Real-World Implementation of PRISMA-7 and Clinical Frailty Scale for Frailty Identification and Integrated Care Activation: A Cross-Sectional Study in Northern Italian Primary Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Recruitment of Participants
2.2. Frailty Screening/Assessment and Collection of Data
- Step 1. The GPs were asked to identify all their community-dwelling patients aged ≥75 years from their electronic medical records (EMRs) and to invite them to a face-to-face visit, where the patients were informed about the study procedures and signed informed consent if they agreed to participate. The participating patients autonomously completed the PRISMA-7 questionnaire.
- Step 2. All the patients with a resulting PRISMA-7 score ≥3 were additionally assessed by the GPs using the Clinical Frailty Scale (CFS) during the same or additional face-to-face contact, and by consulting the GPs’ EMR. The available German [17,18] and Italian versions [19,20] of PRISMA-7 and CFS were used, as these are the two main languages spoken in the investigated (bilingual) region. Owing to the need for feasibility in daily practice, the second-step CFS assessment was limited to patients with PRISMA-7 ≥ 3. Therefore, measures of diagnostic accuracy (e.g., sensitivity, specificity, and ROC curves) could not be computed using this study design.
- Step 3. The patients with a CFS score ≥ 5 were potentially eligible for IDC activation if not already receiving structured home-based services. The concerned cases were evaluated by GPs in cooperation with the patients themselves, their relatives/caregivers, and the nurses of the healthcare district. Only newly activated IDC cases were recorded in this study. In uncertain cases, the research team contacted GPs for clarification.
2.3. Statistical Analysis
3. Results
3.1. Study Population and Screening Completion
3.2. Frailty Classification and Agreement Between Tools
3.3. Frailty Classification Patterns and Determinants
3.3.1. Age-Related Patterns
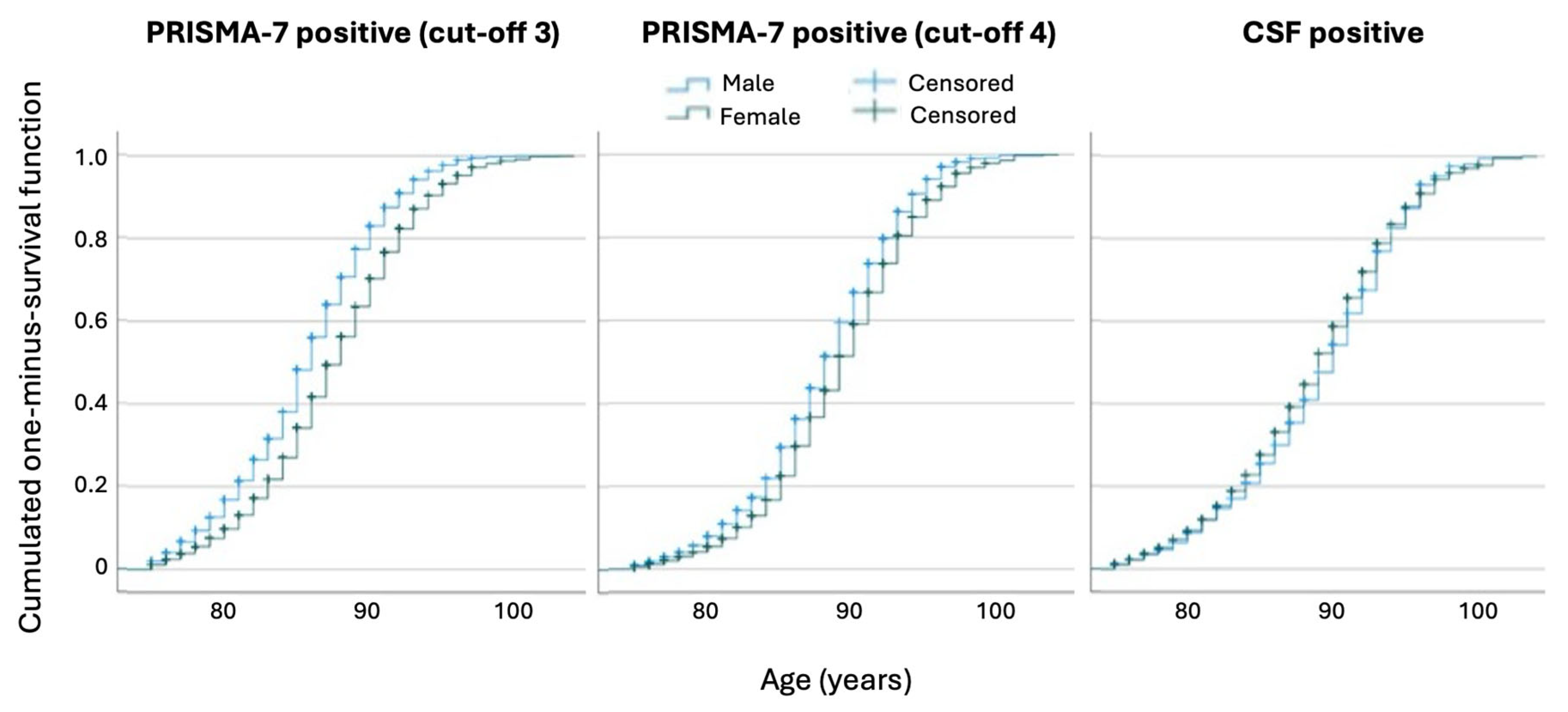
3.3.2. Sex-Related Differences
3.3.3. Geographic Trends
3.4. Clinical Implications: Integrated Care and Variability
3.4.1. Activation of Integrated Care
3.4.2. Variability in Frailty Classification
4. Discussion
4.1. Comparison with Other Studies
4.2. Equity and Implementation Considerations
4.3. Integrated Domiciliary Care (IDC) Insights
4.4. Strengths and Limitations
4.5. Implications for Practice and Future Research
- Validation studies with complete PRISMA-7 and CFS assessments across the full sample will allow for ROC analyses and more robust cut-off calibration.
- Longitudinal outcome studies examining frailty progression, care responsiveness, hospitalisation, institutionalisation, and mortality by frailty group and screening tools.
- Equity-focused analyses of tool performance by sex, age, language, or social determinants can inform the refinement of the existing tools.
- Implementation science approaches to identify the barriers and enablers of IDC activation and explore provider-level factors (e.g., GP engagement and readiness for interprofessional collaboration).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFS | Clinical Frailty Scale |
| CI | confidence interval |
| EMR | electronic medical record |
| GP(s) | general practitioner(s) |
| IDC | integrated domiciliary care |
| IQR | interquartile range |
| OR | odds ratio |
| PRISMA-7 | Program of Research to Integrate Services for the Maintenance of Autonomy 7 items |
| ROC | Receiver operating characteristic (curve) |
References
- Hoogendijk, E.O.; van der Horst, H.E.; Deeg, D.J.H.; Frijters, D.H.M.; Prins, B.A.H.; Jansen, A.P.D.; Nijpels, G.; van Hout, H.P.J. The Identification of Frail Older Adults in Primary Care: Comparing the Accuracy of Five Simple Instruments. Age Ageing 2013, 42, 262–265. [Google Scholar] [CrossRef]
- Doody, P.; Lord, J.M.; Greig, C.A.; Whittaker, A.C. Frailty: Pathophysiology, Theoretical and Operational Definition(s), Impact, Prevalence, Management and Prevention, in an Increasingly Economically Developed and Ageing World. Gerontology 2023, 69, 927–945. [Google Scholar] [CrossRef]
- Boucher, E.L.; Gan, J.M.; Rothwell, P.M.; Shepperd, S.; Pendlebury, S.T. Prevalence and Outcomes of Frailty in Unplanned Hospital Admissions: A Systematic Review and Meta-Analysis of Hospital-Wide and General (Internal) Medicine Cohorts. eClinicalMedicine 2023, 59, 101947. [Google Scholar] [CrossRef]
- Kojima, G.; Liljas, A.E.M.; Iliffe, S. Frailty Syndrome: Implications and Challenges for Health Care Policy. Risk Manag. Health Policy 2019, 12, 23–30. [Google Scholar] [CrossRef]
- Somagutta, M.R.; Uday, U.; Bathula, N.R.; Pendyala, S.; Mahadevaiah, A.; Jain, M.S.; Mahmutaj, G.; Gad, M.; Jean Baptiste, J. Diagnosing Frailty in Primary Care Practice. Cureus 2022, 14, 23329. [Google Scholar] [CrossRef]
- Mauro, M.; Giancotti, M. The 2022 Primary Care Reform in Italy: Improving Continuity and Reducing Regional Disparities? Health Policy 2023, 135, 104862. [Google Scholar] [CrossRef]
- Karunananthan, S.; Bergman, H. Managing Frailty in Primary Care: Evidence Gaps Cannot Be Ignored. CMAJ 2018, 190, E1122–E1123. [Google Scholar] [CrossRef]
- Roller-Wirnsberger, R.; Lindner, S.; Liew, A.; O’Caoimh, R.; Koula, M.L.; Moody, D.; Espinosa, J.M.; Durme, T.; Dimitrov, P.; Benjak, T. European Collaborative and Interprofessional Capability Framework for Prevention and Management of Frailty—A Consensus Process Supported by the Joint Action for Frailty Prevention (ADVANTAGE) and the European Geriatric Medicine Society (EuGMS). Aging Clin. Exp. Res. 2020, 32, 561–570. [Google Scholar] [CrossRef]
- Soulis, G.; Kyriakopoulou, E.; Leventouri, A.; Zigkiri, E.; Efthymiou, V.; Kentros, Z.; Koutsouri, A. Pilot Testing of Useful Tools’ Validity for Frailty Assessment in Greece: Translated PRISMA-7 Tool, Modified Fried Criteria and Clinical Frailty Scale. Healthcare 2024, 12, 930. [Google Scholar] [CrossRef]
- Sukkriang, N.; Punsawad, C. Comparison of Geriatric Assessment Tools for Frailty among Community Elderly. Heliyon 2020, 6, e04797. [Google Scholar] [CrossRef]
- Raiche, M.; Hebert, R.; Dubois, M.F. PRISMA-7: A Case-Finding Tool to Identify Older Adults with Moderate to Severe Disabilities. Arch. Gerontol. Geriatr. 2008, 47, 9–18. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 210–215. [Google Scholar] [CrossRef]
- Vo, N.T.; Tu, Y.-K.; Lin, K.-C.; Chiu, H.-Y.; Huang, H.-C. Diagnostic Accuracy of the FRAIL Scale, Groningen Frailty Indicator, Tilburg Frailty Indicator, and PRISMA-7 for Frailty Screening Among Older Adults in Community Settings: A Systematic Review and Network Meta-Analysis. Gerontologist 2024, 64, gnae008. [Google Scholar] [CrossRef]
- Abbasi, M.; Khera, S.; Dabravolskaj, J.; Garrison, M.; King, S. Identification of Frailty in Primary Care: Feasibility and Acceptability of Recommended Case Finding Tools Within a Primary Care Integrated Seniors’ Program. Gerontol. Geriatr. Med. 2019, 5, 2333721419848153. [Google Scholar] [CrossRef]
- Clegg, A.; Rogers, L.; Young, J. Diagnostic Test Accuracy of Simple Instruments for Identifying Frailty in Community-Dwelling Older People: A Systematic Review. Age Ageing 2015, 44, 148–152. [Google Scholar] [CrossRef]
- Vetrano, D.L. Rend di Fragilità e Long-Term Care in Italia; Società Italiana di Medicina Generale e delle Cure Primarie: Firenze, Italy, 2023. [Google Scholar]
- Braun, T.; Grüneberg, C.; Thiel, C. German Translation, Cross-Cultural Adaptation and Diagnostic Test Accuracy of Three Frailty Screening Tools: PRISMA-7, FRAIL Scale and Groningen Frailty Indicator. Z. Gerontol. Geriatr. 2018, 51, 282–292. [Google Scholar] [CrossRef]
- Kaeppeli, T.; Rueegg, M.; Dreher-Hummel, T.; Brabrand, M.; Kabell-Nissen, S.; Carpenter, C.R.; Bingisser, R.; Nickel, C.H. Validation of the Clinical Frailty Scale for Prediction of Thirty-Day Mortality in the Emergency Department. Ann. Emerg. Med. 2020, 76, 291–300. [Google Scholar] [CrossRef]
- Aziende del Servizio Sanitario Regionale. PRISMA-7—Progetto di Screening per Individuare la Fragilità Nelle Persone Anziane; Promozione Della Salute e Prevenzione; Regione Autonoma Friuli Venezia Giulia: Trieste, Italia, 2022. [Google Scholar]
- Butzlaff, M.; Nonnenkamp, J.P. Polypharmacy in Chronic Diseases—Scala Clinica per La Fragilità, PRIMA, Ed.; Private Universität Witten/Herdecke: Witten, Germany.
- Abbasi, M.; Rolfson, D.; Khera, A.S.; Dabravolskaj, J.; Dent, E.; Xia, L. Identification and Management of Frailty in the Primary Care Setting. CMAJ 2018, 190, E1134–E1140. [Google Scholar] [CrossRef]
- Torun, E.; Az, A.; Akdemir, T.; Solakoglu, G.A.; Aciksari, K.; Gungorer, B. Evaluation of the Risk Factors for Falls in the Geriatric Population Presenting to the Emergency Department. Ulus. Travma Acil Cerrahi Derg. 2023, 29, 897–903. [Google Scholar] [CrossRef]
- Ambagtsheer, R.C.; Thompson, M.Q.; Archibald, M.M.; Casey, M.G.; Schultz, T.J. Diagnostic Test Accuracy of Self-Reported Screening Instruments in Identifying Frailty in Community-Dwelling Older People: A Systematic Review. Geriatr. Gerontol. Int. 2020, 20, 14–24. [Google Scholar] [CrossRef]
- User Guide for the PRISMA-7 Questionnaire to Identify Older Adults with Significant Disabilities. 2021. Available online: https://www.expertise-sante.com/wp-content/uploads/2024/03/PRISMA-7-User-guide_International_2021-10-01.pdf (accessed on 10 May 2025).
- Landesinstitut für Statistik ASTAT. Bevölkerung in Südtirol; Landesinstitut für Statistik ASTAT: Bozen, Italy, 2023; Available online: https://astat.provinz.bz.it/de/publikationen/die-dauerzaehlung-der-bevoelkerung-in-suedtirol-2023 (accessed on 10 May 2025).
- Thillainadesan, J.; Scott, I.A.; Le Couteur, D.G. Frailty, a Multisystem Ageing Syndrome. Age Ageing 2020, 49, 758–763. [Google Scholar] [CrossRef]
- Gordon, E.H.; Hubbard, R.E. Frailty: Understanding the Difference between Age and Ageing. Age Ageing 2022, 51, afac185. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of Frailty in 62 Countries across the World: A Systematic Review and Meta-Analysis of Population-Level Studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef]
- Salminen, M.; Viljanen, A.; Eloranta, S.; Viikari, P.; Wuorela, M.; Vahlberg, T.; Isoaho, R.; Kivela, S.L.; Korhonen, P.; Irjala, K. Frailty and Mortality: An 18-Year Follow-up Study among Finnish Community-Dwelling Older People. Aging Clin. Exp. Res. 2020, 32, 2013–2019. [Google Scholar] [CrossRef]
- Galluzzo, L.; Noale, M.; Maggi, S.; Feraldi, A.; Baldereschi, M.; Carlo, A.; Onder, G.; the ILSA Working Group. Frailty Prevalence, Incidence, and Association with Incident Disability in the Italian Longitudinal Study on Aging. Gerontology 2023, 69, 249–260. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, 198398. [Google Scholar] [CrossRef]
- Liotta, G.; O’Caoimh, R.; Gilardi, F.; Proietti, M.G.; Rocco, G.; Alvaro, R.; Scarcella, P.; Molloy, D.W.; Orlando, S.; Mancinelli, S. Assessment of Frailty in Community-Dwelling Older Adults Residents in the Lazio Region (Italy): A Model to Plan Regional Community-Based Services. Arch. Gerontol. Geriatr. 2017, 68, 1–7. [Google Scholar] [CrossRef]
- Ji, D.; Guo, H.; Qiu, S.; Dong, L.; Shen, Y.; Shen, Z.; Xu, J. Screening for Frailty and Its Association with Activities of Daily Living, Cognitive Impairment, and Falls among Community-Dwelling Older Adults in China. BMC Geriatr. 2024, 24, 576. [Google Scholar] [CrossRef]
- Cohen, S.A.; Ahmed, N.; Brown, M.J.; Meucci, M.R.; Greaney, M.L. Rural-Urban Differences in Informal Caregiving and Health-Related Quality of Life. J. Rural. Health 2022, 38, 442–456. [Google Scholar] [CrossRef]
- Groenewegen, P.P.; Bosmans, M.W.G.; Boerma, W.G.W.; Spreeuwenberg, P. The Primary Care Workforce in Europe: A Cross-Sectional International Comparison of Rural and Urban Areas and Changes between 1993 and 2011. Eur. J. Public. Health 2020, 30, iv12–iv17. [Google Scholar] [CrossRef]
- Chevillard, G.; Mousquès, J. Medically Underserved Areas: Are Primary Care Teams Efficient at Attracting and Retaining General Practitioners? Soc. Sci. Med. 2021, 287, 114358. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.; Pohontsch, N.J.; Bole, L.; Schäfer, I.; Scherer, M. Regional Variations of Perceived Problems in Ambulatory Care from the Perspective of General Practitioners and Their Patients—An Exploratory Focus Group Study in Urban and Rural Regions of Northern Germany. BMC Fam. Pract. 2017, 18, 68. [Google Scholar] [CrossRef]
- Sutorius, F.L.; Hoogendijk, E.O.; Prins, B.A.H.; van Hout, H.P.J. Comparison of 10 Single and Stepped Methods to Identify Frail Older Persons in Primary Care: Diagnostic and Prognostic Accuracy. BMC Fam. Pr. 2016, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.T.; Gan, Y.; Teo, S.Q.; Lim, K.Q.; Leow, H.X.; Jbabdi, M.; Raiche, M.; Yang, M. The Chinese Translation and Cross-Cultural Adaptation of PRISMA-7 Questionnaire: An Observational Study to Establish the Accuracy, Reliability and Validity. BMC Geriatr. 2024, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Saenger, A.L.F.; Caldas, C.P.; Raîche, M.; da Motta, L.B. Identifying the Loss of Functional Independence of Older People Residing in the Community: Validation of the PRISMA-7 Instrument in Brazil. Arch. Gerontol. Geriatr. 2018, 74, 62–67. [Google Scholar] [CrossRef]
- Yaman, H.; Ünal, Z. The Validation of the PRISMA-7 Questionnaire in Community-Dwelling Elderly People Living in Antalya, Turkey. Electron. Phys. 2018, 10, 7266–7272. [Google Scholar] [CrossRef]
- Battaggia, A.; Scalisi, A.; Franco Novelletto, B.; Fusello, M.; Michieli, R.; Cancian, M. Prevalence of frailty in older people in Veneto (Italy). J. Drug Assess. 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Battaggia, A.; Scalisi, A.; Magliozzo, F.; Franco, B.N.; Fusello, M.; Michieli, R.; Cancian, M. The Burden of Frailty in Older People Visiting GPs in Veneto and Sicily, Italy. J. Drug Assess. 2019, 8, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Tan, J.K.; Ismail, A.H.; Ibrahim, R.; Hassan, N.H. Factors Associated with Frailty in Older Adults in Community and Nursing Home Settings: A Systematic Review with a Meta-Analysis. J. Clin. Med. 2024, 13, 8. [Google Scholar] [CrossRef]
- Roppolo, M.; Mulasso, A.; Gobbens, R.J.; Mosso, C.O.; Rabaglietti, E. A Comparison between Uni- and Multidimensional Frailty Measures: Prevalence, Functional Status, and Relationships with Disability. Clin. Interv. Aging 2015, 10, 1669–1678. [Google Scholar]
- Perna, S.; Francis, M.D.; Bologna, C.; Moncaglieri, F.; Riva, A.; Morazzoni, P.; Allegrini, P.; Isu, A.; Vigo, B.; Guerriero, F. Performance of Edmonton Frail Scale on Frailty Assessment: Its Association with Multi-Dimensional Geriatric Conditions Assessed with Specific Screening Tools. BMC Geriatr. 2017, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, G.; Midão, L.; Paúl, C.; Cena, C.; Duarte, M.; Costa, E. Prevalence of Frailty Status among the European Elderly Population: Findings from the Survey of Health, Aging and Retirement in Europe. Geriatr. Gerontol. Int. 2019, 19, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Blakeman, T.; Marwijk, H.; Reeves, D. Identification and Management of Frail Patients in English Primary Care: An Analysis of the General Medical Services 2018/2019 Contract Dataset. BMJ Open 2021, 11, 041091. [Google Scholar] [CrossRef] [PubMed]
- Job, J.; Nicholson, C.; Clark, D.; Arapova, J.; Jackson, C. The Feasibility, Acceptability and Appropriateness of Screening for Frailty in Australians Aged 75 Years and over Attending Australian General Practice. Aust. J. Prim. Health 2024, 30, PY23173. [Google Scholar] [CrossRef]
- Celik, S.; Turkdogan, O.; Erdogan, T.; Celik, P. PRISMA-7 Is a Predictor of Intensive Care Unit Admission and Mortality in Older Patients in an Emergency Department. Geriatr. Gerontol. Int. 2025, 25, 61–66. [Google Scholar] [CrossRef]
- Perez Coello, L.; Bouza Bellas, L.; Veras Castro, R. Prevalence of Frailty in a Population Aged 70 and Older Who Pay a Visit to Health Center and Its Detection in Primary Care. Rev. Esp. Geriatr. Gerontol. 2024, 59, 101454. [Google Scholar]
- Ausserhofer, D.; Mahlknecht, A.; Barbieri, V.; Engl, A.; Piccoliori, G.; Wiedermann, C.J. Comparing the PRISMA-7 and a Modified Version (PRISMA-6) for Frailty Screening: Addressing Sex Bias in Community-Dwelling Older Adults. Geriatrics 2025, 10, 9. [Google Scholar] [CrossRef]
- Turner, G.; Clegg, A.; British Geriatrics Society; Age UK; Royal College of General Practioners. Best Practice Guidelines for the Management of Frailty: A British Geriatrics Society, Age UK and Royal College of General Practitioners Report. Age Ageing 2014, 43, 744–747. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A Simple Frailty Questionnaire (FRAIL) Predicts Outcomes in Middle Aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef]
- Luo, J.; Liao, X.; Zou, C.; Zhao, Q.; Yao, Y.; Fang, X.; Spicer, J. Identifying Frail Patients by Using Electronic Health Records in Primary Care: Current Status and Future Directions. Front. Public Health 2022, 10, 901068. [Google Scholar] [CrossRef]
- Swart, K.M.; Heijden, A.A.; Blom, M.T.; Overbeek, J.A.; Nijpels, G.; Hout, H.P.; Elders, P.J.; Herings, R.M. Identification of Frailty in Primary Care: Accuracy of Electronically Derived Measures. Br. J. Gen. Pr. 2023, 73, 735, 752–759. [Google Scholar] [CrossRef] [PubMed]

| Variables | n (%) | Median (IQR) |
|---|---|---|
| GPs, n = 142 | ||
| Age, years | – | 47 (39–58) |
| Female sex | 72 (50.7) | – |
| Location of GP office | ||
| Urban area | 75 (52.8) | – |
| Rural area | 67 (47.2) | – |
| Patients, n = 19,501 | ||
| Age, years | – | 81 (78–85) |
| Female sex | 11,203 (60.0) | – |
| Number of screened patients (PRISMA-7) | 18,658 | 122 (3–173) 1 |
| Instrument | Measure | Value |
|---|---|---|
| PRISMA-7 (cut-off ≥ 3) | Frail patients, n (%) | 8582 (46.0) |
| Median score—frail | 4.0 (3.0–5.0) | |
| Median score—non-frail | 1.0 (1.0–2.0) | |
| PRISMA-7 (cut-off ≥ 4) | Frail patients | 5372 (28.8) |
| Median score—frail | 5.0 (4.0–6.0) | |
| Median score—non-frail | 2.0 (1.0–2.0) | |
| CFS | Screened patients | 7970 |
| Frail (score ≥ 5) | 3852 (48.3) | |
| Mild (score 5) | 1526 (19.1) | |
| Moderate (score 6) | 1326 (16.6) | |
| Severe+ (7–9) | 1000 (12.5) | |
| Median score—frail | 6.0 (5.0–7.0) | |
| Median score—non-frail | 3.0 (3.0–4.0) |
| Combined Frailty Status | PRISMA-7 Cut-Off ≥ 3 n (%) | PRISMA-7 Cut-Off ≥ 4 n (%) |
|---|---|---|
| Not frail (PRISMA-7 negative) | 10,034 (55.7) | 12,910 (71.4) |
| Frail by PRISMA-7, not frail by CFS | 4118 (22.9) | 1640 (9.1) |
| Frail by both PRISMA-7 and CFS | 3852 (21.4) | 3518 (19.5) |
| Predictor | PRISMA-7 ≥ 3 | PRISMA-7 ≥ 4 | CFS ≥ 5 |
|---|---|---|---|
| n | 18,003 | 17,669 | 7969 |
| Nagelkerke R2 | 0.327 | 0.315 | 0.109 |
| Age (per year) | 1.27 (1.26–1.28) *** | 1.27 (1.26–1.28) *** | 1.09 (1.08–1.10) *** |
| Female sex | 0.54 (0.51–0.58) *** | 0.82 (0.76–0.88) *** | 1.88 (1.72–2.07) *** |
| Rural GP office | 1.19 (1.11–1.28) *** | n.s. | 0.83 (0.76–0.91) *** |
| Constant | −19.79 | −20.45 | −7.80 |
| A | ||||
| Variable | Value | |||
| Eligible patients (PRISMA-7 ≥ 3 and CFS ≥ 5) | 3701 | |||
| Patients with newly activated IDC | 526 (14.2%) | |||
| Median number of IDC activations per GP | 3 (IQR 1–5) 1 | |||
| Minimum–Maximum IDC activations per GP | 0–18 | |||
| GPs excluded from IDC analysis due to implausible values | 3 GPs (n = 151 patients) | |||
| B | ||||
| Characteristic | Group | IDC Activated n (%) | IDC Not Activated n (%) | p-Value 1 |
| Patient age | 75–84 years | 174 (12.4) | 1230 (87.6) | 0.013 |
| ≥85 years | 352 (15.3) | 1945 (84.7) | ||
| Patient sex | Male | 209 (14.8) | 1201 (85.2) | 0.410 |
| Female | 317 (13.8) | 1973 (86.2) | ||
| GP office location | Urban | 283 (12.4) | 1999 (87.6) | <0.001 |
| Rural | 243 (17.1) | 1176 (82.9) | ||
| C | ||||
| Variable | Model with PRISMA-7 | Model with CFS | ||
| Nagelkerke’s R2 | 0.076 | 0.149 | ||
| PRISMA-7 score (per point) | OR 1.65 (1.50–1.81), p < 0.001 | – | ||
| CFS score (per point) | – | OR 2.29 (2.07–2.54), p < 0.001 | ||
| Rural GP office | OR 1.40 (1.16–1.70), p < 0.001 | OR 1.47 (1.21–1.79), p < 0.001 | ||
| GP age (per year) | OR 0.98 (0.97–0.98), p < 0.001 | OR 0.97 (0.96–0.98), p < 0.001 | ||
| Patient age/sex | Not significant | Not significant | ||
| GP sex | Not included | Not included | ||
| Constant term | −3.43, p < 0.001 | −5.75, p < 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahlknecht, A.; Wiedermann, C.J.; Barbieri, V.; Ausserhofer, D.; Engl, A.; Piccoliori, G. Real-World Implementation of PRISMA-7 and Clinical Frailty Scale for Frailty Identification and Integrated Care Activation: A Cross-Sectional Study in Northern Italian Primary Practice. J. Clin. Med. 2025, 14, 3431. https://doi.org/10.3390/jcm14103431
Mahlknecht A, Wiedermann CJ, Barbieri V, Ausserhofer D, Engl A, Piccoliori G. Real-World Implementation of PRISMA-7 and Clinical Frailty Scale for Frailty Identification and Integrated Care Activation: A Cross-Sectional Study in Northern Italian Primary Practice. Journal of Clinical Medicine. 2025; 14(10):3431. https://doi.org/10.3390/jcm14103431
Chicago/Turabian StyleMahlknecht, Angelika, Christian J. Wiedermann, Verena Barbieri, Dietmar Ausserhofer, Adolf Engl, and Giuliano Piccoliori. 2025. "Real-World Implementation of PRISMA-7 and Clinical Frailty Scale for Frailty Identification and Integrated Care Activation: A Cross-Sectional Study in Northern Italian Primary Practice" Journal of Clinical Medicine 14, no. 10: 3431. https://doi.org/10.3390/jcm14103431
APA StyleMahlknecht, A., Wiedermann, C. J., Barbieri, V., Ausserhofer, D., Engl, A., & Piccoliori, G. (2025). Real-World Implementation of PRISMA-7 and Clinical Frailty Scale for Frailty Identification and Integrated Care Activation: A Cross-Sectional Study in Northern Italian Primary Practice. Journal of Clinical Medicine, 14(10), 3431. https://doi.org/10.3390/jcm14103431





