uveitis onset and increased ocular complications [
Figure 1]. Early identification of these manifestations may facilitate timely systemic treatment and improve patient outcomes.
Systemic inflammation triggered by IIMU—especially in posterior uveitis—may lead to bilateral sensorineural hearing loss and vestibular dysfunction via contiguous or hematogenous spread to the inner ear.
2. Materials and Methods
2.1. Study Design and Population
This was a retrospective observational study conducted at the Uveitis Unit of the Granada North Health Area (Spain), covering a four-year period from January 2019 to December 2022. The aim was to evaluate audiovestibular manifestations in patients diagnosed with IIMU.
2.2. Participants
All adult patients (≥18 years) diagnosed with IIMU during the study period were considered for inclusion. IIMU was defined as non-infectious, non-neoplastic, and non-traumatic uveitis with no evidence of systemic autoimmune disease, and a favorable response to immunomodulatory therapy (corticosteroids, immunosuppressants, and/or biologics).
Inclusion criteria were as follows:
A confirmed diagnosis of uveitis by a fellowship-trained ophthalmologist.
Non-infectious, non-neoplastic, and non-systemic autoimmune etiology confirmed through comprehensive evaluation.
Positive clinical response to immunomodulatory treatment.
Exclusion criteria included the following:
2.3. Data Collection and Clinical Assessment
Demographic variables, medical history, and relevant clinical findings were recorded. Demographic data, medical history, and clinical characteristics were extracted from electronic health records. Ophthalmologic examination included best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, intraocular pressure measurement, and optical coherence tomography (OCT). Uveitis was categorized by anatomical localization (anterior or non-anterior: intermediate, posterior or panuveitis), laterality, onset (sudden or insidious), duration (limited or persistent), and course (acute, recurrent, or chronic), according to SUN (Standardization of Uveitis Nomenclature) criteria. Acute uveitis has sudden onset and lasts <3 months, recurrent uveitis involves episodes with ≥3 months of inactivity, and chronic uveitis persists with recurrences within 3 months after treatment discontinuation.
Deteriorating ophthalmologic activity was defined as any of the following: increased intraocular inflammation, reduction in BCVA attributable to inflammatory activity, or progression of uveitic complications over serial visits.
2.3.1. Audivestibular Evaluation
All patients underwent a comprehensive audiovestibular assessment by neurotology specialists blinded to the ophthalmologic findings. The protocol included detailed medical history, neurotological examination, tympanometry, pure tone audiometry (PTA), video head impulse test (vHIT), and cervical vestibular-evoked myogenic potentials (cVEMP).
2.3.2. PTA
Audiometric testing was performed using an Interacoustic AC40 audiometer (Intercoustics, Assens, Denmark) with TDH39-P supra-aural headphones (Telephonics, Farmingdale, NY, USA) inside a calibrated Sibelmed S-40 soundproof booth (Sibel, Sabadell, Spain). Air and bone conduction thresholds were measured from 125 to 8000 Hz. Masking was applied when appropriate. SNHL was defined according to the BIAP classification [
11]. Normal corresponds to ≤20 dB, mild to 21–40 dB, moderate to 41–70 dB, severe to 71–90 dB, profound to 91–119 dB, and total or cochlear deafness to ≥120 dB.
2.3.3. vHIT Assessment
vHIT was performed using ICS Impulse (GN Otometrics, Natus Medical, Taastrup, Denmark). Only horizontal semicircular canal function was assessed due to device limitations. Subjects fixated on a target 1 m away; 20 rapid, passive head impulses (15° to each side, 150–200°/s) were administered. Normal vestibulo-ocular reflex (VOR) gain was defined as 0.8–1.2, based on reference standards [
12]. Abnormal vHIT indicates vestibulo-ocular reflex gain in at least one semicircular canal.
The test was always performed by the same neurotologist to minimize interobserver variability. Tests that yielded inconclusive or non-physiological data were labeled as “non-evaluable” and included in the tables as missing values.
2.3.4. cVEMP Evaluation
cVEMP testing was performed with Eclipse EP 25 (Intercoustics, Assens, Denmark) and insert earphones (IP-30 ABR, Radioear, Middelfart, Denmark). Surface electrodes (Ambu Neuroline 720, Ambu, Ballerup, Denmark) were positioned on the sternocleidomastoid muscle, sternoclavicular junction, and forehead (ground). Impedance was maintained at <5 kΩ (inter-electrode <3 kΩ). Patients sat upright and rotated their head contralaterally to ensure muscle activation. Tone bursts (500 Hz, 6 ms) were delivered at 100 dB nHL. Myogenic activity was maintained between 49.9 µV and 150.6 µV RMS, with artifact rejection ±800 µV. A response was considered absent if P1–N1 waves were not detected. The amplitude asymmetry ratio (AAR) was calculated as follows:
(AL − AS)/(AL + AS) × 100, where AL and AS denote the larger and smaller peak-to-peak amplitudes. cVEMP primarily evaluates the function of the saccule and the inferior vestibular nerve. Abnormal cVEMP was defined as absent response or AAR > 35%. Tests that yielded inconclusive or non-physiological data were labeled as “non-evaluable” and included in the tables as missing values.
2.4. Statistical Analysis
A descriptive analysis of the collected variables was performed, using measures of central tendency and dispersion for continuous variables and absolute and relative frequencies for categorical variables. The normality of continuous variables was assessed using the Shapiro–Wilk test.
To compare patients with and without bilateral sensorineural hearing loss (B-SNHL) and those with and without vestibular involvement, bivariate statistical tests were employed. Continuous variables were analyzed using the independent-samples t-test. Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, as required. A p-value < 0.05 was considered statistically significant.
All statistical analyses were performed using STATA software, version 16.1 (StataCorp LLC, College Station, TX, USA).
3. Results
Demographics, clinical findings, characteristics of uveitis, and details regarding the neurotological examination of patients are listed in
Table 1 and
Table 2.
A total of 34 patients with IIMU were included, of whom 79.41% were women. The mean age at uveitis onset was 34 ± 15.99 years. Most cases presented with bilateral uveitis (85.29%), insidious onset (70.59%), and a persistent (89.24%) and chronic course (73.53%). A family history of SNHL was present in 23.53% of cases. Relevant systemic or neurological symptoms included headache (23.53%), subjective hearing loss (26.47%), vertigo (20.59%), and dizziness (20.59%). The most common cardiovascular risk factors were hypertension (38.24%) and smoking (23.53%). The predominant anatomical type of uveitis was non-anterior (64.70%). Antinuclear antibodies (ANAs) were positive in 44.12% of patients, with positivity defined as a titer of ≥1:160. Immunomodulatory treatments included corticosteroids (35.29%) and methotrexate (41.18%). At final follow-up, ophthalmologic activity had improved in 58.82% of cases and remained inactive in 26.47%, and ocular complications were present in 67.65%.
Audiovestibular Assessment
PTA revealed SNHL in 41.18% of patients, to mild (29.41%), moderate (8.82%), and severe (2.94%) degrees. B-SNHL was the most frequent finding (35.29%), while unilateral hearing loss was observed in the right ear in 2.94% and in the left ear in 2.94%.
Abnormal results were identified in 14.71% of vHIT and 32.35% of cVEMP tests.
A comparison between patients with and without B-SNHL is shown in
Table 3.
Patients with B-SNHL had a significantly later age at uveitis onset (52.33 ± 14.39 years) compared to those without B-SNHL (35.91 ± 13.92 years; p = 0.003). No statistically significant differences were found regarding sex distribution, family history, or cardiovascular risk factors.
Worsening ophthalmologic activity was significantly more frequent among patients with B-SNHL (25% vs. 0%; p = 0.037). Although ocular complications were more common in the B-SNHL group (83.33% vs. 59.09%), the difference did not reach statistical significance (p = 0.252).
A comparison between patients with and without vestibular dysfunction is shown in
Table 4.
Vestibular dysfunction was also associated with significantly later uveitis onset (51.00 ± 17.41 years) compared to patients without vestibular involvement (35.95 ± 12.22 years; p = 0.006). No significant differences were observed in sex, family history, or cardiovascular risk factors.
Worsening ophthalmologic activity was more frequent in patients with vestibular dysfunction (15.38% vs. 4.67%; p = 0.544), as were ocular complications (76.92% vs. 61.90%; p = 0.465).
4. Discussion
This study highlights a high prevalence of audiovestibular manifestations among patients with IIMU, with over 40% exhibiting SNHL and more than one-third demonstrating vestibular dysfunction upon objective testing [
13,
14]. Notably, vHIT was limited to horizontal semicircular canals, which may underestimate the full extent of vestibular impairment. These findings are consistent with the previous literature on autoimmune uveitis [
15,
16] and systemic autoimmune disorders such as Vogt–Koyanagi–Harada and Cogan’s syndrome [
17,
18], supporting the hypothesis that shared immune-mediated mechanisms may affect both the eye and inner ear. In this context, IIMU should be considered a potentially systemic inflammatory condition with multi-organ involvement.
Clinically, audiovestibular dysfunction in IIMU was associated with a higher frequency of ocular complications and persistent inflammation. While not all associations reached statistical significance, the observed trends suggest potentially meaningful clinical correlations. This underscores the importance of early screening, interdisciplinary collaboration, and the timely initiation of immunosuppressive therapy.
A particularly relevant observation was the trend toward increased audiovestibular involvement in patients with non-anterior uveitis. Although the association was not statistically significant, it may reflect a broader or more systemic inflammatory phenotype. Prior research supports this hypothesis, suggesting that posterior segment inflammation may be more likely to affect the inner ear due to anatomical proximity and possible hematogenous dissemination [
19,
20,
21].
Differential diagnoses must include infectious etiologies such as syphilis or tuberculosis, which may mimic immune-mediated uveitis with concurrent inner-ear involvement. While these were excluded in our cohort, their consideration remains crucial due to their distinct treatment implications.
Additionally, biologic therapies appeared to be associated with lower rates of audiovestibular dysfunction, supporting previous findings that early immunomodulation may preserve sensory function in autoimmune settings [
22]. Although our study design precludes causal inference, this observation warrants further prospective evaluation. The nature of SNHL in autoimmune conditions has been described as fluctuating, progressive, or irreversible if left untreated [
23]. While our cross-sectional design limited longitudinal evaluation, these data emphasize the need for early detection and management to prevent permanent deficits.
Interestingly, the low number of patients with severe SNHL may reflect referral or selection bias—patients with mild symptoms might be more likely to undergo ophthalmologic rather than otologic evaluation. Future studies incorporating systematic audiovestibular screening could yield more accurate prevalence estimates.
Overall, our results support the integration of routine audiovestibular assessment in the evaluation of patients with IIMU, particularly those with chronic or non-anterior forms. This may facilitate earlier intervention, reduce the risk of irreversible damage, and improve long-term functional outcomes.
5. Conclusions
This study emphasizes the importance of a multidisciplinary approach in the management of idiopathic immune-mediated uveitis. Audiovestibular dysfunction—particularly bilateral SNHL and vestibular abnormalities—is frequent in this population and may represent an underrecognized manifestation of systemic immune dysregulation. Periodic audiovestibular evaluation, especially in patients with non-anterior or chronic uveitis, should be considered part of standard care. Early identification enables timely, personalized treatment strategies, including immunosuppressive and potentially biologic therapies. These findings also advocate for the development of consensus diagnostic criteria for immune-mediated audiovestibular disease (IMAVD) and the inclusion of neurotological screening in future clinical guidelines.
Limitations
This study has several limitations. First, the sample size was relatively small, limiting statistical power to detect some associations. Second, its cross-sectional design precludes the determination of temporal or causal relationships between uveitis and inner-ear dysfunction. Third, potential referral bias may have influenced the composition of the audiological cohort, with more symptomatic or complex cases more likely to be referred. The underreporting of vestibular symptoms could also lead to an underestimation of true prevalence.
The vestibular assessment was limited to horizontal canal vHIT and cVEMP, potentially overlooking dysfunction in vertical canals or utricle. Additional testing (e.g., vertical vHIT, caloric tests, oVEMP) would offer a more comprehensive assessment. Furthermore, the absence of standardized biomarkers for immune-mediated inner-ear disease complicates diagnosis and may contribute to underrecognition.
To address these limitations, future studies should employ prospective longitudinal designs and standardized audiovestibular protocols. This would enable the assessment of disease progression, treatment efficacy, and long-term outcomes. Despite these constraints, this study provides important insights into the interplay between uveitis and inner-ear involvement, reinforcing the need for integrated diagnostic and therapeutic approaches.
Author Contributions
Conceptualization, A.B.-M. and N.N.-N.; Methodology, A.B.-M., J.M.E.-S. and N.N.-N.; Validation, A.B.-M., J.D.-P., J.M.E.-S. and N.N.-N.; Formal Analysis, A.B.-M. and M.d.C.O.-P.; Writing—Original Draft Preparation, A.B.-M.; A.E.-G., J.M.E.-S. and N.N.-N.; Writing—Review and Editing, A.B.-M., M.d.C.O.-P., A.E.-G., J.M.E.-S. and N.N.-N.; Visualization, A.B.-M., J.D.-P., J.M.E.-S. and N.N.-N.; Supervision, A.B.-M., A.E.-G., J.M.E.-S. and N.N.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee for Biomedical Research of the Province of Granada (CEIM/CEI GRANADA) (Ethics Portal Code: 0393-N-23; approval date: 20/07/2023).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
De-identified data are available on request preceded by a signed data access agreement form.
Acknowledgments
Antonio Bustos-Merlo is a PhD student on the Biomedicine Program at the School of Health Sciences at the University of Granada and this work is part of his doctoral thesis.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAR | Amplitude asymmetry ratio |
| ANA | Antinuclear antibodies |
| ANCA | Antineutrophil Cytoplasmic Antibodies |
| APL | Antiphospholipid Antibodies |
| B-SNHL | Bilateral sensorineural hearing loss |
| cVEMPS | Cervical vestibular-evoked myogenic potentials |
| DM2 | Diabetes mellitus type 2 |
| ECA | Angiotensin-Converting Enzyme |
| ENA | Extractable Nuclear Antigen |
| HLA | Human Leukocyte Antigen |
| IGRA | Interferon-Gamma Release Assays |
| IIMU | Idiopathic immune-mediated uveitis |
| SNHL | Sensorineural hearing loss |
| vHIT | Video head impulse testing |
References
- Gritz, D.C.; Wong, I.G. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004, 111, 491–500. [Google Scholar] [CrossRef]
- Durrani, O.M.; Meads, C.A.; Murray, P.I. Uveitis: A potentially blinding disease. Ophthalmologica 2004, 218, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A. Uveitis in developing countries. Indian J. Ophthalmol. 2013, 61, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.R. A look at autoimmunity and inflammation in the eye. J. Clin. Investig. 2010, 120, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Thorne, J.E.; Flaxel, C.J.; Jain, N.; Kim, S.J.; Maguire, M.G.; Patel, S.; Weng, C.Y.; Yeh, S.; Kim, L.A. Treatment of Noninfectious Uveitic Macular Edema with Periocular and Intraocular Corticosteroid Therapies: A Report by the American Academy of Ophthalmology. Ophthalmology 2024, 131, 1107–1120. [Google Scholar] [CrossRef]
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of uveitis nomenclature (SUN) for reporting clinical data. Results of the first international workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Molnár, A.; Maihoub, S.; Mavrogeni, P.; Tamás, L.; Szirmai, A. Depression scores and quality of life of vertiginous patients, suffering from different vestibular disorders. Eur. Arch. Otorhinolaryngol. 2022, 279, 5173–5179. [Google Scholar] [CrossRef]
- Keithley, E.M. Inner ear immunity. Hear. Res. 2022, 419, 108518. [Google Scholar] [CrossRef]
- Shah, S.; Chidarala, S.; Jeong, S.; Zhang, K.; Nguyen, S.A.; Wilkinson, R.; Ward, C.; Rizk, H. Secondary autoimmune immune ear disease (AIED): A systematic review and meta-analysis on vestibular manifestations of systemic autoimmune and inflammatory disorders. Clin. Rheumatol. 2023, 42, 2747–2759. [Google Scholar] [CrossRef]
- Breslin, N.K.; Varadarajan, V.V.; Sobel, E.S.; Haberman, R.S. Autoimmune inner ear disease: A systematic review of management. Laryngoscope Investig. Otolaryngol. 2020, 5, 1217–1226. [Google Scholar] [CrossRef]
- Molnár, A.; Maihoub, S.; Tamás, L.; Szirmai, Á. Comparison between caloric and video-head impulse tests in Ménière’s disease and vestibular neuritis. Int. J. Audiol. 2023, 62, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Bureau International d’Audiophonologie. BIAP Recommendation 02/1 Bis: Audiometric Classification of Hearing Impairments. Lisbon: BIAP. 1997. Available online: https://www.biap.org/en/recommendations/65-ct-2-classification-of-hearing-losses/10-biap-recommendation-02-1-bis (accessed on 1 October 2024).
- Hassan, M.A.; Mansour, S.K.; El-Kholy, A.E.; Ahmed, H.; Mohamed, R.; Soliman, A. Clinical manifestations of autoimmune uveitis with cochlear and vestibular dysfunction. Ophthalmology 2020, 127, 512–518. [Google Scholar] [CrossRef]
- Király, M.; Kovács, A.; Sáry, T. Sensorineural hearing loss in autoimmune diseases: Pathogenesis and therapeutic implications. Autoimmun. Rev. 2018, 17, 853–860. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Liu, X.; Li, Y.; Wang, Z.; Zhao, J. Immune-mediated uveitis and the impact on audiovestibular function: A systemic review. J. Autoimmun. 2020, 107, 102357. [Google Scholar] [CrossRef]
- Sang, T.T.; Lee, D.C.; Cho, Y.W.; Kim, H.S.; Park, H.J.; Jeong, M.H. Vestibular dysfunction in autoimmune diseases: A clinical review of immune-mediated vestibulitis. Autoimmun. Rev. 2021, 20, 102510. [Google Scholar] [CrossRef]
- Greco, A.; Fusconi, M.; Gallo, A.; Turchetta, R.; Marinelli, C.; Macri, G.F.; De Virgilio, A.; de Vincentiis, M. Vogt-Koyanagi-Harada syndrome. Autoimmun. Rev. 2013, 12, 1033–1038. [Google Scholar] [CrossRef]
- Jeong, S.S.; Rizk, H.G. Audiovestibular involvement in Cogan’s syndrome: A retrospective study of 60 patients. Autoimmun. Rev. 2019, 18, 132–137. [Google Scholar] [CrossRef]
- Bovo, R.; Aimoni, C.; Martini, A. Immune-mediated inner ear disease. Acta Otolaryngol. 2006, 126, 1012–1021. [Google Scholar] [CrossRef]
- Chen, M.; Qiu, Y.; Zhang, P.; Yang, Y.; Liu, J.; Huang, Z. Cochleovestibular dysfunction in autoimmune diseases: Pathogenesis and treatment outcomes. Otol. Neurotol. 2019, 40, 702–711. [Google Scholar] [CrossRef]
- Di Mauro, P.; La Mantia, I.; Cocuzza, S.; Sciancalepore, P.I.; Rasà, D.; Maniaci, A.; Ferlito, S.; Tundo, I.; Anzivino, R. Acute Vertigo After COVID-19 Vaccination: Case Series and Literature Review. Front. Med. 2022, 8, 790931. [Google Scholar] [CrossRef]
- Mellor, D.H.; Dawes, P.; Hearn, R.E.; Parry, R.A.; Matthews, J.S.; Booth, S.L. Audiovestibular manifestations in uveitis patients: Implications for clinical practice. Ophthalmic Physiol. Opt. 2021, 41, 512–520. [Google Scholar] [CrossRef]
- Bovo, R.; Ciorba, A.; Martini, A. The diagnosis of autoimmune inner ear disease: Evidence and critical pitfalls. Eur. Arch. Otorhinolaryngol. 2009, 266, 37–40. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).