1. Introduction
Primary ciliary dyskinesia (PCD) is a hereditary disease with an autosomal recessive inheritance pattern, although sporadic cases of dominant and sex-linked inheritance have also been described [
1]. Its prevalence is estimated to be approximately 1 in 7500–10,000 live births [
2], although it is likely underdiagnosed in the general population. The condition is characterized by cilia immotility or the dyskinesia [
3] (abnormal and ineffective ciliary movement) of motile cilia, which, in the case of respiratory cilia, leads to mucus stasis in the airways. This leads to bacterial proliferation and the following associated complications:
- (a)
Chronic bronchitis with bronchiectasis and progressive deterioration of lung function;
- (b)
Chronic sinusitis with frequent exacerbations, as well as sinus hypoplasia and persistent pansinusitis;
- (c)
Secretory otitis media, which can progress to chronic otitis and hearing loss over time, particularly in childhood [
4].
The disease presents clinically from birth, with persistent rhinorrhea and a lifelong productive cough with mucopurulent secretions [
4]. Neonatal respiratory distress of unknown cause is also common [
5]. Approximately 46% of patients exhibit situs inversus, while 12% present with heterotaxy [
6], both resulting from the impaired motility of embryonic nodal cilia. Male patients are often infertile due to sperm flagellar immotility, while female patients experience subfertility due to defective ciliary movement in the fallopian tubes, which hinders ovum transport [
7]. The diagnosis of PCD is complex and relies on the following [
8,
9,
10]:
- (a)
Assessment of ciliary motility using high-speed video microscopy DHSV—Digital High-Speed Leica DMI3000B inverted microscope (Leica, Berlin, Germany) in nasal (more accessible) or bronchial epithelial samples. This technique enables a detailed analysis of the ciliary beat pattern. Variations in patterns have been previously associated with specific genetic findings [
11]. Also, this study allows for the measurement of ciliary beat frequency (CBF), which typically ranges from 7 to 16 Hz in healthy individuals under physiological conditions [
12]. It is important to note that CBF values are influenced by environmental factors such as temperature and humidity;
- (b)
Evaluation of ciliary ultrastructure through electron microscopy (EM) in the same samples, although no structural abnormalities are detected in 30% of patients;
- (c)
Genetic analysis, with over 50 genes associated with PCD identified to date, explaining 65–70% of cases [
13].
The lack of a functional mucociliary clearance system complicates treatment, as neither medical nor surgical therapies can restore normal nasosinusal or otological physiology, resulting in lifelong disease persistence. At the pulmonary level, progressive lung damage develops, leading to bronchiectasis, atelectasis, and eventually respiratory failure. In some patients, lung transplantation remains the only viable option for survival [
14], though its success depends on whether the transplanted lung maintains a permanently functional mucociliary system.
The hypothesis underlying this study posits that the transplanted lung preserves the donor’s normal ciliary function, leading to a favorable prognosis for the recipient by improving lung function and quality of life. Conversely, if mucociliary function is lost over time, the transplanted lung would progressively deteriorate, ultimately leading to respiratory failure.
The primary objective of this research is to determine ciliary motility and ultrastructure in the bronchial epithelium of transplanted lungs in patients with PCD. This will assess whether the mucociliary function of the organ remains preserved despite being implanted in an individual affected by this genetic disorder. The findings aim to contribute to scientific knowledge and provide valuable insights regarding the impact of lung transplantation on mucociliary function and the prognosis of patients with PCD undergoing this procedure.
2. Materials and Methods
A descriptive observational study was conducted on two cases of bilateral lung transplantation in patients diagnosed with PCD and severe disease progression. The study population consisted of two female patients (aged 60 and 63 years) with advanced lung deterioration, who met the eligibility criteria for bilateral lung transplantation due to extensive bilateral bronchiectasis and pulmonary function decline confirmed through respiratory tests. Patient selection followed the established lung transplantation protocols [
15]. Ethical approval was granted by the local Ethics Committee of Hospital Universitario y Politécnico La Fe (Registry No.: 2022-291-1, approval date: 29 March 2023). Written informed consent was obtained from all participants.
Ciliated cell samples for functional and structural analysis were obtained nasally via curettage of the middle turbinate mucosa under rhinoscopic guidance (
Figure 1), while bronchial samples were collected through brushing during bronchoscopy (
Figure 2). Cells were collected in DMEM high-glucose medium with streptomycin at 10% to preserve cellular integrity and viability. The ciliary motility of the nasal and bronchial epithelial samples from the transplanted lungs was assessed using high-speed, high-precision videomicroscopy immediately after collection. The temperature was maintained at 24 °C (room temperature). Multiple fields per sample were analyzed to identify regions containing continuous epithelial strips and isolated cells. Images were acquired from both the lateral and top views. The samples were visualized using a Leica DMI3000B inverted microscope (Leica, Berlin, Germany) with a 63× objective lens, providing a total magnification of 630× (
Figure 3). Images were recorded using a Basler acA1300-200 um digital video camera with an ON-Semiconductor PYTHON 1300 CMOS sensor (Basler AG, Ahrensburg, Germany) attached to the microscope. All recordings and image processing were performed using the Sisson–Ammons Video Analysis (SAVA) system [
16], which allowed for the determination of ciliary motion patterns and ciliary beat frequency (CBF). CBF was measured for each patient throughout the entire follow-up period.
The ciliary ultrastructure of the nasal and bronchial samples from the transplanted lungs was assessed in accordance with the International Consensus Guideline for reporting transmission electron microscopy results in the diagnosis of Primary Ciliary Dyskinesia (BEAT PCD TEM Criteria) (Eur Respir J 2020) [
17]. Cells were fixed in 2.5% phosphate-buffered glutaraldehyde (pH 7.3) for 24 h, then gently centrifuged at 1500 rpm for two minutes. The resulting pellet was processed as previously reported [
18]. A total of 50 axonemes were analyzed from both the nasal and bronchial ciliated epithelium of the transplanted lungs. Ciliary sections were selected near healthy ciliated cells to minimize the presence of secondary ciliary defects. Transmission electron microscopy (TEM) analysis was performed using a Hitachi HT7700 electron microscope (Hitachi High-Tech Corporation, Tokyo, Japan). Axonemal defects were classified according to the international consensus guidelines.
Patients underwent preoperative evaluations and post-transplant follow-up visits at different time points due to the chronological difference in their transplant years (2021 and 2022, respectively). The first patient was evaluated at 1, 3, 6, 12, 18, 24, and 30 months post-transplant, while the second patient was evaluated at 1, 3, 6, 12, and 18 months. During these visits, variables related to ciliary motility and ultrastructure, as previously described, were recorded and analyzed. Additionally, post-transplant follow-up by the lung transplant unit included pulmonary function tests, imaging studies, blood tests, lung rejection assessments, microbiological cultures, and quality-of-life monitoring.
3. Results
The first patient included in this study was a 63-year-old woman who was diagnosed with PCD around the age of 50; however, symptoms associated with the disease had been present since birth. A significant family history finding was that her younger sister had also been diagnosed with PCD. The patient exhibited a compatible clinical phenotype [
4], with severe neonatal respiratory distress and chronic symptoms, including productive cough, mucopurulent rhinorrhea, recurrent pneumonias, bronchiectasis, recurrent otitis with tympanic myringosclerosis, and moderate bilateral mixed hearing loss. No laterality defects were observed.
Genetic testing using DNA from peripheral blood identified the following two heterozygous mutations in different genes: RSPH1 with a nucleotide change c.85G > T and an amino acid change p.Glu29*; and HYDIN with a nucleotide change c.6889G > T and an amino acid change p.Glu2297*. These same mutations were present in her sister with PCD.
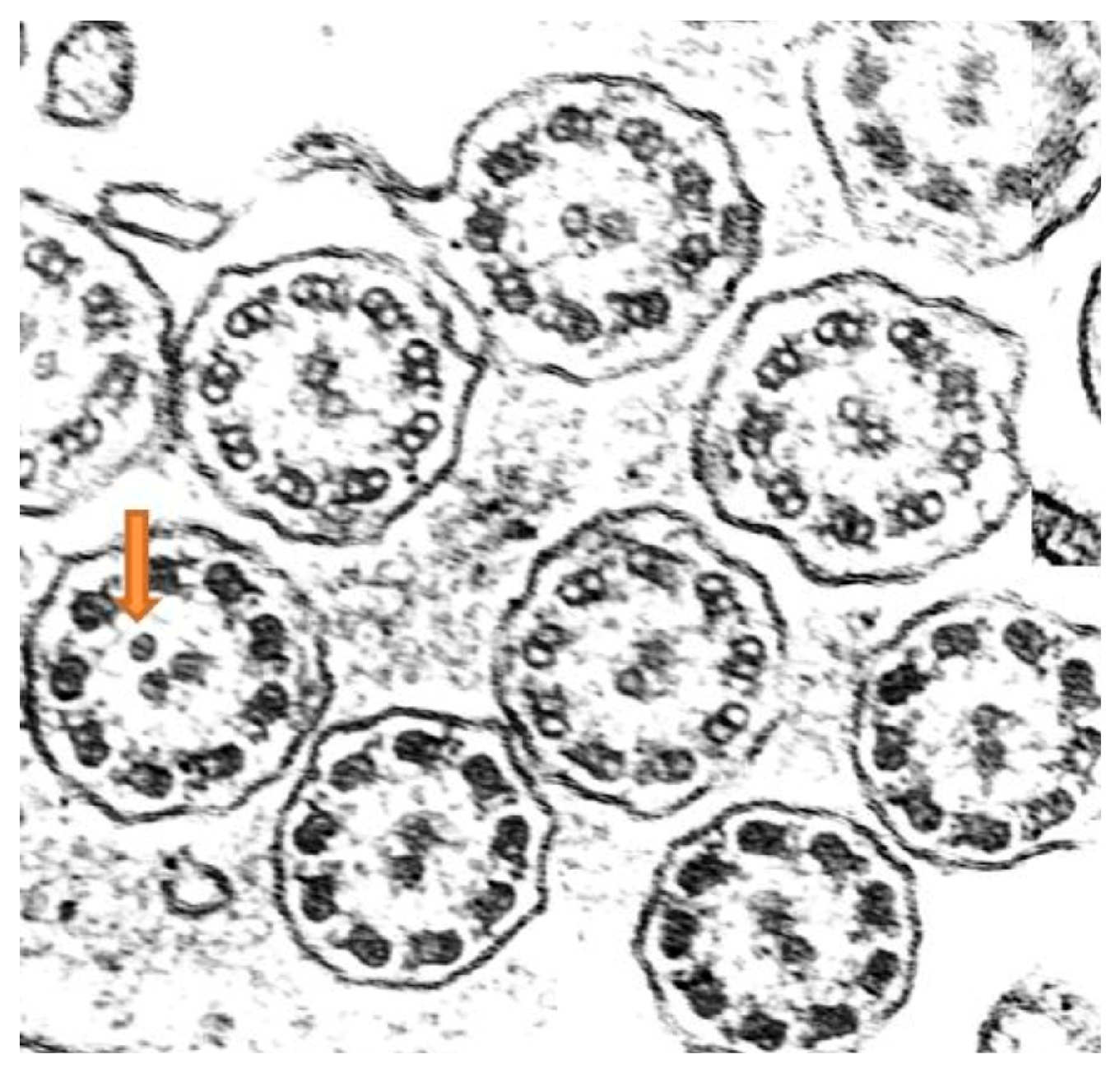
Ciliary ultrastructure analysis revealed that both the bronchial and nasal samples preserved normal ultrastructure in 92% of the axonemes, while 8% presented abnormalities, specifically supernumerary microtubules, indicating overall preserved ciliary morphology [
19] (
Figure 4).
High-speed videomicroscopy analysis of nasal ciliary motility revealed a dyskinetic ciliary movement pattern, primarily characterized by immobile cilia, as well as some residual uncoordinated or vibratory ciliary activity. These findings were consistent with the diagnosis of PCD [
3,
20].
The second patient was a 60-year-old woman with symptoms present since birth but no known family history of the disease. She exhibited a similar clinical phenotype, with persistent symptoms including productive cough, recurrent otitis with conductive hearing loss, pneumonias, bronchiectasis, atelectasis, and additional findings such as agenesis of the frontal sinus.
Genetic analysis identified the following two heterozygous mutations in the RSPH1 gene: c.275-2A > C splicing; and c.85G > T with an amino acid change p.Glu29*.
Ciliary ultrastructure analysis showed that 62% of the axonemes maintained normal structure, while 38% presented defects in the central complex, corresponding to a class 2 defect and indicating altered ciliary morphology [
19].
Nasal ciliary motility analysis revealed a dyskinetic pattern characterized by limited ciliary movement in a single phase and the presence of rigid cilia (See
Supplementary Video S1). Additionally, some cells exhibited completely immobile cilia, consistent with the diagnosis of PCD.
Both patients underwent bilateral lung transplantation due to the unfavorable progression of their condition despite conventional treatments [
19], including respiratory physiotherapy, azithromycin three times a week, and inhaled colistin.
Post-transplant ciliary motility and ultrastructure studies followed the same protocol for both patients, although they were conducted in different years according to each transplant date. The sample collection schedule was adapted to each patient’s clinical bronchoscopy requirements. The first patient was assessed at 3, 6, 12, 18, 24, and 30 months post-transplant, showing immotile cilia in the nasal epithelium, whereas the bronchial ciliary function remained normal, displaying the two characteristic phases of coordinated ciliary movement (See
Supplementary Video S2). The mean ciliary beat frequency in the bronchial epithelial samples during the follow-up period was 9 Hz, with a range of 8.5–12 Hz.
The second patient, who underwent transplantation a year later, was assessed at 3, 6, 12, and 18 months post-transplant, with findings similar to the first patient; the nasal cilia remained dyskinetic, while the bronchial ciliary function was normal (See
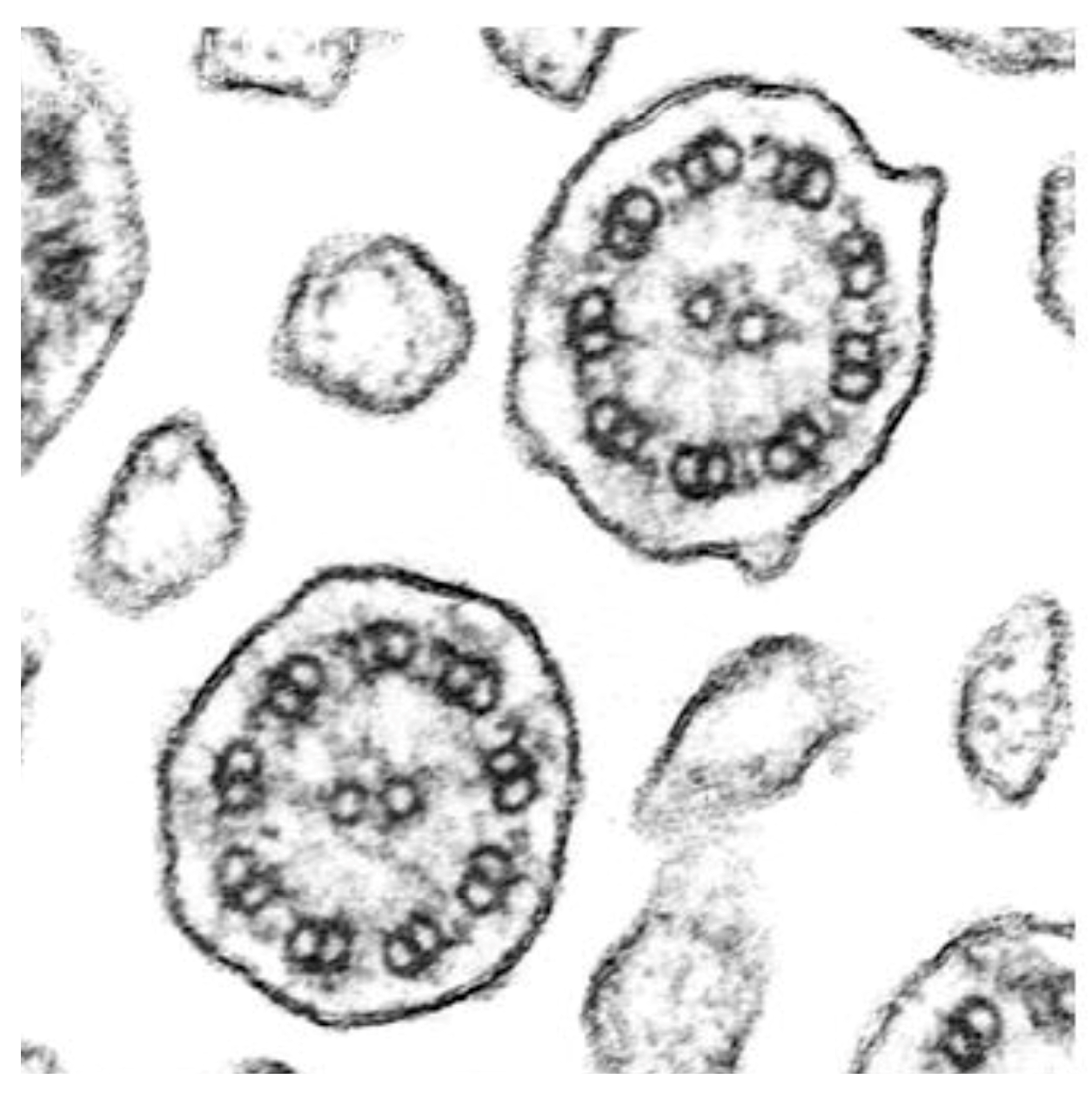
Supplementary Video S3), suggesting stable bronchial ciliary function in the transplanted lungs. The mean ciliary beat frequency in the bronchial epithelial samples was 9.6 Hz, with a range of 8.5–12 Hz. Importantly, transmission electron microscopy analysis of the biopsy samples from the transplanted lungs in both patients showed normal ciliary ultrastructure (
Figure 5).
4. Discussion
In patients with PCD undergoing lung transplantation, preserving the functionality of bronchial mucociliary clearance is crucial for ensuring the long-term success of the transplanted organ. Understanding whether the respiratory ciliated epithelium retains the donor’s functional characteristics or if the recipient’s genetic alterations impact ciliary function is essential.
The results of our study demonstrated that, at 30 months post-transplant for patient 1 and 18 months for patient 2, the bronchial cilia of the transplanted lungs maintained normal motility. These findings suggest that lung transplantation can preserve normal ciliary function in the lower airways of the transplanted organ, despite the patient’s genetic alterations causing ciliary dysfunction in other tissues. This is an encouraging sign for both graft survival and the patient’s prognosis. The transplanted lung functions as an “island” within the body, free from the recipient’s genetic defect.
These results are consistent with the previous clinical studies demonstrating the benefits of lung transplantation in patients with advanced-stage PCD and progressive lung deterioration [
14,
15,
16,
18,
19,
20,
21]. However, we did not find any prior studies in the literature specifically evaluating ciliary function in the transplanted lungs of patients with PCD. It is noteworthy that a recent multicenter study, which represents the largest cohort to date of lung transplant recipients with PCD, retrospectively collected data from 36 patients who underwent lung transplantation between 1995 and 2020. The findings confirmed that, despite surgical challenges associated with laterality disorders in some patients, lung transplantation is a viable therapeutic option for patients with PCD with end-stage disease. However, this study did not include specific assessments of ciliary motility and ultrastructure in the bronchial epithelium, relying instead on clinical evaluation through pulmonary function tests, survival analysis, and the absence of chronic lung allograft dysfunction.
These findings are promising for PCD research and suggest that lung transplantation can be a feasible therapeutic option in this population. Additionally, they provide a foundation for future investigations and improvements in therapeutic approaches for this disease. Nevertheless, additional studies with larger sample sizes and longer follow-up periods are needed to confirm and generalize these results.
The main limitation of our study is the small sample size. However, given the rarity of the disease, the low number of patients with PCD requiring lung transplantation, and the consistency of findings in both cases, we believe these results provide valuable clinical insights.
5. Conclusions
This study contributes new scientific knowledge on PCD and provides relevant insights into the impact of lung transplantation on ciliary function in these patients. The results support the hypothesis that lung transplantation preserves bronchial ciliary motility, a critical factor for long-term transplant success. These findings establish a solid foundation for further research and continued improvement in therapeutic strategies for patients with PCD.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/jcm14103439/s1, Video S1: Bronchial cells from a transplanted lung with normal cilliary motility, 6 months post-transplant. Video S2: Bronchial cells from a transplanted lung with normal cilliary motility, 12 months post-transplant. Video S3: Bronchial cells from a transplanted lung with normal cilliary motility, 18 months post-transplant.
Author Contributions
Conceptualization, M.A.; methodology, M.A., C.B., L.C.-V., R.B.-M., N.M.-F., E.C. and T.J.; formal analysis, M.A. and L.C.-V.; investigation, M.A., C.B., L.C.-V., R.B.-M., J.M.M. and T.J.; writing—original draft preparation, M.A.; writing—review and editing, L.C.-V. and S.A.; funding acquisition, M.A. and T.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III, grant numbers PI19/00949 and PI22/01010.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee of Hospital Universitario y Politécnico La Fe (Registry No.: 2022-291-1, approval date: 29 March 2023).
Informed Consent Statement
Written informed consent was obtained from all patients for the use of their data and images in this publication, in accordance with the established ethical principles.
Data Availability Statement
The data in the study can be obtained by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PCD | Primary Ciliary Dyskinesia |
References
- Werner, C.; Onnebrink, J.G.; Omran, H. Diagnosis and management of primary ciliary dyskinesia. Cilia 2015, 4, 2. [Google Scholar] [CrossRef]
- Hannah, W.B.; Seifert, B.A.; Truty, R.; Zariwala, M.A.; Ameel, K.; Zhao, Y.; Nykamp, K.; Gaston, B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: A genetic database analysis. Lancet Respir. Med. 2022, 10, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Armengot, M.; Bonet, M.; Carda, C.; Milara, J.; Cortijo, J. Development and validation of a method of cilia motility analysis for the early diagnosis of primary ciliary dyskinesia. Acta Otorrinolaringol. Esp. 2012, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Armengot-Carceller, M.; Reula, A.; Mata-Roig, M.; Pérez-Panadés, J.; Milian-Medina, L.; Carda-Batalla, C. Understanding Primary Ciliary Dyskinesia: Experience from a Mediterranean Diagnostic Reference Centre. J. Clin. Med. 2020, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Mullowney, T.; Manson, D.; Kim, R.; Stephens, D.; Shah, V.; Dell, S. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics 2014, 134, 1160–1166. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Tolleson-Rinehart, S.; Zariwala, M.A.; Knowles, M.R.; Leigh, M.W. The prevalence of clinical features associated with primary ciliary dyskinesia in a heterotaxy population: Results of a web-based survey. Cardiol. Young 2014, 25, 752–759. [Google Scholar] [CrossRef]
- Vanaken, G.J.; Bassinet, L.; Boon, M.; Mani, R.; Honoré, I.; Papon, J.-F.; Cuppens, H.; Jaspers, M.; Lorent, N.; Coste, A.; et al. Infertility in an adult cohort with primary ciliary dyskinesia: Phenotype–gene association. Eur. Respir. J. 2017, 50, 1700314. [Google Scholar] [CrossRef]
- Behan, L.; Dimitrov, B.D.; Kuehni, C.E.; Hogg, C.; Carroll, M.; Evans, H.J.; Goutaki, M.; Harris, A.; Packham, S.; Walker, W.T.; et al. PICADAR: A diagnostic predictive tool for primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 1103–1112. [Google Scholar] [CrossRef]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2016, 49, 1601090. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef]
- Raidt, J.; Wallmeier, J.; Hjeij, R.; Onnebrink, J.G.; Pennekamp, P.; Loges, N.T.; Olbrich, H.; Häffner, K.; Dougherty, G.W.; Omran, H.; et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur. Respir. J. 2014, 44, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.C.; Chen, J.J.; Chou, L.; Wong, B.J.F.; Chen, Z. Visualization and Detection of Ciliary Beating Pattern and Frequency in the Upper Airway using Phase Resolved Doppler Optical Coherence Tomography. Sci. Rep. 2017, 7, 8522. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Davis, S.D.; Omran, H.; Shoemark, A. Primary ciliary dyskinesia in the genomics age. Lancet Respir. Med. 2020, 8, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Marro, M.; Leiva-Juárez, M.M.; D’ovidio, F.; Chan, J.; Van Raemdonck, D.; Ceulemans, L.J.; Moreno, P.; Kindelan, A.A.; Krueger, T.; Koutsokera, A.; et al. Lung Transplantation for Primary Ciliary Dyskinesia and Kartagener Syndrome: A Multicenter Study. Transpl. Int. 2023, 36, 10819. [Google Scholar] [CrossRef]
- Román, A.; Ussetti, P.; Solé, A.; Zurbano, F.; Borro, J.M.; Vaquero, J.M.; de Pablo, A.; Morales, P.; Blanco, M.; Bravo, C.; et al. Normativa para la selección de pacientes candidatos a trasplante pulmonar. Arch. Bronconeumol. 2011, 47, 303–309. [Google Scholar] [CrossRef]
- Sisson, J.H.; Stoner, J.A.; Ammons, B.A.; Wyatt, T.A. All-digital image capture and whole-field analysis of ciliary beat frequency. J. Microsc. 2003, 211 Pt 2, 103–111. [Google Scholar] [CrossRef]
- Shoemark, A.; Boon, M.; Brochhausen, C.; Bukowy-Bieryllo, Z.; De Santi, M.M.; Goggin, P.; Griffin, P.; Hegele, R.G.; Hirst, R.A.; Leigh, M.W.; et al. International consensus guideline for reporting transmission electron microscopy results in the diagnosis of primary ciliary dyskinesia (BEAT PCD TEM Criteria). Eur. Respir. J. 2020, 55, 1900725. [Google Scholar] [CrossRef]
- Blanco-Máñez, R.; Armengot-Carceller, M.; Jaijo, T.; Vera-Sempere, F. Axonemal Symmetry Break, a New Ultrastructural Diagnostic Tool for Primary Ciliary Dyskinesia? Diagnostics 2022, 12, 129. [Google Scholar] [CrossRef]
- Knowles, M.R.; Ostrowski, L.E.; Leigh, M.W.; Sears, P.R.; Davis, S.D.; Wolf, W.E.; Hazucha, M.J.; Carson, J.L.; Olivier, K.N.; Sagel, S.D.; et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am. J. Respir. Crit. Care Med. 2014, 189, 707–717. [Google Scholar] [CrossRef]
- Rubbo, B.; Shoemark, A.; Jackson, C.L.; Hirst, R.; Thompson, J.; Hayes, J.; Frost, E.; Copeland, F.; Hogg, C.; O’callaghan, C.; et al. Accuracy of high-speed video analysis to diagnose primary ciliary dyskinesia. Chest 2019, 155, 1008–1017. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X.; Jiang, W.; Huang, J.; Chen, J.; Kreisel, D.; Danguilan, J.L.J.; Hsin, M.; Lin, H. Double lung transplantation for end-stage Kartagener syndrome: A case report and literature review. J. Thorac. Dis. 2020, 12, 1588–1594. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).