Comparison of Neurodevelopmental Therapy with Standard Therapy for the Treatment of Patients with Spasticity After Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Standards
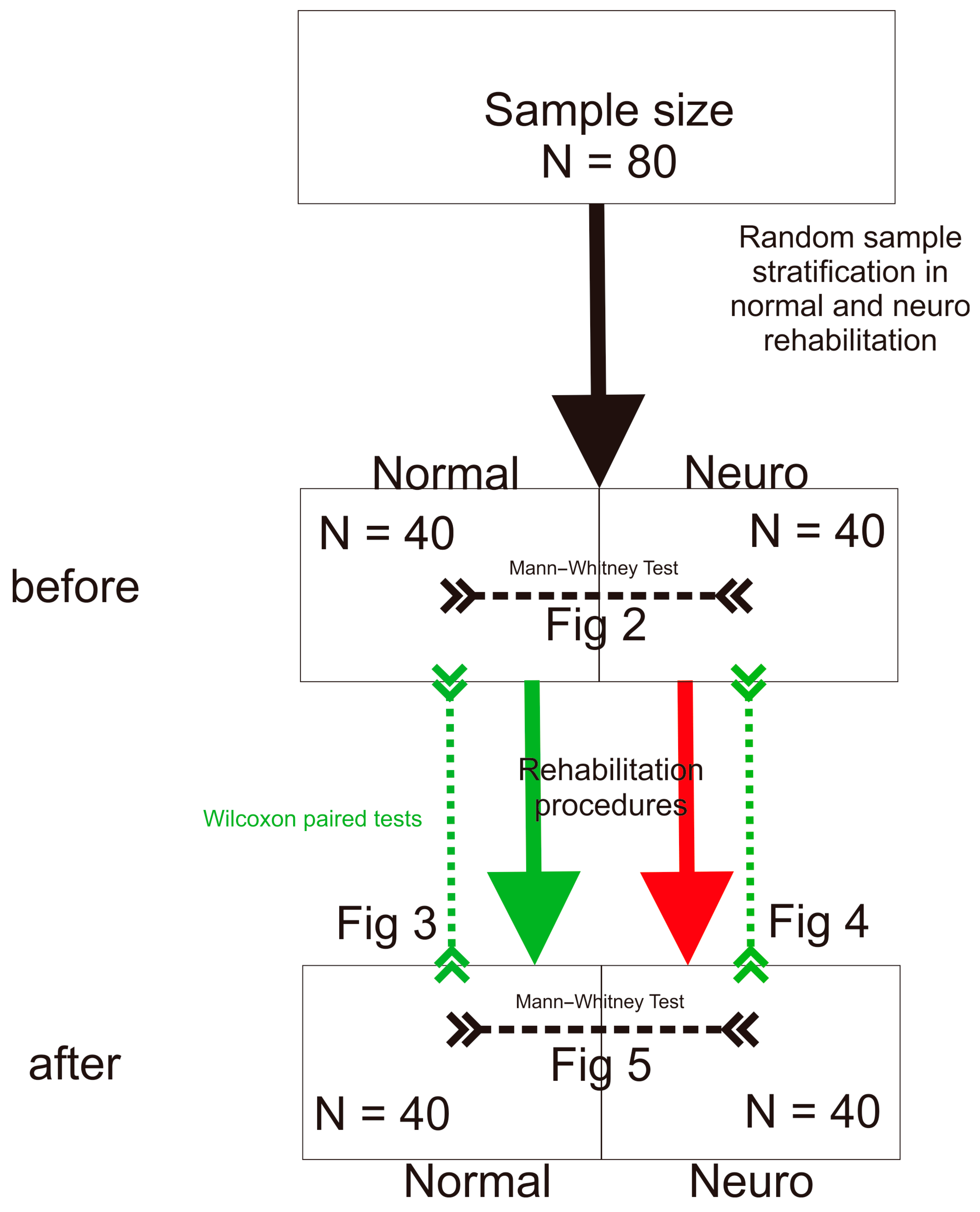
2.2. Participants
Independent Variables
2.3. Procedures
Statistical Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Curra, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: Implications for neurorehabilitation. BioMed Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cuaresma, L.; Lucena-Anton, D.; Gonzalez-Medina, G.; Martin-Vega, F.J.; Galan-Mercant, A.; Luque-Moreno, C. Effectiveness of Stretching in Post-Stroke Spasticity and Range of Motion: Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151. [Google Scholar] [CrossRef]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Kilrane, M.; Greig, C.A.; Brazzelli, M.; Mead, G.E. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2016, 3, Cd003316. [Google Scholar] [CrossRef] [PubMed]
- Bobath, B. Adult Hemiplegia: Evaluation and Treatment, 3rd ed.; Heinemann Medical Books: Oxford, UK, 1990. [Google Scholar]
- Wolny, T.; Saulicz, E.; Gnat, R.; Kokosz, M.; Myśliwiec, A.; Kuszewski, M. Influence of proprioceptiveneuromuscular facilitation (PNF) on the degree of spasticity in late-stage stroke patients. Fizjoterapia Pol. 2011, 11, 1–8. [Google Scholar]
- Pathak, A.; Gyanpuri, V.; Dev, P.; Dhiman, N.R. The Bobath Concept (NDT) as rehabilitation in stroke patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 3983–3990. [Google Scholar] [CrossRef]
- Hindle, K.B.; Whitcomb, T.J.; Briggs, W.O.; Hong, J. Proprioceptive Neuromuscular Facilitation (PNF): Its Mechanisms and Effects on Range of Motion and Muscular Function. J. Hum. Kinet. 2012, 31, 105–113. [Google Scholar] [CrossRef]
- Elzib, H.; Pawloski, J.; Ding, Y.; Asmaro, K. Antidepressant pharmacotherapy and poststroke motor rehabilitation: A review of neurophysiologic mechanisms and clinical relevance. Brain Circ. 2019, 5, 62–67. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, J.Y.; Borlongan, C.V.; Tajiri, N. A brief physical activity protects against ischemic stroke. Brain Circ. 2019, 5, 112–118. [Google Scholar] [CrossRef]
- Ostrowska, P.; Hansdorfer-Korzon, R.; Studnicki, R.; Spychała, D. Use of the posturography platform as a tool for quantitative assessment of imbalance and postural control in post-stroke patients in chronic phase. Fizjoter. Pol. 2023, 1, 142–163. [Google Scholar] [CrossRef]
- Paolucci, T.; Agostini, F.; Mussomeli, E.; Cazzolla, S.; Conti, M.; Sarno, F.; Bernetti, A.; Paoloni, M.; Mangone, M. A rehabilitative approach beyond the acute stroke event: A scoping review about functional recovery perspectives in the chronic hemiplegic patient. Front. Neurol. 2023, 14, 1234205. [Google Scholar] [CrossRef] [PubMed]
- Guiu-Tula, F.X.; Cabanas-Valdes, R.; Sitja-Rabert, M.; Urrutia, G.; Gomara-Toldra, N. The Efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef]
- Wang, R.Y.; Chen, H.I.; Chen, C.Y.; Yang, Y.R. Efficacy of Bobath versus orthopaedic approach on impairment and function at different motor recovery stages after stroke: A randomized controlled study. Clin. Rehabil. 2005, 19, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Krukowska, J.; Bugajski, M.; Sienkiewicz, M.; Czernicki, J. The influence of NDT-Bobath and PNF methods on the field support and total path length measure foot pressure (COP) in patients after stroke. Neurol. Neurochir. Pol. 2016, 50, 449–454. [Google Scholar] [CrossRef]
- Gunning, E.; Uszynski, M.K. Effectiveness of the Proprioceptive Neuromuscular Facilitation Method on Gait Parameters in Patients With Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 980–986. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Chou, L.W.; Hsieh, Y.L. Proprioceptive Neuromuscular Facilitation-Based Physical Therapy on the Improvement of Balance and Gait in Patients with Chronic Stroke: A Systematic Review and Meta-Analysis. Life 2022, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. In The Corsini Encyclopedia of Psychology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; p. 1. [Google Scholar]
- Wilcoxon Test. The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; pp. 575–578. [Google Scholar]
- Smith, G.V.; Silver, K.H.; Goldberg, A.P.; Macko, R.F. “Task-oriented” exercise improves hamstring strength and spastic reflexes in chronic stroke patients. Stroke 1999, 30, 2112–2118. [Google Scholar] [CrossRef]
- Studnicki, R.; Studzińska, K.; Adamczewski, T.; Hansdorfer-Korzon, R.; Krawczyk, M. Analyzing the Impact of Rehabilitation Utilizing Neurofunctional Exercises on the Functional Status of Stroke Patients. J. Clin. Med. 2024, 13, 6271. [Google Scholar] [CrossRef]
- Ada, L.; Dorsch, S.; Canning, C.G. Strengthening interventions increase strength and improve activity after stroke: A systematic review. Aust. J. Physiother. 2006, 52, 241–248. [Google Scholar] [CrossRef]
- Allison, R.; Shenton, L.; Bamforth, K.; Kilbride, C.; Richards, D. Incidence, Time Course and Predictors of Impairments Relating to Caring for the Profoundly Affected arm After Stroke: A Systematic Review. Physiother. Res. Int. 2016, 21, 210–227. [Google Scholar] [CrossRef]
- Hesse, S.; Bertelt, C.; Jahnke, M.T.; Schaffrin, A.; Baake, P.; Malezic, M.; Mauritz, K.H. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke 1995, 26, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Choi, J. Person-centered rehabilitation care and outcomes: A systematic literature review. Int. J. Nurs. Stud. 2019, 93, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Korner-Bitensky, N. When does stroke rehabilitation end? Int. J. Stroke 2013, 8, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Veluswamy, S.K.; Hombali, A.; Mullick, A.; Manikandan, N.; Solomon, J.M. Effect of Transcutaneous Electrical Nerve Stimulation on Spasticity in Adults with Stroke: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 751–768. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Pérez-Bellmunt, A.; Llurda-Almuzara, L.; Plaza-Manzano, G.; De-la-Llave-Rincón, A.I.; Navarro-Santana, M.J. Is Dry Needling Effective for the Management of Spasticity, Pain, and Motor Function in Post-Stroke Patients? A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 131–141. [Google Scholar] [CrossRef]
- Brusola, G.; Garcia, E.; Albosta, M.; Daly, A.; Kafes, K.; Furtado, M. Effectiveness of physical therapy interventions on post-stroke spasticity: An umbrella review. NeuroRehabilitation 2023, 52, 349–363. [Google Scholar] [CrossRef]
- Pollock, A.S.; Legg, L.; Langhorne, P.; Sellars, C. Barriers to achieving evidence-based stroke rehabilitation. Clin. Rehabil. 2000, 14, 611–617. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studnicki, R.; Krawczyk, M.; Hansdorfer-Korzon, R.; Zubrzycki, I.Z.; Wiacek, M. Comparison of Neurodevelopmental Therapy with Standard Therapy for the Treatment of Patients with Spasticity After Stroke. J. Clin. Med. 2025, 14, 3450. https://doi.org/10.3390/jcm14103450
Studnicki R, Krawczyk M, Hansdorfer-Korzon R, Zubrzycki IZ, Wiacek M. Comparison of Neurodevelopmental Therapy with Standard Therapy for the Treatment of Patients with Spasticity After Stroke. Journal of Clinical Medicine. 2025; 14(10):3450. https://doi.org/10.3390/jcm14103450
Chicago/Turabian StyleStudnicki, Rafał, Maciek Krawczyk, Rita Hansdorfer-Korzon, Igor Z. Zubrzycki, and Magdalena Wiacek. 2025. "Comparison of Neurodevelopmental Therapy with Standard Therapy for the Treatment of Patients with Spasticity After Stroke" Journal of Clinical Medicine 14, no. 10: 3450. https://doi.org/10.3390/jcm14103450
APA StyleStudnicki, R., Krawczyk, M., Hansdorfer-Korzon, R., Zubrzycki, I. Z., & Wiacek, M. (2025). Comparison of Neurodevelopmental Therapy with Standard Therapy for the Treatment of Patients with Spasticity After Stroke. Journal of Clinical Medicine, 14(10), 3450. https://doi.org/10.3390/jcm14103450