Distinct Gut Microbiota Profiles in Unruptured and Ruptured Intracranial Aneurysms: Focus on Butyrate-Producing Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Fecal Sample Collection
2.3. DNA Isolation
2.4. Metagenomic Analysis
3. Results
3.1. Patient Characteristics
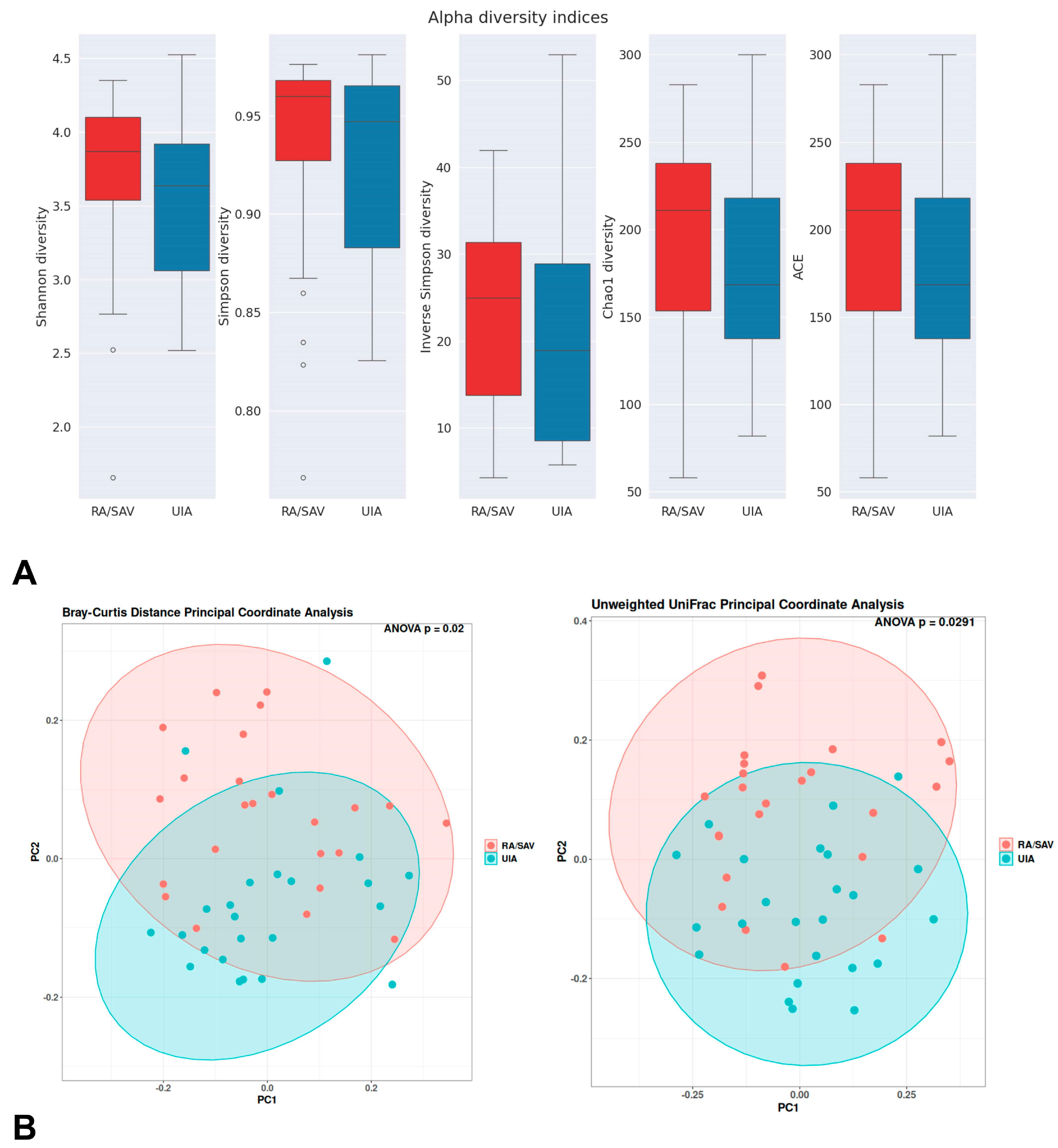
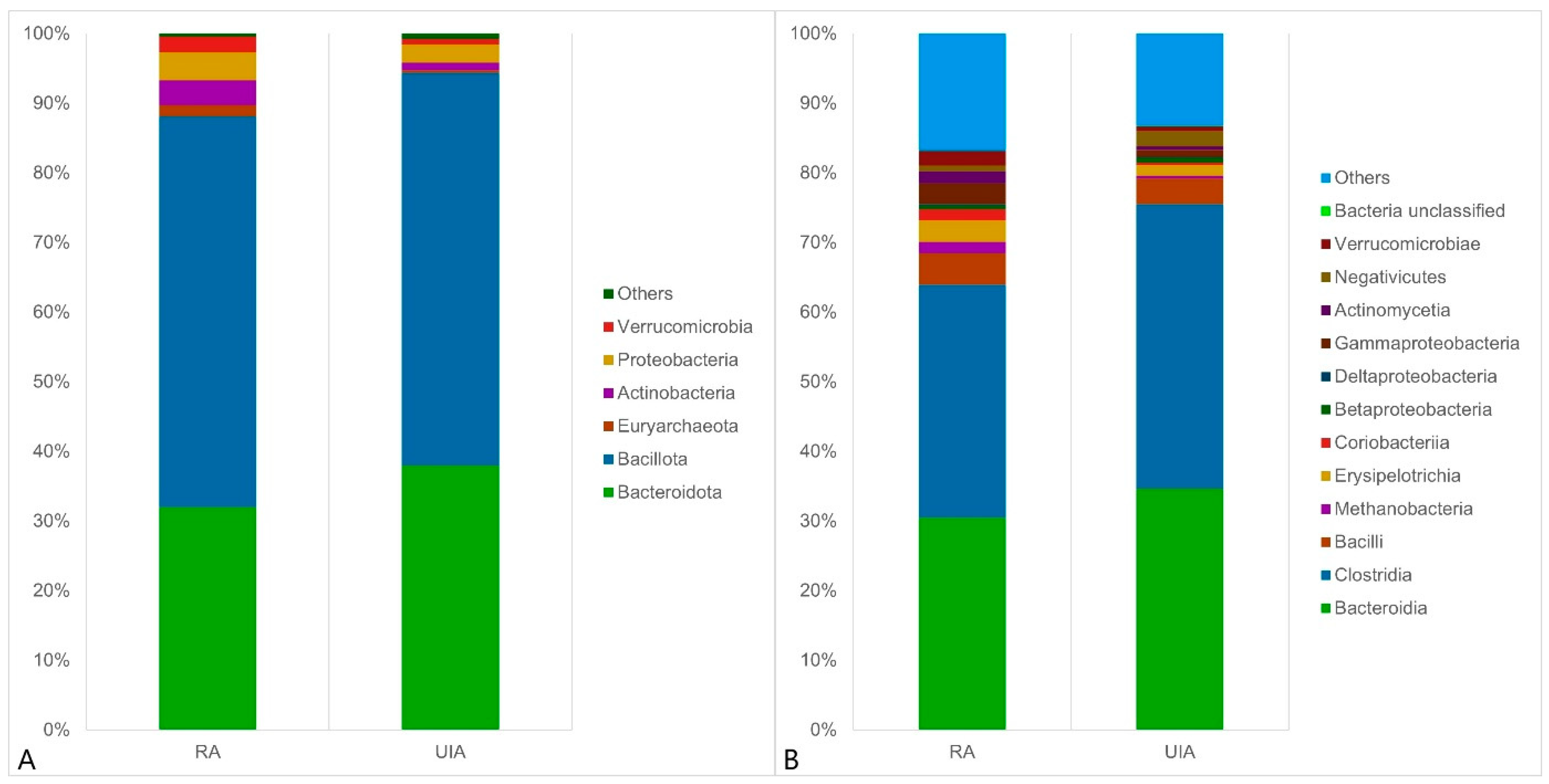
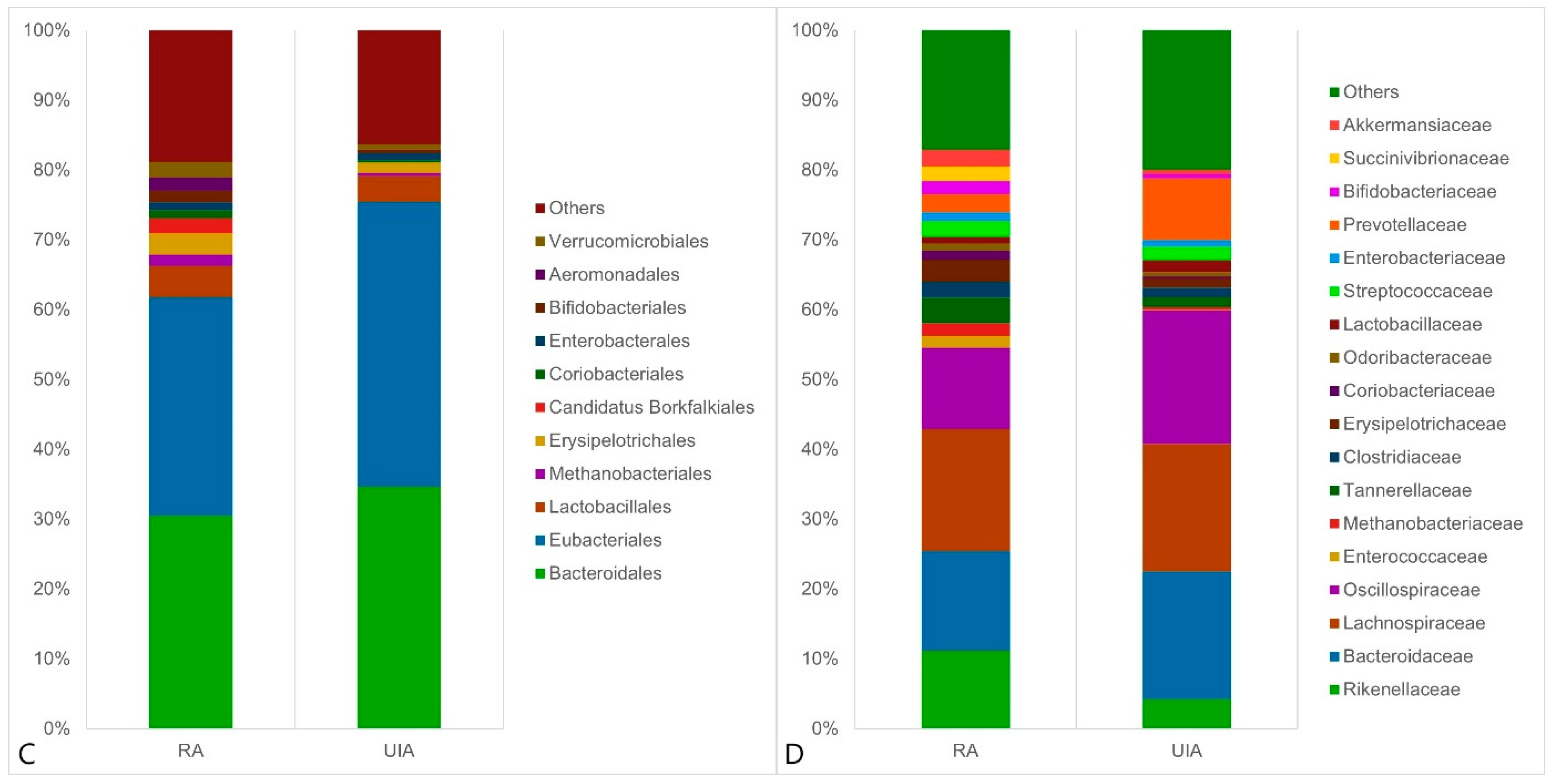
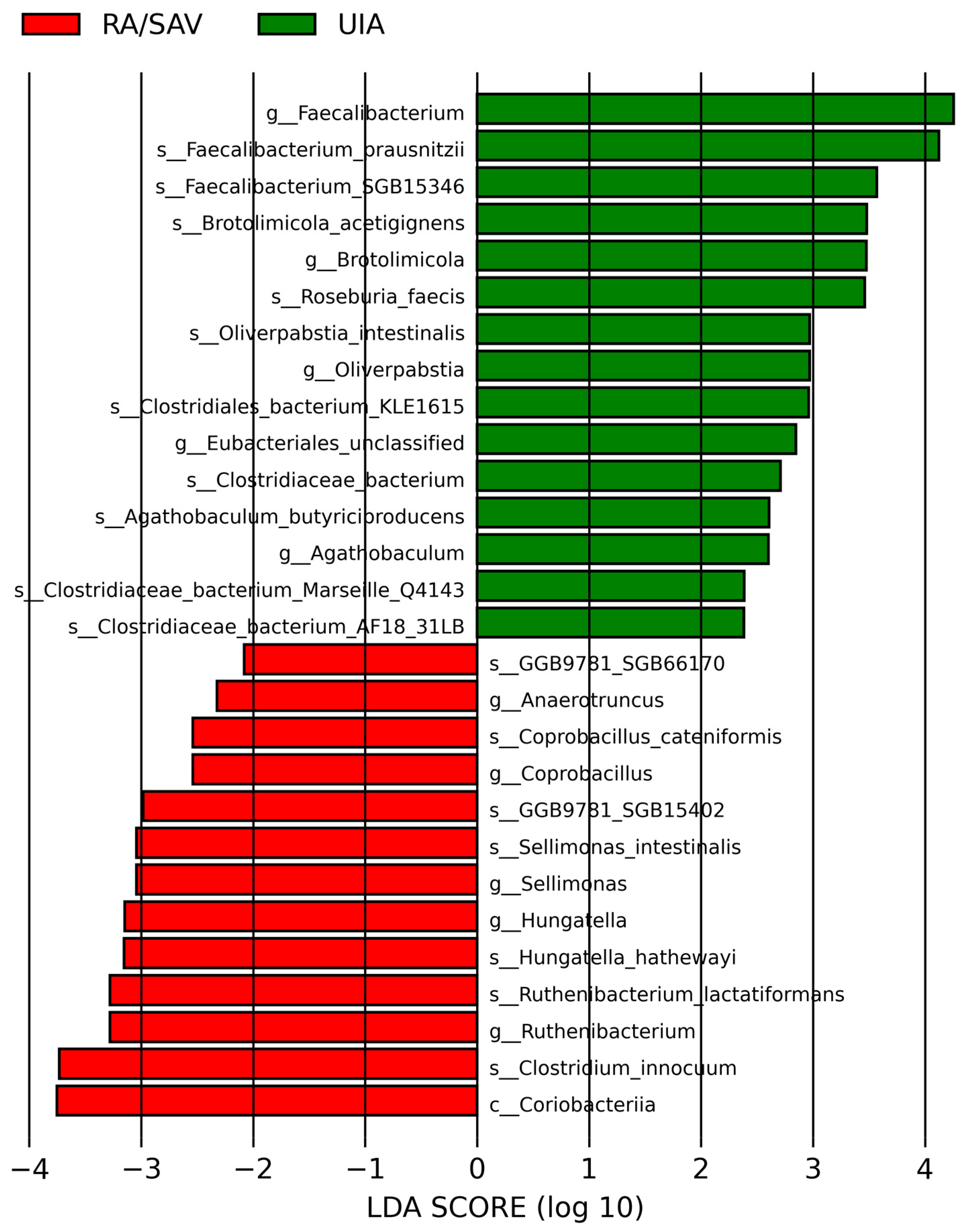
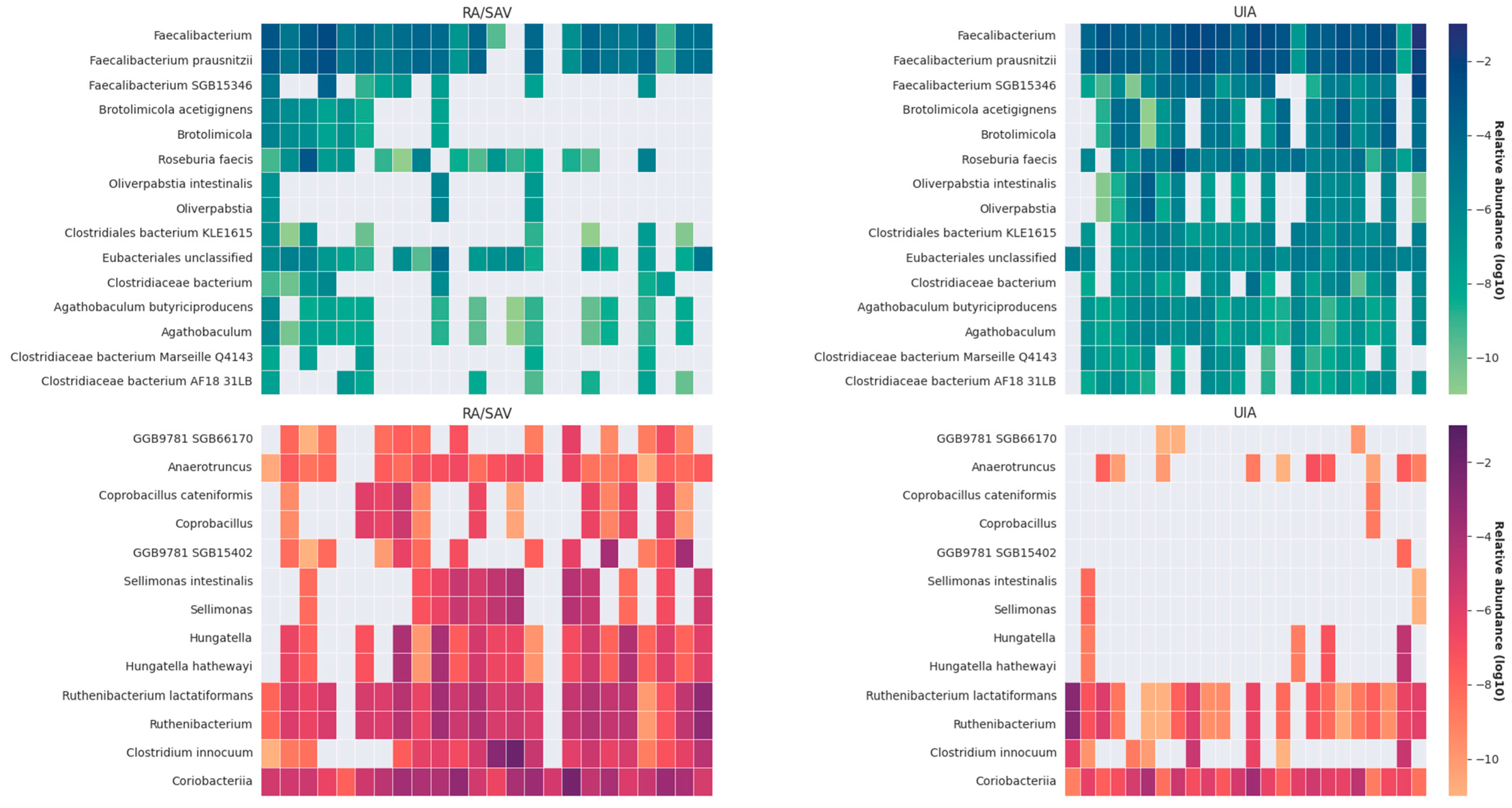
3.2. Gut Microbiome Analysis in the RA and UIA Groups
4. Discussion
4.1. Gut Microbiota in Intracranial Aneurysm (IA) Patients
4.2. Gut Microbiota in Other Intracranial and Neurological Conditions
4.3. Mechanistic Implications and Future Directions
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-Hydroxydopamine |
| AD | Alzheimer’s Disease |
| AIS | Acute Ischemic Stroke |
| ANOVA | Analysis of Variance |
| aSAH | Aneurysmal Subarachnoid Hemorrhage |
| CRP | C-Reactive Protein |
| CSF | Cerebrospinal Fluid |
| DCI | Delayed Cerebral Ischemia |
| DNBS | Deoxynivalenol-Sulfate |
| DSS | Dextran Sodium Sulfate |
| IBD | Inflammatory Bowel Disease |
| IGF-1 | Insulin-like Growth Factor-1 |
| IHD | Ischemic Heart Disease |
| IQR | Interquartile Range |
| LDA | Linear Discriminant Analysis |
| LEfSe | Linear Discriminant Analysis Effect Size |
| LPS | Lipopolysaccharide |
| log10 | Logarithm Base 10 |
| MetaPhlAn | Metagenomic Phylogenetic Analysis |
| mFS | Modified Fisher Score |
| NGS | Next-Generation Sequencing |
| NF-κB | Nuclear Factor Kappa B |
| OTUs | Operational Taxonomic Units |
| PCoA | Principal Coordinate Analysis |
| RA | Ruptured Aneurysm |
| SAH | Subarachnoid Hemorrhage |
| SCFAs | Short-Chain Fatty Acids |
| UIA | Unruptured Intracranial Aneurysm |
| WFNS | World Federation of Neurological Societies Score |
References
- Asikainen, A.; Korja, M.; Kaprio, J.; Rautalin, I. Case Fatality in Patients with Aneurysmal Subarachnoid Hemorrhage in Finland: A Nationwide Register-Based Study. Neurology 2023, 100, e348–e356. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Lee, J.Y.; Heo, N.H.; Han, J.J.; Lee, E.C.; Hong, D.Y.; Lee, D.H.; Lee, M.R.; Oh, J.S. Short- and Long-Term Mortality of Subarachnoid Hemorrhage According to Hospital Volume and Severity Using a Nationwide Multicenter Registry Study. Front. Neurol. 2022, 13, 952794. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of Gut-Brain Axis, Gut Microbial Composition, and Probiotic Intervention in Alzheimer’s Disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ito, M.; Ishida, T.; Hamaguchi, T.; Maeda, T.; Kashihara, K.; Tsuboi, Y.; Ueyama, J.; Shimamura, T.; Mori, H.; et al. Meta-Analysis of Gut Dysbiosis in Parkinson’s Disease. Mov. Disord. 2020, 35, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Ding, D.; Zhu, H.; Wang, R.; Su, F.; Wu, W.; Xiao, Z.; Liang, X.; Zhao, Q.; Hong, Z.; et al. Disturbed Microbial Ecology in Alzheimer’s Disease: Evidence from the Gut Microbiota and Fecal Metabolome. BMC Microbiol. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.F.; Walker, A.W.; Louis, P.; Parkhill, J.; Vermeiren, J.; Bosscher, D.; Duncan, S.H.; Flint, H.J. Modulation of the Human Gut Microbiota by Dietary Fibres Occurs at the Species Level. BMC Biol. 2016, 14, 3. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism from the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Lee, D.W.; Ryu, Y.K.; Chang, D.H.; Park, H.Y.; Go, J.; Maeng, S.Y.; Hwang, D.Y.; Kim, B.C.; Lee, C.H.; Kim, K.S. Agathobaculum butyriciproducens Shows Neuroprotective Effects in a 6-OHDA-Induced Mouse Model of Parkinson’s Disease. J. Microbiol. Biotechnol. 2022, 32, 1168–1177. [Google Scholar] [CrossRef]
- Breyner, N.M.; Michon, C.; de Sousa, C.S.; Vilas Boas, P.B.; Chain, F.; Azevedo, V.A.; Langella, P.; Chatel, J.M. Microbial Anti-Inflammatory Molecule (MAM) from Faecalibacterium prausnitzii Shows a Protective Effect on DNBS and DSS-Induced Colitis Model in Mice Through Inhibition of NF-κB Pathway. Front. Microbiol. 2017, 8, 114. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii Prevents Physiological Damages in a Chronic Low-Grade Inflammation Murine Model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.-J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermúdez-Humarán, L.G.; Langella, P. The Commensal Bacterium Faecalibacterium prausnitzii is Protective in DNBS-Induced Chronic Moderate and Severe Colitis Models. Inflamm. Bowel Dis. 2014, 20, 417–430. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, J.; Ran, Z.H. Association Between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Håkansson, F.; Postma, E.; Keita, Å. Faecalibacterium prausnitzii Supernatant Improves Intestinal Barrier Function in Mice DSS Colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W. Gut Microbiota Dysbiosis in Patients with Ischemic Stroke and the Associated Metabolite Alterations. Front. Cell. Infect. Microbiol. 2021, 11, 573636. [Google Scholar]
- Luo, Y.; Chang, G.; Yu, G.; Lin, Y.; Zhang, Q.; Wang, Z.; Han, J. Unveiling the Negative Association of Faecalibacterium prausnitzii with Ischemic Stroke Severity, Impaired Prognosis, and Pro-Inflammatory Markers. Heliyon 2024, 10, e26651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y.; Yu, M.; Tian, Y. Gut Microbiota Dysbiosis in Patients with Aneurysmal Subarachnoid Hemorrhage and Its Potential Association with Inflammation: A Pilot Study. Brain Res. Bull. 2019, 153, 59–68. [Google Scholar] [CrossRef]
- Sun, K.; Cao, Y.; Chen, Y.; Peng, Q.; Xie, Y.; Luo, Y.; Tian, H.; Li, X.; Zeng, M.; Zhang, X.; et al. Altered Gut Microbiomes Are Associated with the Symptomatic Status of Unruptured Intracranial Aneurysms. Front. Neurosci. 2022, 16, 1056785. [Google Scholar] [CrossRef]
- Hatanaka, N.; Shimizu, A.; Somroop, S.; Li, Y.; Asakura, M.; Nagita, A.; Awasthi, S.P.; Hinenoya, A.; Yamasaki, S. High Prevalence of Campylobacter ureolyticus in Stool Specimens of Children with Diarrhea in Japan. Jpn. J. Infect. Dis. 2017, 70, 455–457. [Google Scholar] [CrossRef]
- Kawabata, S.; Takagaki, M.; Nakamura, H.; Oki, H.; Motooka, D.; Nakamura, S.; Nishida, T.; Terada, E.; Izutsu, N.; Takenaka, T.; et al. Dysbiosis of Gut Microbiome Is Associated with Rupture of Cerebral Aneurysms. Stroke 2022, 53, 895–903. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, Q.; Xu, Z.; Long, S.; Luo, G.; Chen, J.; Wei, W.; Li, Z.; Li, X. Multiple Omics Reveal Link Between the Microbiota-Gut-Brain Axis and Intracranial Aneurysm Rupture. iScience 2024, 27, 111184. [Google Scholar] [CrossRef]
- Hoh, B.L.; Ko, N.U.; Amin-Hanjani, S.; Chou, S.H.-Y.; Cruz-Flores, S.; Dangayach, N.S.; Derdeyn, C.P.; Du, R.; Hänggi, D.; Hetts, S.W.; et al. 2023 Guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2023, 54, e314–e370, Erratum in Stroke 2023, 54, e516. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage as an Outcome Event in Clinical Trials and Observational Studies: Proposal of a Multidisciplinary Research Group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 December 2024).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Blanco-Míguez, A.; Beghini, F.; Cumbo, F.; Mclver, L.J.; Thompson, K.N.; Zolfo, M.; Manghi, P.; Dubois, L.; Huang, K.D.; Thomas, A.M.; et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. 2023, 41, 1633–1644. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. (2012–2019). Microbiome R Package. Available online: https://bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 20 December 2024).
- Smith, D.P. (n.d.). Rbiom R Package. Available online: https://cran.r-project.org/web/packages/rbiom/index.html (accessed on 20 December 2024).
- Paradis, E.; Blomberg, S.; Bolker, B.; Brown, J.; Claramunt, S.; Claude, J.; Cuong, H.S.; Desper, R.; Didier, G.; Durand, B.; et al. (n.d.). Ape R Package. Available online: https://cran.r-project.org/web/packages/ape/index.html (accessed on 20 December 2024).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Chang, D.H.; Ryu, Y.K.; Park, H.Y.; Lee, I.B.; Noh, J.R.; Hwang, D.Y.; Kim, B.C.; Kim, K.S.; Lee, C.H. Human Gut Microbiota Agathobaculum butyriciproducens Improves Cognitive Impairment in LPS-Induced and APP/PS1 Mouse Models of Alzheimer’s Disease. Nutr. Res. 2021, 86, 96–108. [Google Scholar] [CrossRef]
- Bai, L.; Li, T.; Zhang, M.; Wang, S.; Gan, S.; Jia, X. Association of Gut Microbiota with Cerebral Cortex and Cerebrovascular Abnormality in Human Mild Traumatic Brain Injury. bioRxiv 2020. [Google Scholar] [CrossRef]
- Taraskina, A.; Ignatyeva, O.; Lisovaya, D.; Ivanov, M.; Ivanova, L.; Golovicheva, V.; Baydakova, G.; Silachev, D.; Popkov, V.; Ivanets, T.; et al. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells 2022, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, G.; Zhao, J.; Zhu, H.; Mo, H.; Xiong, Z.; Zhao, Z.; Chen, J.; Ning, W. The Impact of Dysbiosis in Oropharyngeal and Gut Microbiota on Systemic Inflammatory Response and Short-Term Prognosis in Acute Ischemic Stroke with Preceding Infection. Front. Microbiol. 2024, 15, 1432958. [Google Scholar] [CrossRef]
- Dai, X.C.; Yu, Y.; Zhou, S.Y.; Yu, S.; Xiang, M.X.; Ma, H. Assessment of the Causal Relationship Between Gut Microbiota and Cardiovascular Diseases: A Bidirectional Mendelian Randomization Analysis. BioData Min. 2024, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zang, C.; Li, P.; Sheng, D.; Xiao, Z.; Xiao, B.; Xia, J.; Zhou, L. Investigating the Role of Gut Microbiota in Hemorrhagic Stroke: Evidence from Causal Analysis. J. Stroke Cerebrovasc. Dis. 2025, 34, 108131. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Huang, Z.Y.; Zhou, W.W.; Li, Z.Y.; Li, X.P.; Chen, S.S.; Ma, J.K. Uncovering the Characteristics of the Gut Microbiota in Patients With Ischemic Stroke and Hemorrhagic Stroke. Sci. Rep. 2024, 14, 11776. [Google Scholar] [CrossRef]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.E.; Zhou, M.J.; Cohen, M.E.; Annavajhala, M.K.; Khan, S.; Moscoso, D.I.; Brooks, C.; Whittier, S.; Chong, D.H.; Uhlemann, A.-C.; et al. Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 2018, 44, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.T.; Fukumori, C.; Ferreira, C.M. New Insights into Therapeutic Strategies for Gut Microbiota Modulation in Inflammatory Diseases. Clin. Transl. Immunol. 2016, 5, e87. [Google Scholar] [CrossRef]
- Ahn, S.; Jin, T.E.; Chang, D.H.; Rhee, M.S.; Kim, H.J.; Lee, S.J.; Park, D.S.; Kim, B.C. Agathobaculum butyriciproducens gen. nov. sp. nov., a Strict Anaerobic, Butyrate-Producing Gut Bacterium Isolated from Human Faeces and Reclassification of Eubacterium desmolans as Agathobaculum desmolans comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 3656–3661. [Google Scholar] [CrossRef] [PubMed]
| Variable | RA (n = 24) | UIA (n = 24) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 59 ± 14 | 63 ± 10 | 0.39 |
| Female, N (%) | 16 (67) | 17 (71) | 0.5 |
| Hypertension, N (%) | 15 (63) | 20 (83) | 0.09 |
| Diabetes, N (%) | 3 (13) | 7 (29) | 0.14 |
| IHD, N (%) | 12 (50) | 6 (25) | 0.07 |
| Smoking, N (%) | 14 (58) | 10 (42) | 0.19 |
| WFNS, median (IQR) | 1.5 (1–4) | N/A | N/A |
| Fisher score, median (IQR) | 3 (1.5–4) | N/A | N/A |
| Loss of consciousness during ictus, N (%) | 14 (58) | N/A | N/A |
| Aneurysm location | 0.36 | ||
| anterior circulation | 18 (75) | 20 (83) | |
| posterior circulation | 6 (25) | 4 (17) | |
| Size of aneurysm, mm | 6 (5–8) | 6 (5–10) | 0.76 |
| Lumbal drain, N (%) | 18 (75) | N/A | N/A |
| Mechanical ventilation, N (%) | 8 (33) | N/A | N/A |
| Decompressive craniotomy, N (%) | 1 (4) | N/A | N/A |
| Extraventricular drainage, N (%) | 3 (13) | N/A | N/A |
| Delayed cerebral ischemia, N (%) | 3 (13) | N/A | N/A |
| Infection, N (%) | 9 (38) | N/A | N/A |
| 3-month mRS score | 2 (1–3) | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csecsei, P.; Takacs, B.; Pasitka, L.; Varnai, R.; Peterfi, Z.; Orban, B.; Czabajszki, M.; Olah, C.; Schwarcz, A. Distinct Gut Microbiota Profiles in Unruptured and Ruptured Intracranial Aneurysms: Focus on Butyrate-Producing Bacteria. J. Clin. Med. 2025, 14, 3488. https://doi.org/10.3390/jcm14103488
Csecsei P, Takacs B, Pasitka L, Varnai R, Peterfi Z, Orban B, Czabajszki M, Olah C, Schwarcz A. Distinct Gut Microbiota Profiles in Unruptured and Ruptured Intracranial Aneurysms: Focus on Butyrate-Producing Bacteria. Journal of Clinical Medicine. 2025; 14(10):3488. https://doi.org/10.3390/jcm14103488
Chicago/Turabian StyleCsecsei, Peter, Bertalan Takacs, Lídia Pasitka, Reka Varnai, Zoltan Peterfi, Brigitta Orban, Mate Czabajszki, Csaba Olah, and Attila Schwarcz. 2025. "Distinct Gut Microbiota Profiles in Unruptured and Ruptured Intracranial Aneurysms: Focus on Butyrate-Producing Bacteria" Journal of Clinical Medicine 14, no. 10: 3488. https://doi.org/10.3390/jcm14103488
APA StyleCsecsei, P., Takacs, B., Pasitka, L., Varnai, R., Peterfi, Z., Orban, B., Czabajszki, M., Olah, C., & Schwarcz, A. (2025). Distinct Gut Microbiota Profiles in Unruptured and Ruptured Intracranial Aneurysms: Focus on Butyrate-Producing Bacteria. Journal of Clinical Medicine, 14(10), 3488. https://doi.org/10.3390/jcm14103488







