Diagnostic and Therapeutic Challenges Between Peripartum and Influenza-Induced Inflammatory Cardiomyopathy—A Case Report and Literature Review
Abstract
:1. Background
2. Objectives
- To discuss the differential diagnosis of peripartum cardiomyopathy and inflammatory cardiomyopathy, highlighting key diagnostic tools.
- To describe a clinical case of peripartum cardiomyopathy and the challenges in diagnosing and managing the condition.
- To emphasize the importance of long-term monitoring and preventive strategies for PPCM patients.
3. Materials and Methods
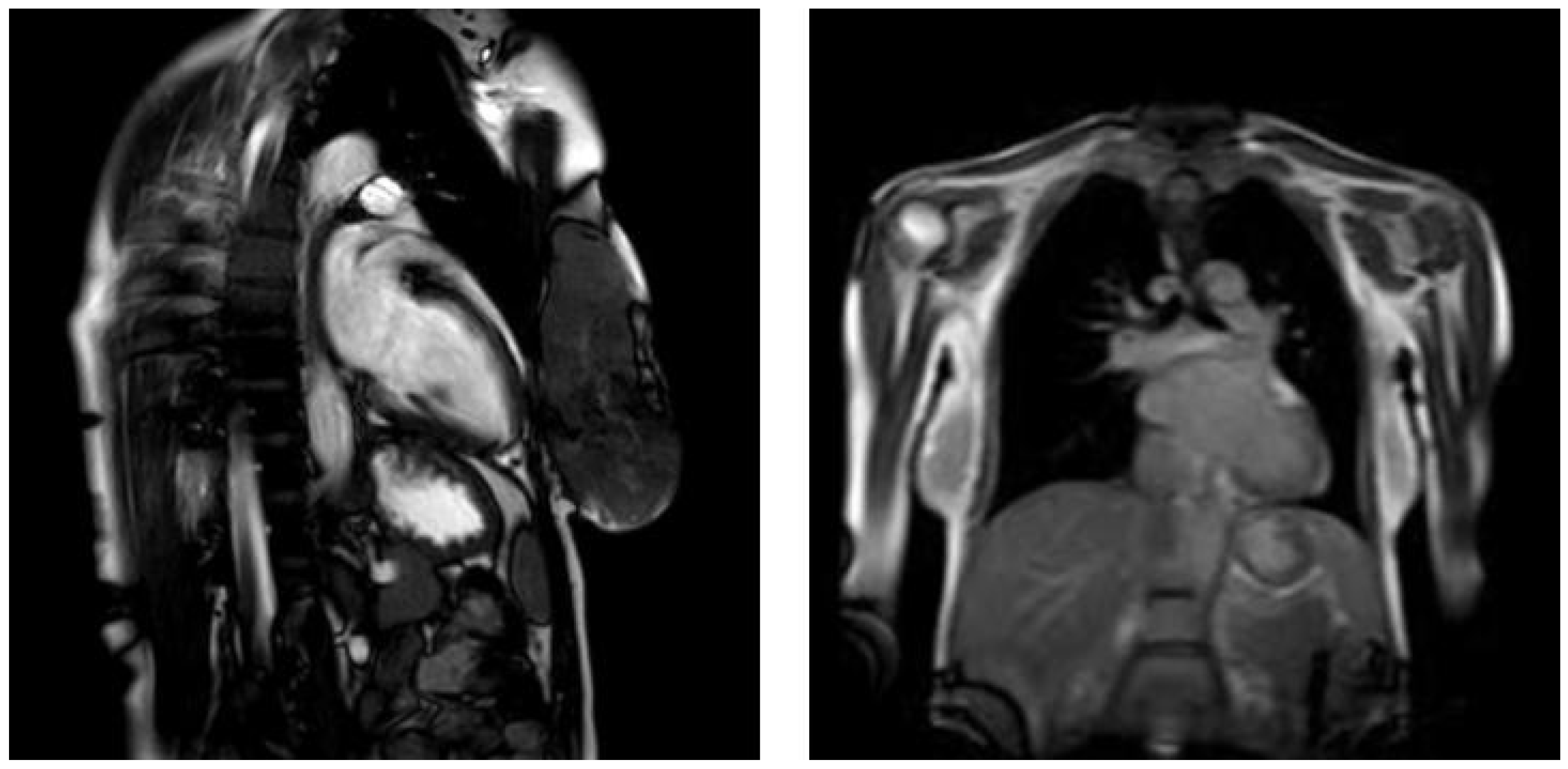
4. Case Presentation
5. Discussion with a Literature Review
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Mogos, M.F.; Piano, M.R.; McFarlin, B.L.; Salemi, J.L.; Liese, K.L.; Briller, J.E. Heart Failure in Pregnant Women. Circ. Heart Fail. 2018, 11, e004005. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Haythe, J.H.; Walsh, M.N.; Kittleson, M.M. Intersection of Heart Failure and Pregnancy: Beyond Peripartum Cardiomyopathy. Circ. Heart Fail. 2021, 14, e008223. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Macartney, M.; Brooksbank, K.; Brown, C.; Dawson, D.; Francis, M.; Japp, A.; Lennie, V.; Leslie, S.J.; Martin, T.; et al. A 20-year population study of peripartum cardiomyopathy. Eur. Heart J. 2023, 44, 5128–5141. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Estabragh, Z.R.; Mamas, M.A. The cardiovascular manifestations of influenza: A systematic review. Int. J. Cardiol. 2013, 167, 2397–2403. [Google Scholar] [CrossRef]
- Kenney, A.D.; Aron, S.L.; Gilbert, C.; Kumar, N.; Chen, P.; Eddy, A.; Zhang, L.; Zani, A.; Vargas-Maldonado, N.; Speaks, S.; et al. Influenza virus replication in cardiomyocytes drives heart dysfunction and fibrosis. Sci. Adv. 2022, 8, eabm5371. [Google Scholar] [CrossRef]
- Filgueiras-Rama, D.; Vasilijevic, J.; Jalife, J.; Noujaim, S.F.; Alfonso, J.M.; Nicolas-Avila, J.A.; Gutierrez, C.; Zamarreño, N.; Hidalgo, A.; Bernabé, A.; et al. Human influenza A virus causes myocardial and cardiac-specific conduction system infections associated with early inflammation and premature death. Cardiovasc. Res. 2021, 117, 876–889. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cifkova, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Rev. Esp. Cardiol. 2019, 72, 161. [Google Scholar] [CrossRef]
- Sliwa, K.; Bauersachs, J.; Arany, Z.; Spracklen, T.F.; Hilfiker-Kleiner, D. Peripartum cardiomyopathy: From genetics to management. Eur. Heart J. 2021, 42, 3094–3102. [Google Scholar] [CrossRef]
- Sliwa, K.; Mebazaa, A.; Hilfiker-Kleiner, D.; Petrie, M.C.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van der Meer, P.; et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur. J. Heart Fail. 2017, 19, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Goland, S.; Modi, K.; Bitar, F.; Janmohamed, M.; Mirocha, J.M.; Czer, L.S.C.; Illum, S.; Hatamizadeh, P.; Elkayam, U. Clinical Profile and Predictors of Complications in Peripartum Cardiomyopathy. J. Card. Fail. 2009, 15, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C.; et al. A Cathepsin D-Cleaved 16 kDa Form of Prolactin Mediates Postpartum Cardiomyopathy. Cell 2007, 128, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Damp, J.; Givertz, M.M.; Semigran, M.; Alharethi, R.; Ewald, G.; Felker, G.M.; Bozkurt, B.; Boehmer, J.; Haythe, J.; Skopicki, H.; et al. Relaxin-2 and Soluble Flt1 Levels in Peripartum Cardiomyopathy: Results of the Multicenter IPAC Study. JACC Heart Fail. 2016, 4, 380–388. [Google Scholar] [CrossRef]
- Sliwa, K.; Förster, O.; Libhaber, E.; Fett, J.D.; Sundstrom, J.B.; Hilfiker-Kleiner, D.; Ansari, A.A. Peripartum cardiomyopathy: Inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur. Heart J. 2006, 27, 441–446. [Google Scholar] [CrossRef]
- Haghikia, A.; Kaya, Z.; Schwab, J.; Westenfeld, R.; Ehlermann, P.; Bachelier, K.; Oettl, R.; von Kaisenberg, C.S.; Katus, H.A.; Bauersachs, J.; et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res. Cardiol. 2015, 110, 60. [Google Scholar] [CrossRef]
- Hoes, M.F.; Arany, Z.; Bauersachs, J.; Hilfiker-Kleiner, D.; Petrie, M.C.; Sliwa, K.; van der Meer, P. Pathophysiology and risk factors of peripartum cardiomyopathy. Nat. Rev. Cardiol. 2022, 19, 555–565. [Google Scholar] [CrossRef]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; DePalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.E.; et al. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef]
- Ware, J.S.; Li, J.; Mazaika, E.; Yasso, C.M.; DeSouza, T.; Cappola, T.P.; Tsai, E.J.; Hilfiker-Kleiner, D.; Kamiya, C.A.; Mazzarotto, F.; et al. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N. Engl. J. Med. 2016, 374, 233–241. [Google Scholar] [CrossRef]
- Petryka-Mazurkiewicz, J.; Kryczka, K.; Mazurkiewicz, Ł.; Miłosz-Wieczorek, B.; Śpiewak, M.; Marczak, M.; Henzel, J.; Grzybowski, J.; Demkow, M.; Dzielińska, Z. Cardiovascular Magnetic Resonance in Peripartum Cardiomyopathy: Comparison with Idiopathic Dilated Cardiomyopathy. Diagnostics 2021, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Honigberg, M.C.; Elkayam, U.; Rajagopalan, N.; Modi, K.; Briller, J.E.; Drazner, M.H.; Wells, G.L.; McNamara, D.M.; Givertz, M.M.; IPAC Investigators. Electrocardiographic findings in peripartum cardiomyopathy. Clin. Cardiol. 2019, 42, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Mbakwem, A.C.; Bauersachs, J.; Viljoen, C.; Hoevelmann, J.; van der Meer, P.; Petrie, M.C.; Mebazaa, A.; Goland, S.; Karaye, K.; Laroche, C.; et al. Electrocardiographic features and their echocardiographic correlates in peripartum cardiomyopathy: Results from the ESC EORP PPCM registry. ESC Heart Fail. 2021, 8, 879–889. [Google Scholar] [CrossRef]
- Joudar, I.; Aichouni, N.; Nasri, S.; Kamaoui, I.; Skiker, I. Diagnostic criteria for myocarditis on cardiac magnetic resonance imaging: An educational review. Ann. Med. Surg. 2023, 85, 3960–3964. [Google Scholar] [CrossRef]
- Polte, C.L.; Bobbio, E.; Bollano, E.; Bergh, N.; Polte, C.; Himmelman, J.; Lagerstrand, K.M.; Gao, S.A. Cardiovascular Magnetic Resonance in Myocarditis. Diagnostics 2022, 12, 399. [Google Scholar] [CrossRef]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef]
- Isaak, A.; Ayub, T.H.; Merz, W.M.; Faron, A.; Endler, C.; Sprinkart, A.M.; Pieper, C.C.; Kuetting, D.; Dabir, D.; Attenberger, U.; et al. Peripartum Cardiomyopathy: Diagnostic and Prognostic Value of Cardiac Magnetic Resonance in the Acute Stage. Diagnostics 2022, 12, 378. [Google Scholar] [CrossRef]
- Behrouzi, B.; Bhatt, D.L.; Cannon, C.P.; Vardeny, O.; Lee, D.S.; Solomon, S.D.; Udell, J.A. Association of Influenza Vaccination With Cardiovascular Risk: A Meta-analysis. JAMA Netw. Open 2022, 5, e228873. [Google Scholar] [CrossRef]
- Simionescu, A.A.; Streinu-Cercel, A.; Popescu, F.D.; Stanescu, A.M.A.; Vieru, M.; Danciu, B.M.; Miron, V.D.; Săndulescu, O. Comprehensive Overview of Vaccination during Pregnancy in Europe. J. Pers. Med. 2021, 11, 1196. [Google Scholar] [CrossRef]
- Hilfiker-Kleiner, D.; Haghikia, A.; Berliner, D.; Vogel-Claussen, J.; Schwab, J.; Franke, A.; Schwarzkopf, M.; Ehlermann, P.; Pfister, R.; Michels, G.; et al. Bromocriptine for the treatment of peripartum cardiomyopathy: A multicentre randomized study. Eur. Heart J. 2017, 38, 2671–2679. [Google Scholar] [CrossRef]
- Kearney, L.; Wright, P.; Fhadil, S.; Thomas, M. Postpartum Cardiomyopathy and Considerations for Breastfeeding. Card. Fail. Rev. 2018, 4, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Radakrishnan, A.; Dokko, J.; Pastena, P.; Kalogeropoulos, A.P. Thromboembolism in peripartum cardiomyopathy: A systematic review. J. Thorac. Dis. 2024, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, K.; Brunisholz, K.; Budge, D.; Horne, B.D.; Alharethi, R.; Folsom, J.; Connolly, J.J.; Stehlik, J.; Kfoury, A. Peripartum cardiomyopathy: Post-transplant outcomes from the United Network for Organ Sharing Database. J. Heart Lung Transplant. 2012, 31, 180–186. [Google Scholar] [CrossRef]
- Bauersachs, J.; König, T.; van der Meer, P.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mbakwem, A.; Hamdan, R.; Jackson, A.M.; Forsyth, P.; de Boer, R.A.; et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 827–843. [Google Scholar] [CrossRef]
- Goland, S.; George, J.; Elkayam, U.; Shimoni, S.; Fugenfirov, I.; Vaisbuch, E.; Arad, M.; Freimark, D.; Simchen, M.; Kuperstein, R. Contemporary outcome of subsequent pregnancies in patients with previous peripartum cardiomyopathy. ESC Heart Fail. 2022, 9, 4262–4270. [Google Scholar] [CrossRef]
- Codsi, E.; Rose, C.H.; Blauwet, L.A. Subsequent Pregnancy Outcomes in Patients With Peripartum Cardiomyopathy. Obstet. Gynecol. 2018, 131, 322–327. [Google Scholar] [CrossRef]
- Ma’ayeh, M.; Slivnick, J.A.; McKiever, M.E.; Garrett, Z.D.; Lim, W.; Cackovic, M.; Rood, K.M.; Bradley, E.A. Imaging-Based Risk Stratification for Recurrence Risk in Women with a History of Peripartum Cardiomyopathy. Am. J. Perinatol. 2022, 39, 225–231. [Google Scholar] [CrossRef]

| Feature | Peripartum Cardiomyopathy | Influenza-Induced Inflammatory Cardiomyopathy |
|---|---|---|
| Typical onset | Last month of pregnancy or within 6 months postpartum | During as well as shortly post-viral infection |
| Aetiology | Idiopathic in most cases, genetics 10% | Influenza infection:
|
| Risk factors | Multiparity, multiple pregnancy, advanced maternal age, obesity or nutritional deficiencies, hypertension, pre-eclampsia, diabetes, cigarette smoking, autoimmune diseases | Immunosuppression, lack of influenza vaccination |
| Symptoms | Fatigue, dyspnea, syncope, oedema | Similar to PPCM, however often accompanied by viral infection symptoms, like fever, myalgia, cough, sore throat |
| Cardiac magnetic resonance | Reduced LVEF, LV dilatation, LV hypertrophy, often oedema | Reduced LVEF, LV dilatation, oedema, hyperaemia, capillary leak, necrosis, and fibrosis |
| Definite diagnostic tools | Diagnosis of exclusion | Endomyocardial biopsy “golden standard” |
| Treatment | Standard HF therapy, bromocriptine (postpartum) | Standard HF therapy + antiviral therapy, immunosuppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachyra, K.; Zasztowt-Sternicka, M.; Litwinska, M.; Litwinska-Korcz, E.; Walasik-Szewczyk, I.; Jabiry-Zieniewicz, Z.; Szpotanska-Sikorska, M. Diagnostic and Therapeutic Challenges Between Peripartum and Influenza-Induced Inflammatory Cardiomyopathy—A Case Report and Literature Review. J. Clin. Med. 2025, 14, 3440. https://doi.org/10.3390/jcm14103440
Stachyra K, Zasztowt-Sternicka M, Litwinska M, Litwinska-Korcz E, Walasik-Szewczyk I, Jabiry-Zieniewicz Z, Szpotanska-Sikorska M. Diagnostic and Therapeutic Challenges Between Peripartum and Influenza-Induced Inflammatory Cardiomyopathy—A Case Report and Literature Review. Journal of Clinical Medicine. 2025; 14(10):3440. https://doi.org/10.3390/jcm14103440
Chicago/Turabian StyleStachyra, Karolina, Monika Zasztowt-Sternicka, Magdalena Litwinska, Ewelina Litwinska-Korcz, Izabela Walasik-Szewczyk, Zoulikha Jabiry-Zieniewicz, and Monika Szpotanska-Sikorska. 2025. "Diagnostic and Therapeutic Challenges Between Peripartum and Influenza-Induced Inflammatory Cardiomyopathy—A Case Report and Literature Review" Journal of Clinical Medicine 14, no. 10: 3440. https://doi.org/10.3390/jcm14103440
APA StyleStachyra, K., Zasztowt-Sternicka, M., Litwinska, M., Litwinska-Korcz, E., Walasik-Szewczyk, I., Jabiry-Zieniewicz, Z., & Szpotanska-Sikorska, M. (2025). Diagnostic and Therapeutic Challenges Between Peripartum and Influenza-Induced Inflammatory Cardiomyopathy—A Case Report and Literature Review. Journal of Clinical Medicine, 14(10), 3440. https://doi.org/10.3390/jcm14103440






