Evaluation of Acute Pancreatitis Severity and Prognosis Using the Aggregate Systemic Inflammation Index (AISI) as a New Marker: A Comparison with Other Inflammatory Indices
Abstract
1. Introduction
2. Methods
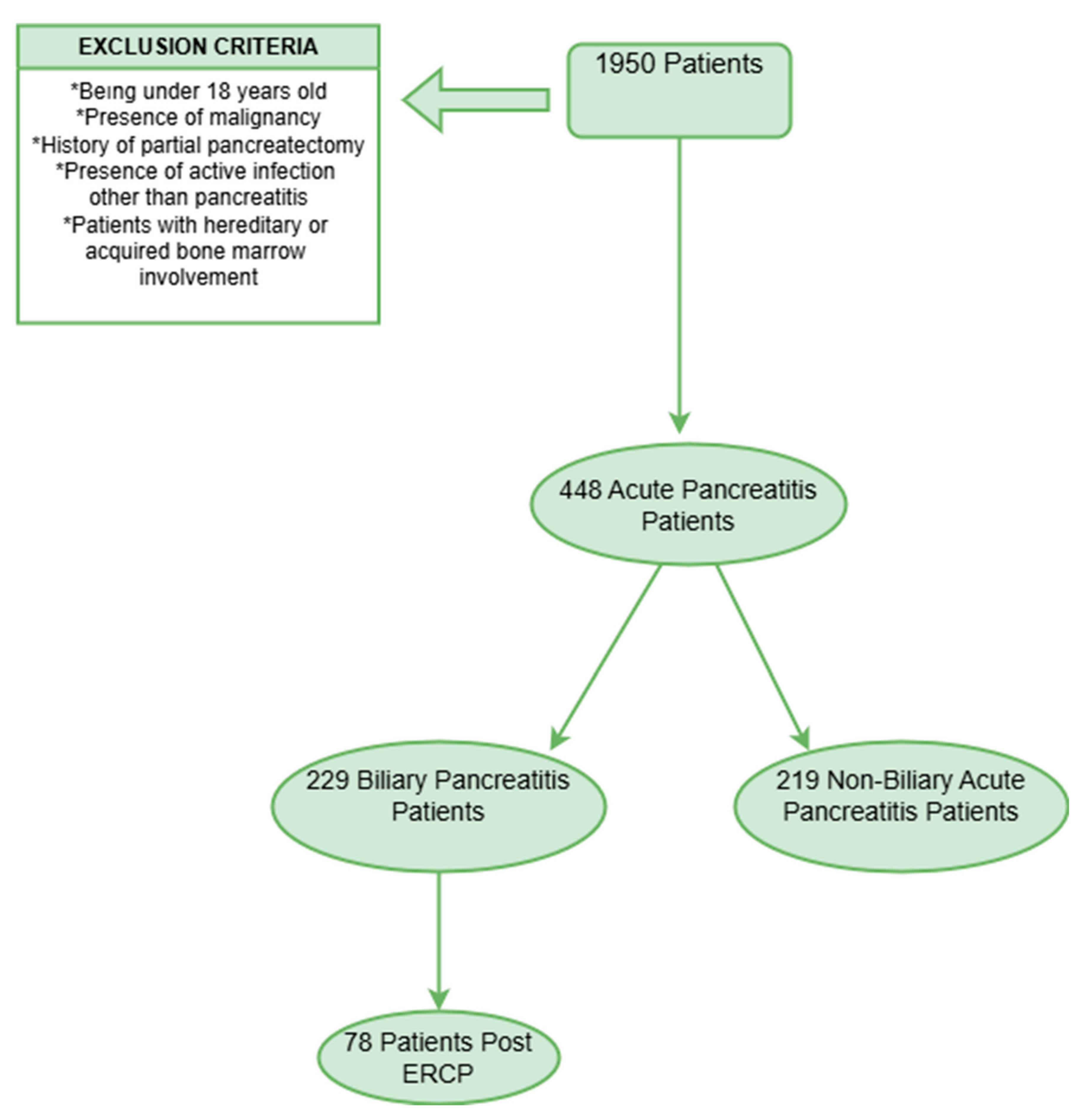
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Inflammatory Index Calculations
2.4. ERCP Procedure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AISI | Aggregate Systemic Inflammation Index |
| ALT | Alanine Aminotransferase |
| AP | Acute Pancreatitis |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| AST | Aspartate Aminotransferase |
| BISAP | Bedside Index for Severity in Acute Pancreatitis |
| BUN | Blood Urea Nitrogen |
| CBC | Complete Blood Count |
| CI | Confidence Interval |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| ERCP | Endoscopic Retrograde Cholangiopancreatography |
| ERCP History | ERCP was performed during hospitalization |
| GGT | Gamma-Glutamyl Transferase |
| HAPS | Harmless Acute Pancreatitis Score |
| IL | Interleukin |
| LDH | Lactate Dehydrogenase |
| LMR | Lymphocyte-to-Monocyte Ratio |
| LOS | Length of Stay |
| MRI | Magnetic Resonance Imaging |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| NLRP | Neutrophil-to-Lymphocyte*Platelet Ratio |
| PLR | Platelet-to-Lymphocyte Ratio |
| PCT | Procalcitonin |
| PPV | Positive Predictive Value |
| RDW | Red Cell Distribution Width |
| ROC | Receiver Operating Characteristic |
| SD | Standard Deviation |
| SIRI | Systemic Inflammatory Response Index |
| SIRS | Systemic Inflammatory Response Syndrome |
| TNF-α | Tumor Necrosis Factor-alpha |
| WBC | White Blood Cell |
References
- Lee, P.J.; Papachristou, G.I.; Speake, C.; Lacy-Hulbert, A. Immune markers of severe acute pancreatitis. Curr. Opin. Gastroenterol. 2024, 40, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Zerem, E. Treatment of severe acute pancreatitis and its complications. World J. Gastroenterol. 2014, 20, 13879–13892. [Google Scholar] [CrossRef]
- Lee, D.W.; Cho, C.M. Predicting Severity of Acute Pancreatitis. Medicina. 2022, 58, 787. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Yeung, Y.P.; Lam, B.Y.; Yip, A.W. APACHE system is better than Ranson system in the prediction of severity of acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2006, 5, 294–299. [Google Scholar] [PubMed]
- Wu, B.U.; Johannes, R.S.; Sun, X.; Tabak, Y.; Conwell, D.L.; Banks, P.A. The early prediction of mortality in acute pancreatitis: A large population-based study. Gut 2008, 57, 1698–1703. [Google Scholar] [CrossRef]
- Vincent, A. CAS Predicting Severity of Acute Pancreatitis-Evaluation of Neutrophil-to-Lymphocyte Count Ratio as Emerging Biomarker: A Retrospective Analytical Study. Cureus 2024, 16, e74881. [Google Scholar]
- Xiu, J.; Lin, X.; Chen, Q.; Yu, P.; Lu, J.; Yang, Y.; Chen, W.; Bao, K.; Wang, J.; Zhu, J.; et al. The aggregate index of systemic inflammation (AISI): A novel predictor for hypertension. Front. Cardiovasc. Med. 2023, 10, 1163900. [Google Scholar] [CrossRef]
- Zinellu, A.; Collu, C.; Nasser, M.; Paliogiannis, P.; Mellino, S.; Zinellu, E.; Traclet, J.; Ahmad, K.; Mangoni, A.A.; Carru, C.; et al. The Aggregate Index of Systemic Inflammation (AISI): A Novel Prognostic Biomarker in Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2021, 10, 4134. [Google Scholar] [CrossRef]
- Dancu, G.M.; Popescu, A.; Sirli, R.; Danila, M.; Bende, F.; Tarta, C.; Sporea, I. The BISAP score, NLR, CRP, or BUN: Which marker best predicts the outcome of acute pancreatitis? Medicine 2021, 100, e28121. [Google Scholar] [CrossRef]
- Jenne, C.N.; Kubes, P. Platelets in inflammation and infection. Platelets 2015, 26, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F. Role of Serum Biomarkers in Differentiating Periprosthetic Joint Infections from Aseptic Failures after Total Hip Arthroplasties. J. Clin. Med. 2024, 13, 5716. [Google Scholar] [CrossRef] [PubMed]
- Pando, E.; Alberti, P.; Hidalgo, J.; Vidal, L.; Dopazo, C.; Caralt, M.; Blanco, L.; Gómez-Gavara, C.; Bilbao, I.; Balsells, J.; et al. The role of extra-pancreatic infections in the prediction of severity and local complications in acute pancreatitis. Pancreatology 2018, 18, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Tilea, I.; Varga, A.; Serban, R.C. Past, Present, and Future of Blood Biomarkers for the Diagnosis of Acute Myocardial Infarction-Promises and Challenges. Diagnostics 2021, 11, 881. [Google Scholar] [CrossRef]
- Xia, D.; Yao, R.; Zhou, P.; Wang, C.; Xia, Y.; Xu, S. LncRNA NEAT1 reversed the hindering effects of miR-495-3p/STAT3 axis and miR-211/PI3K/AKT axis on sepsis-relevant inflammation. Mol. Immunol. 2020, 117, 168–179. [Google Scholar] [CrossRef]
- Velichko, A.; Huyut, M.T.; Belyaev, M.; Izotov, Y.; Korzun, D. Machine Learning Sensors for Diagnosis of COVID-19 Disease Using Routine Blood Values for Internet of Things Application. Sensors 2022, 22, 7886. [Google Scholar] [CrossRef]
- Gunjaca, I.; Zunic, J.; Gunjaca, M.; Kovac, Z. Circulating cytokine levels in acute pancreatitis-model of SIRS/CARS can help in the clinical assessment of disease severity. Inflammation 2012, 35, 758–763. [Google Scholar] [CrossRef]
- Zerem, E.; Kurtcehajic, A.; Kunosić, S.; Zerem Malkočević, D.; Zerem, O. Current trends in acute pancreatitis: Diagnostic and therapeutic challenges. World J. Gastroenterol. 2023, 29, 2747–2763. [Google Scholar] [CrossRef]
- Matta, B.; Gougol, A.; Gao, X.; Reddy, N.; Talukdar, R.; Kochhar, R.; Goenka, M.K.; Gulla, A.; Gonzalez, J.A.; Singh, V.K.; et al. Worldwide Variations in Demographics, Management, and Outcomes of Acute Pancreatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 1567–1575.e2. [Google Scholar] [CrossRef]
- Goyal, H.; Awad, H.; Hu, Z.D. Prognostic value of admission red blood cell distribution width in acute pancreatitis: A systematic review. Ann. Transl. Med. 2017, 5, 342. [Google Scholar] [CrossRef]
- Hong, W.; Pan, J.; Goyal, H.; Zippi, M. Editorial: Acute pancreatitis infection: Epidemiology, prevention, clinical characteristics, treatment, and prediction. Front. Cell. Infect. Microbiol. 2023, 13, 1175195. [Google Scholar] [CrossRef] [PubMed]
- Şenol, K.; Saylam, B.; Kocaay, F.; Tez, M. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am. J. Emerg. Med. 2013, 31, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Dumnicka, P.; Maduzia, D.; Ceranowicz, P.; Olszanecki, R.; Drożdż, R.; Kuśnierz-Cabala, B. The Interplay between Inflammation, Coagulation and Endothelial Injury in the Early Phase of Acute Pancreatitis: Clinical Implications. Int. J. Mol. Sci. 2017, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Santamaría, D.M.; Taxonera, C.; Giner, M. Update on pathogenesis and clinical management of acute pancreatitis. World J. Gastrointest. Pathophysiol. 2012, 3, 60–70. [Google Scholar] [CrossRef][Green Version]
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef]
- Granger, J.; Remick, D. Acute pancreatitis: Models, markers, and mediators. Shock 2005, 24 (Suppl. 1), 45–51. [Google Scholar] [CrossRef]
- Jafari, T.; Feizi, A.; Askari, G.; Fallah, A.A. Parenteral immunonutrition in patients with acute pancreatitis: A systematic review and meta-analysis. Clin. Nutr. 2015, 34, 35–43. [Google Scholar] [CrossRef]
- Gürleyik, G.; Zahidullahoğlu Cirpici, O.; Aktekin, A.; Sağlam, A. Akut pankreatit şiddetinin erken tanısında Ranson ve APACHE II skorlarının, serum interlökin-6 ve C-reaktif protein düzeylerinin rolü. Ulus. Travma Acil Cerrahi Derg. 2004, 10, 83–88. [Google Scholar]
- Suppiah, A.; Malde, D.; Arab, T.; Hamed, M.; Allgar, V.; Smith, A.M.; Morris-Stiff, G. The prognostic value of the neutrophil-lymphocyte ratio (NLR) in acute pancreatitis: Identification of an optimal, N.L.R. J. Gastrointest Surg. 2013, 17, 675–681. [Google Scholar] [CrossRef]
- Park, H.S.; In, S.G.; Yoon, H.J.; Lee, W.J.; Woo, S.H.; Kim, D. Predictive values of neutrophil-lymphocyte ratio as an early indicator for severe acute pancreatitis in the emergency department patients. J. Lab. Physicians. 2019, 11, 259–264. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, T.; Dong, X.; Sun, L.; Wu, Q.; Liu, J.; Sun, X. Systemic Immune-Inflammation Index for Predicting the Prognosis of Critically Ill Patients with Acute Pancreatitis. Int. J. Gen. Med. 2021, 14, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; He, Y.; Bao, H.; Zhang, W.; Wang, X. Diagnostic Value of Neutrophil-Lymphocyte Ratio for Predicting the Severity of Acute Pancreatitis: A Meta-Analysis. Dis. Markers. 2020, 2020, 9731854. [Google Scholar] [CrossRef] [PubMed]
- Junare, P.R.; Debnath, P.; Nair, S.; Chandnani, S.; Udgirkar, S.; Thange, R.; Jain, S.; Deshmukh, R.; Debnath, P.; Rathi, P.; et al. Complete hemogram: Simple and cost-effective in staging and predicting outcome in acute pancreatitis. Wien. Klin. Wochenschr. 2021, 133, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Jung, S.; Lee, K.J.; Kim, J.W. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. 2018, 18, 18. [Google Scholar] [CrossRef]
- Singh, V.K.; Wu, B.U.; Bollen, T.L.; Repas, K.; Maurer, R.; Johannes, R.S.; Mortele, K.J.; Conwell, D.L.; Banks, P.A. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am. J. Gastroenterol. 2009, 104, 966–971. [Google Scholar] [CrossRef]
- Sahin, A. Neutrophil-Creatinine Index: A New Prognostic Factor for Severity of Acute Pancreatitis. Medicina 2024, 60, 607. [Google Scholar] [CrossRef]
- Meher, S.; Mishra, T.S.; Sasmal, P.K.; Rath, S.; Sharma, R.; Rout, B.; Sahu, M.K. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J. Biomark. 2015, 2015, 519534. [Google Scholar] [CrossRef]
- Riaz, H.M.A.; Islam, Z.; Rasheed, L.; Sarfraz, Z.; Sarfraz, A.; Robles-Velasco, K.; Sarfraz, M.; Cherrez-Ojeda, I. The Evaluation of Inflammatory Biomarkers in Predicting Progression of Acute Pancreatitis to Pancreatic Necrosis: A Diagnostic Test Accuracy Review. Healthcare 2022, 11, 27. [Google Scholar] [CrossRef]
- Akdur, G.; Bardakçı, O.; Das, M.; Akdur, O.; Beyazit, Y. Diagnostic utility of hematological indices in predicting adverse outcomes and severity of acute pancreatitis based on BISAP and modified Glasgow score. Ulus. Travma Acil. Cerrahi. Derg. 2022, 28, 268–275. [Google Scholar] [CrossRef]
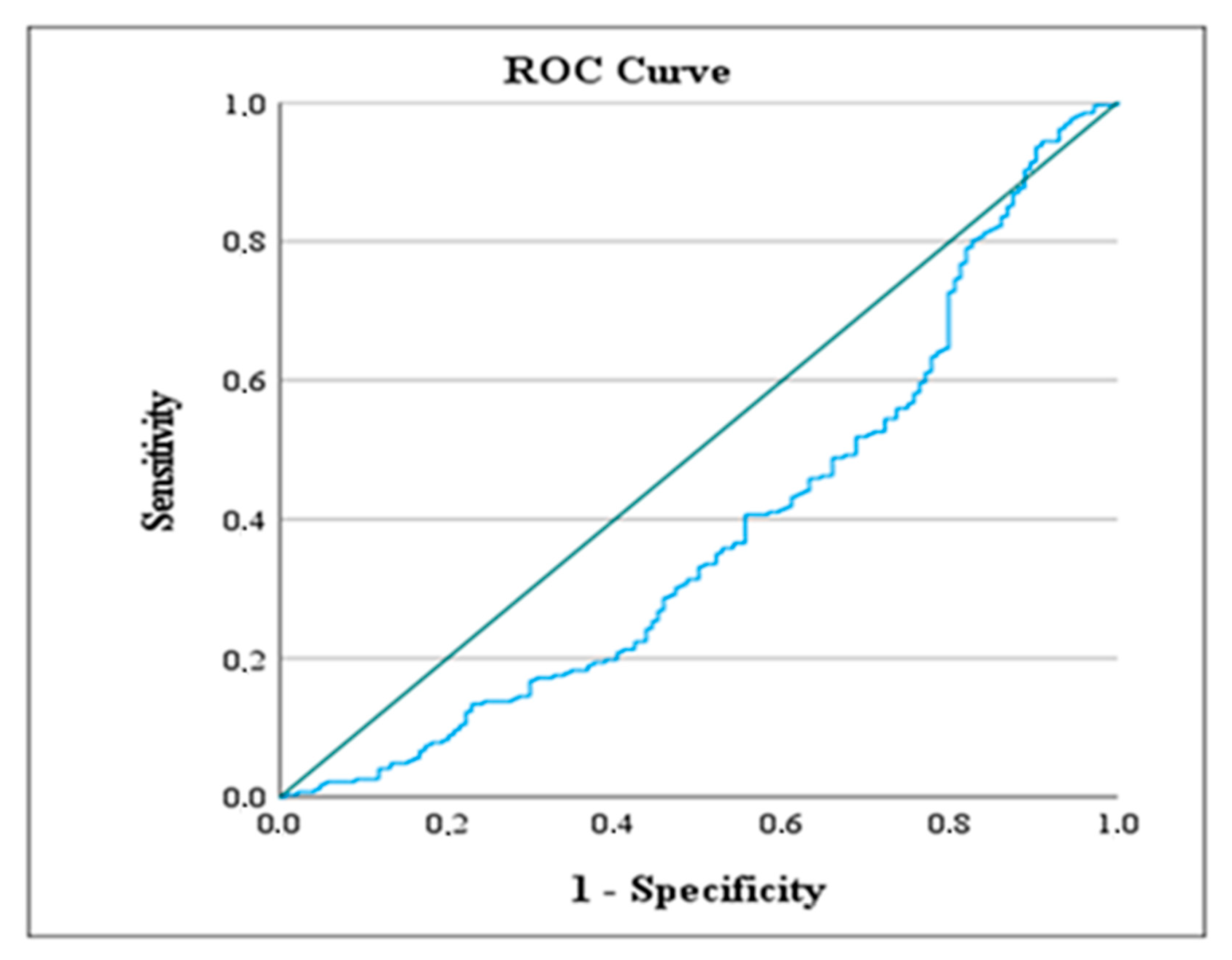
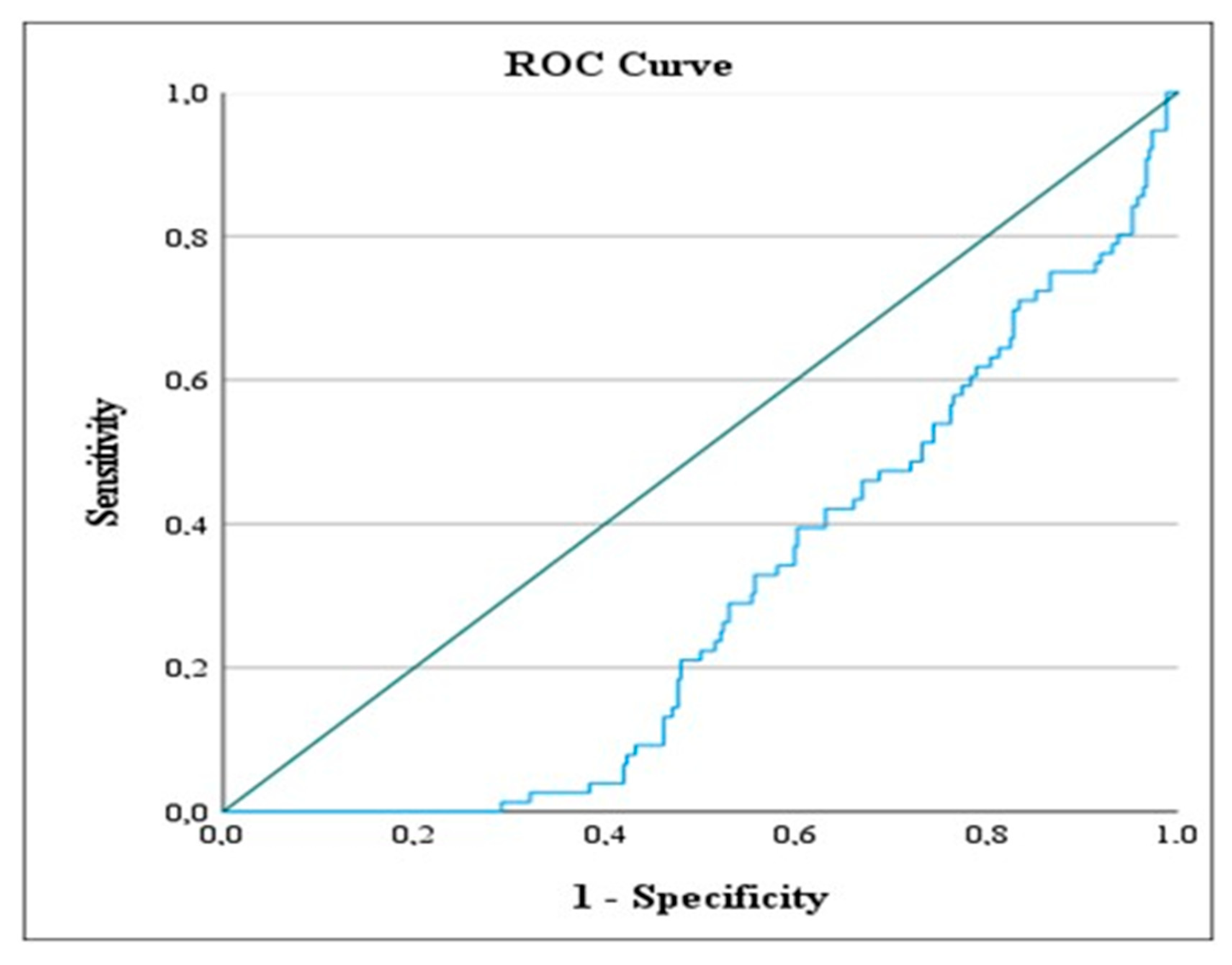

| A. Continuous Variables | |||
| Variable | Mean ± SD (Median) | Min.–Max. | |
| Age | 63.47 ± 17.92 (62) | 23–98 | |
| Hospitalization Time (Days) | 10.60 ± 8.75 (8) | 0–64 | |
| AISI | 1183.89 ± 1067.42 | 61.53–6869.41 | |
| Modified AISI | 2075.35 ± 2629.51 | 83.77–20,608.24 | |
| Glasgow 2 | 1.12 ± 1.08 | 0.00–5.00 | |
| Marshall | 0.28 ± 0.98 | 0.00–12.00 | |
| BISAP | 0.99 ± 1.08 | 0.00–5.00 | |
| RANSON | 1.89 ± 1.42 | 0.00–9.00 | |
| APACHE II | 5.67 ± 4.25 | 0.00–26.00 | |
| HAPS | 0.65 ± 0.63 | 0.00–2.00 | |
| B. Categorical Variables | |||
| Variable Category | N (%) | ||
| Gender | |||
| Female | 219 (53.16) | ||
| Male | 193 (46.84) | ||
| Biliary Disease | |||
| No | 200 (48.54) | ||
| Yes | 212 (51.46) | ||
| ERCP History | |||
| No | 239 (58.01) | ||
| Yes | 173 (41.99) | ||
| Systemic Complication | |||
| No | 292 (70.87) | ||
| Yes | 120 (29.13) | ||
| Local Complication | |||
| No | 330 (80.10) | ||
| Yes | 82 (19.90) | ||
| Post-ERCP Pancreatitis | |||
| No | 338 (82.04) | ||
| Yes | 74 (17.96) | ||
| Mortality | |||
| No | 397 (96.36) | ||
| Yes | 15 (3.64) | ||
| Atlanta | Mild | 239.00 | (58.01) |
| Moderate | 131.00 | (31.80) | |
| Severe | 4.00 | (10.19) | |
| Variable | Mean ± SD (Median) | Min.–Max. |
|---|---|---|
| Hemoglobin (g/dL) | 13.41 ± 2.66 (13.40) | 5.10–46.70 |
| Platelet (×109/L) | 268.25 ± 89.75 (257.00) | 80.00–697.00 |
| Leukocyte (×109/L) | 10.59 ± 4.00 (9.80) | 2.41–25.15 |
| Creatinine (mg/dL) | 0.99 ± 1.24 (0.77) | 0.12–20.31 |
| Albumin (g/L) | 21.23 ± 18.52 (4.70) | 1.90–51.00 |
| CRP (g/L) | 47.67 ± 66.97 (16.50) | 0.50–400.00 |
| BUN (mg/dL) | 17.54 ± 11.92 (14.90) | 3.70–92.93 |
| Alanine Aminotransferase (U/L) | 207.10 ± 221.94 (138.00) | 6.00–1297.00 |
| Total-bilirubin (mg/dL) | 2.88 ± 3.36 (1.80) | 0.20–25.80 |
| Direct-bilirubin (mg/dL) | 1.87 ± 2.61 (0.90) | 0.00–19.10 |
| Alkaline Phosphatase (U/L) | 214.84 ± 191.26 (162.50) | 23.00–1936.00 |
| Gamma-glutamyl transferase (U/L) | 343.17 ± 310.75 (276.50) | 5.00–1545.00 |
| Lipase (U/L) | 1910.69 ± 1762.79 (1472.50) | 20.00–12,917.00 |
| Lactate dehydrogenase (U/L) | 349.85 ± 289.05 (276.50) | 123.00–4677.00 |
| Aspartate Aminotransferase (U/L) | 194.92 ± 256.20 (111.50) | 6.00–2224.00 |
| Neutrophil (×109/L) | 8.73 ± 4.04 (7.85) | 1.79–24.75 |
| Lymphocyte (×109/L) | 1.27 ± 0.66 (1.15) | 0.18–5.03 |
| Monocyte (×109/L) | 0.50 ± 0.18 (0.46) | 0.06–1.30 |
| Platelet (×109/L) | 265.72 ± 80.95 (254.00) | 48.00–602.00 |
| Amylase (U/L) | 1239.70 ± 1092.89 (948.50) | 22.00–7461.00 |
| PLR | 260.53 ± 145.99 (222.68) | 26.93–890.91 |
| MLR | 0.48 ± 0.27 (0.41) | 0.08–1.67 |
| NLRP | 0.04 ± 0.05 (0.03) | 0.00–0.41 |
| NLR | 9.54 ± 8.51 (6.89) | 0.78–57.15 |
| SIRI | 4.61 ± 4.28 (3.32) | 0.33–4.28 |
| Laboratory Parameters | Length of Hospital Stay (Day) | Systemic Complication | Local Complication | Mortality | Biliary Pancreatitis | Sex (Male) | History of ERCP | Post-ERCP Pancreatitis | Age |
|---|---|---|---|---|---|---|---|---|---|
| Statistic | r/p | t/p | t/p | t/p | t/p | t/p | t/p | t/p | r/p |
| Alkaline Phosphatase (U/L) | 0.179/0.001 * | −0.705/0.481 | 1.356/0.176 | −0.439/0.661 | −1.412/0.159 | −0.362/0.717 | −5.618/0.001 * | −2.529/0.001 * | 0.125/0.030 |
| Alanine Aminotransferase (U/L) | −0.146/0.001 * | −0.528/0.598 | 0.115/0.909 | 1.148/0.252 | −5.471/0.001 * | 2.994/0.003 * | 1.020/0.308 | 2.412/0.017 * | 0.155/0.002 |
| Aspartate Aminotransferase (U/L) | 0.051/0.030 * | −1.335/0.183 | −0.142/0.887 | 0.111/0.911 | −3.328/0.001 * | 3.786/0.001 * | 2.300/0.022 * | 2.203/0.030 * | 0.042/0.399 |
| Albumin (g/L) | −0.037/0.459 | −6.563/0.001 * | 3.201/0.002 * | 2.226/0.041 * | 0.077/0.938 | 0.120/0.904 | 2.245/0.025 * | 1.783/0.077 | 0.021 */0.669 |
| Amylase (U/L) | 0.152/0.001 * | −0.295/0.768 | −0.281/0.779 | 0.057/0.955 | −3.577/0.001 * | 1.723/0.086 | 2.098/0.036 * | 3.692/0.001 * | 0.133/0.007 |
| BUN (mg/dL) | 0.237/0.001 * | −3.944/0.001 * | −0.603/0.547 | −3.335/0.005 * | 0.078/0.938 | −0.058/0.977 | 0.911/0.322 | 1.311/0.191 | 0.386/0.001 |
| CRP (g/L) | 0.212/0.001 * | −5.023/0.001 * | −2.249/0.027 * | −1.052/0.294 | −0.073/0.942 | −2.925/0.001 * | 0.886/0.376 | 2.531/0.012 * | 0.117/0.017 |
| Creatinine (mg/dL) | 0.130/0.008 * | −2.143/0.034 * | 0.891/0.373 | −2.128/0.051 | 0.792/0.429 | −1.050/0.294 | 0.171/0.865 | 0.113/0.910 | 0.179/0.001 |
| Direct bilirubin (mg/dL) | 0.187/0.001 * | −0.393/0.695 | 2.654/0.009 * | −0.493/0.622 | −3.121/0.001 * | −1.312/0.190 | −5.001/0.001 * | −1.975/0.051 | 0.107/0.030 |
| Gamma-glutamyl transferase (U/L) | −0.005/0.927 | −0.203/0.839 | 0.953/0.341 | −0.260/0.795 | −5.429/0.001 * | −0.104/0.917 | −3.729/0.001 * | −0.830/0.407 | 0.030 */0.543 |
| Hemoglobin (g/dL) | 0.151/0.002 * | 1.865/0.063 | −1.429/0.154 | 2.576/0.010 * | 0.608/0.544 | −5.018/0.001 * | 0.770/0.442 | 0.348/0.728 | 0.247/0.001 |
| Lactate dehydrogenase (U/L) | 0.143/0.001 * | −2.592/0.001 * | 1.105/0.270 | −0.219/0.827 | −2.262/0.024 * | 3.721/0.001 * | 3.209/0.001 * | 3.893/0.001 * | 0.042 */0.397 |
| Leukocyte (×109/L) | 0.084/0.089 | −5.481/0.001 * | −2.068/0.041 * | −0.054/0.957 | −0.164/0.870 | −2.097/0.037 * | 2.458/0.014 * | 4.013/0.001 * | 0.012 */0.807 |
| Lipase (U/L) | 0.139/0.005 | 0.539/0.590 | −0.009/0.993 | 0.369/0.712 | −2.477/0.014 * | 1.751/0.081 | 1.488/0.137 | 0.627/0.531 | 0.156/0.002 |
| Lymphocyte (×109/L) | −0.139/0.005 * | 4.104/0.001 * | 0.829/0.408 | 2.852/0.005 * | 3.684/0.001 * | −0.924/0.356 | 1.848/0.065 | −0.174/0.862 | 0.241/0.001 |
| Monocyte (×109/L) | −0.073/0.137 | −0.977/0.330 | −0.859/0.392 | 0.318/0.755 | −0.824/0.411 | −3.836/0.001 * | 1.084/0.279 | 2.336/0.021 * | 0.068/0.167 |
| Neutrophil (×109/L) | 0.116/0.018 * | −6.492/0.001 * | −2.256/0.025 * | −1.553/0.142 | −0.680/0.497 | −1.699/0.090 | 2.627/0.009 * | 3.826/0.001 * | 0.042 */0.391 |
| Platelet | 0.004/0.930 | −0.421/0.674 | −0.230/0.818 | 2.094/0.037 * | 0.703/0.483 | 3.683/0.001 * | 0.881/0.379 | −0.432/0.666 | 0.140/0.004 |
| Total-bilirubin (mg/dL) | 0.171/0.001 * | −0.538/0.591 | 2.566/0.011 * | −0.517/0.605 | −3.443/0.001 * | −1.295/0.196 | −4.421/0.001 * | −1.398/0.165 | 0.095/0.053 |
| Parameter | Glasgow 2 | Marshall | BISAP | RANSON | Apache II | HAPS |
|---|---|---|---|---|---|---|
| Statistic | r/p | r/p | r/p | r/p | r/p | r/p |
| Alkaline Phosphatase (U/L) | 0.134/0.001 * | 0.009/0.861 | 0.060/0.221 | 0.108/0.028 * | 0.052/0.296 | 0.152/0.001 * |
| Alanine Aminotransferase (U/L) | 0.122/0.013 * | 0.039/0.428 | 0.147/0.001 * | 0.237/0.001 * | 0.165/0.001 * | 0.106/0.032 * |
| Aspartate Aminotransferase (U/L | 0.041/0.412 | 0.057/0.247 | 0.026/0.603 | 0.361/0.001 * | −0.009/0.853 | 0.044/0.368 |
| Albumin (g/L) | 0.019/0.695 | 0.021/0.676 | 0.036/0.465 | 0.017/0.723 | 0.135/0.006 * | 0.139/0.005 * |
| Amylase (U/L) | 0.115/0.020 * | 0.041/0.404 | 0.156/0.001 * | 0.134/0.001 * | −0.134/0.001 * | 0.011/0.082 |
| BUN (mg/dL) | 0.484/0.001 * | 0.480/0.001 * | 0.546/0.001 * | 0.237/0.001 * | 0.625/0.001 * | 0.048/0.331 |
| CRP (g/L) | 0.327/0.001 * | 0.207/0.001 * | 0.349/0.001 * | 0.213/0.001 * | 0.301/0.001 * | 0.089/0.072 |
| Creatinine (mg/dL) | 0.315/0.001 * | 0.611/0.001 * | 0.349/0.001 * | 0.165/0.001 * | 0.530/0.001 * | 0.081/0.102 |
| Direct-bilirubin (mg/dL) | 0.124/0.012 * | 0.017/0.730 | 0.091/0.065 | 0.089/0.072 | 0.072/0.143 | 0.027/0.584 |
| Gamma-glutamyl transferase (U/L) | 0.003/0.946 | 0.029/0.555 | 0.049/0.321 | 0.102/0.038 * | −0.032/0.513 | 0.083/0.093 |
| Hemoglobin (g/dL) | 0.259/0.001 * | 0.177/0.001 * | 0.295/0.001 * | 0.087/0.079 | 0.314/0.001 * | 0.324/0.001 * |
| Lactate dehydrogenase (U/L) | 0.199/0.001 * | 0.298/0.001 * | 0.159/0.001 * | 0.406/0.001 * | 0.208/0.001 * | 0.119/0.016 * |
| Leukocyte (×109/L) | 0.221/0.001 * | 0.056/0.259 | 0.179/0.001 * | 0.262/0.001 * | 0.112/0.023 * | 0.180/0.001 * |
| Lipase (U/L) | 0.145/0.003 * | 0.001/0.989 | 0.173/0.001 * | 0.076/0.126 | −0.179/0.001 * | 0.050 */0.312 |
| Lymphocyte (×109/L) | 0.189/0.001 * | 0.123/0.012 * | 0.241/0.001 * | 0.277/0.001 * | −0.255/0.001 * | 0.021/0.672 |
| Monocyte (×109/L) | 0.123/0.012 * | 0.058/0.243 | 0.045/0.366 | 0.160/0.001 * | 0.053/0.286 | 0.056/0.261 |
| Neutrophil (×109/L) | 0.284/0.001 * | 0.114/0.021 * | 0.255/0.001 * | 0.295/0.001 * | 0.206/0.001 * | 0.145/0.003 * |
| Platelet | 0.148/0.003 * | 0.070/0.158 | 0.170/0.001 * | 0.036/0.465 | −0.139/0.005 * | 0.032/0.512 |
| Total-bilirubin (mg/dL) | 0.100/0.001 * | 0.017/0.738 | 0.092/0.063 | 0.090/0.066 | 0.063/0.201 | 0.007/0.894 |
| A. Correlation Coefficients (r) for Continuous Variables | ||||||
| Variable | PLR | NLRP | NLR | AISI | SIRI | Modified AISI |
| Statistic | r/p | r/p | r/p | r/p | r/p | r/p |
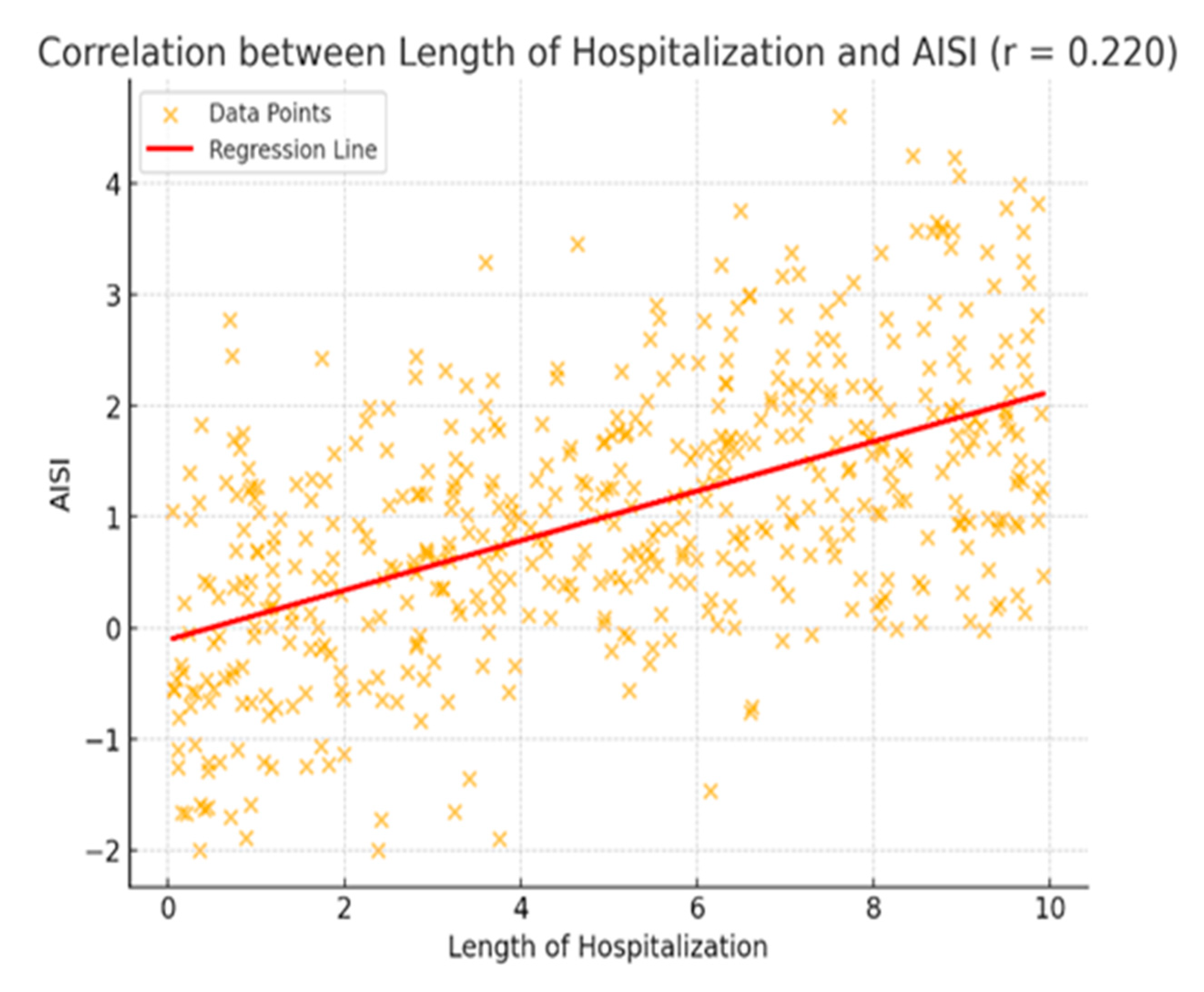
| Hospital Stay | 0.122/0.013 * | 0.141/0.004 * | 0.157/0.001 * | 0.220/0.001 * | 0.239/0.001 * | 0.138/0.005 * |
| Glasgow 2 | 0.158/0.001 * | 0.316/0.001 * | 0.308/0.001 * | 0.315/0.001 * | 0.365/0.001 * | 0.237/0.001 * |
| Marshall | 0.095/0.055 | 0.149/0.001 * | 0.168/0.001 * | 0.160/0.001 * | 0.155/0.001 * | 0.131/0.008 * |
| BISAP | 0.243/0.001 * | 0.430/0.001 * | 0.434/0.001 * | 0.283/0.001 * | 0.368/0.001 * | 0.204/0.001 * |
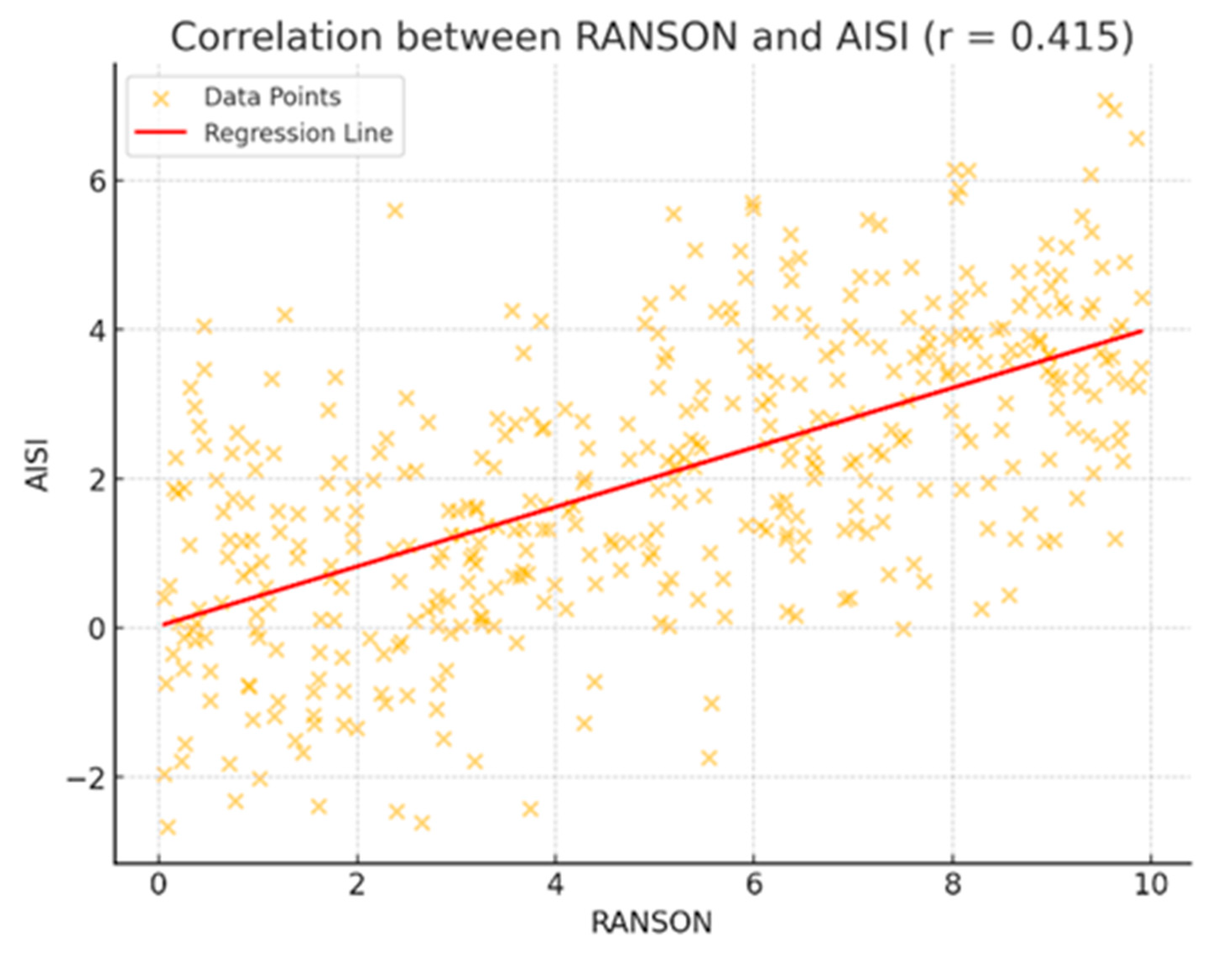
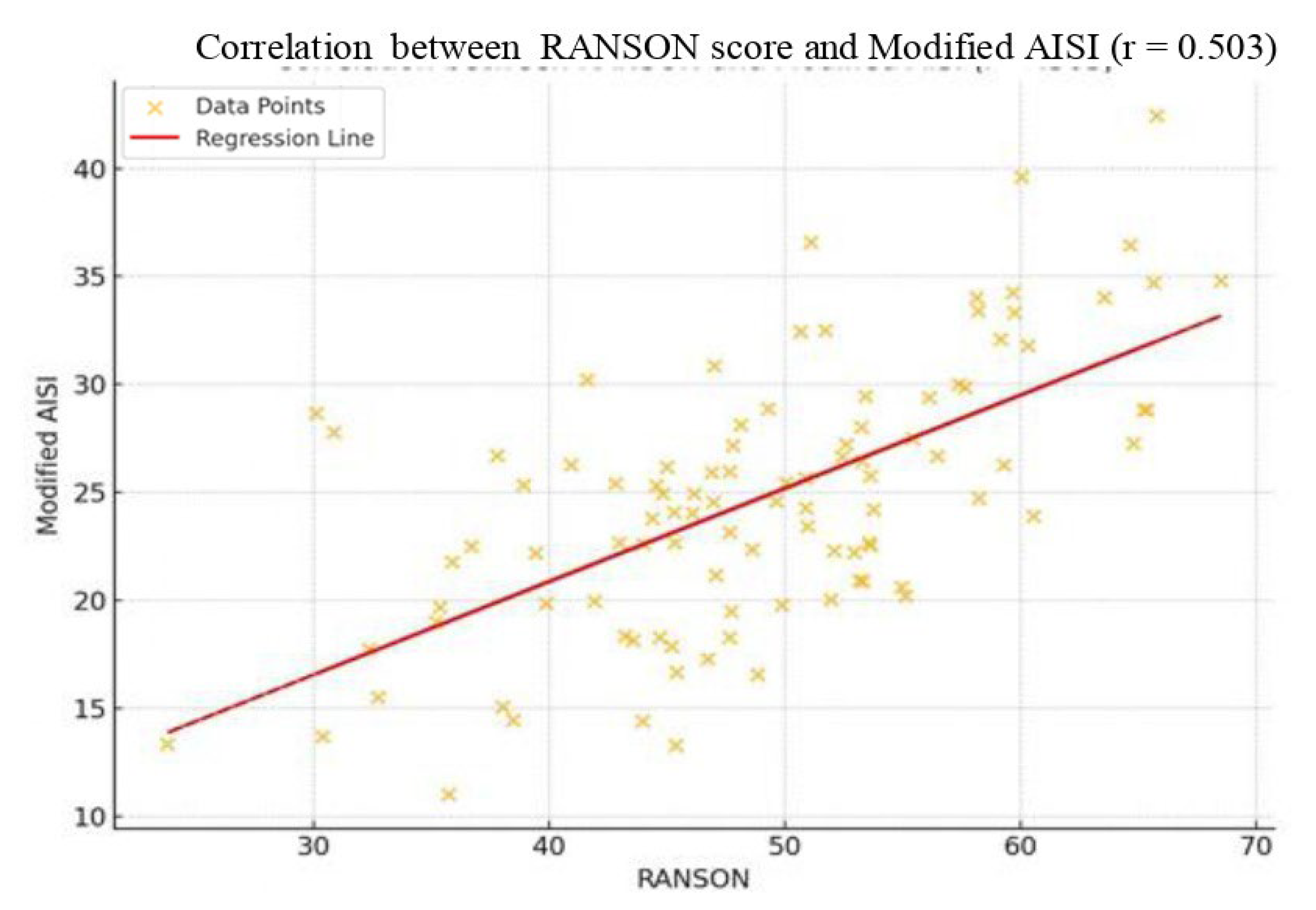
| RANSON | 0.291/0.001 * | 0.271/0.001 * | 0.351/0.001 * | 0.415/0.001 * | 0.403/0.001 * | 0.503/0.001 * |
| APACHE II | 0.179/0.001 * | 0.290/0.001 * | 0.312/0.001 * | 0.272/0.001 * | 0.303/0.001 * | 0.165/0.001 * |
| HAPS Score | −0.004/0.939 | 0.020/0.679 | 0.067/0.176 | 0.117/0.017 * | 0.092/0.061 | 0.174/0.001 * |
| Age | 0.232/0.001 * | 0.272/0.001 * | 0.280/0.001 * | 0.135/0.001 * | 0.181/0.001 * | 0.100/0.042 * |
| B. Independent Sample t-Test Results for Binary Variables | ||||||
| Group Comparison | PLR | NLRP | NLR | AISI | SIRI | Modified AISI |
| Statistic | t/p | t/p | t/p | t/p | t/p | t/p |
| Systemic Complication | −4.777/0.001 * | −5.191/0.001 * | −6.384/0.001 * | −6.214/0.001 * | −6.257/0.001 * | −5.457/0.001 * |
| Local Complication | −1.252/0.191 | −1.722/0.088 | −1.932/0.083 | −2.254/0.025 * | −2.025/0.047 * | −1.783/0.078 |
| Mortality | −0.856/0.393 | −2.824/0.013 * | −2.691/0.017 * | −1.810/0.091 | −2.038/0.043 * | −1.858/0.145 |
| Biliary Pancreatitis | −0.868/0.004 * | −1.940/0.053 | −2.812/0.005 * | −2.762/0.006 * | −3.066/0.002 * | −3.628/0.001 * |
| Sex (Female vs. Male) | −2.352/0.019 * | −1.261/0.208 | −0.639/0.523 | −1.152/0.250 | −2.120/0.033 * | −0.100/0.920 |
| ERCP History | −1.237/0.217 | −0.012/0.990 | −0.115/0.909 | 0.412/0.680 | 0.121/0.904 | 1.344/0.180 |
| Post-ERCP Pancreatitis | 0.288/0.773 | 2.497/0.013 * | −3.904/0.001 * | −3.797/0.001 * | 4.910/0.001 * | 6.878/0.001 * |
| Inflammatory Index | Severity (Group) | Mean | SD | ANOVA (F) | p-Value | Post hoc (Tukey HSD) |
|---|---|---|---|---|---|---|
| PLR | Mild (1) | 236.91 | 125.70 | 13.145 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 273.38 | 156.11 | ||||
| Severe (3) | 354.90 | 178.20 | ||||
| MLR | Mild (1) | 0.43 | 0.24 | 15.297 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 0.52 | 0.27 | ||||
| Severe (3) | 0.66 | 0.35 | ||||
| NLRP | Mild (1) | 0.03 | 0.03 | 19.056 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 0.05 | 0.06 | ||||
| Severe (3) | 0.07 | 0.08 | ||||
| NLR | Mild (1) | 7.29 | 5.59 | 25.082 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 11.86 | 10.23 | ||||
| Severe (3) | 15.17 | 11.58 | ||||
| AISI | Mild (1) | 920.21 | 816.70 | 26.861 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 1382.59 | 1055.16 | ||||
| Severe (3) | 2064.64 | 1641.24 | ||||
| Modified AISI | Mild (1) | 1538.16 | 2034.23 | 23.338 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 2354.62 | 2352.49 | ||||
| Severe (3) | 4261.11 | 4573.60 | ||||
| SIRI | Mild (1) | 3.51 | 3.00 | 26.804 | 0.001 * | 3 > 1, 3 > 2, 2 > 1 |
| Moderate (2) | 5.53 | 4.34 | ||||
| Severe (3) | 7.97 | 7.07 |
| Variable | Category | Predictive Value | Sensitivity | 1-Spesificity | Positive Value | Negative Value | Odds Ratio |
|---|---|---|---|---|---|---|---|
| (Reference) | (CI) | ||||||
| Hospitalization Time (Days) | Under 10 Days (r) | 236.626 * | 94.40% | 91.00% | 65.05% | 34.95% | 0.385 |
| Over 10 Days | (0.326–0.444) | ||||||
| Ranson Situation | 0 (r) | 113.189 * | 100% | 98.80% | 18.45% | 81.55% | 0.295 |
| 1+ | (0.240–0.351) | ||||||
| Ranson Situation | 0 (r) | 99.287 ** | 100% | 99.70% | 18.45% | 81.55% | 0.232 |
| 1+ | (0.183–0.281) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, O.; Göre, B.; Öztürk, O.; Cengiz, A.M.; Güler Kadıoğlu, S.; Asfuroğlu Kalkan, E.; Ateş, İ. Evaluation of Acute Pancreatitis Severity and Prognosis Using the Aggregate Systemic Inflammation Index (AISI) as a New Marker: A Comparison with Other Inflammatory Indices. J. Clin. Med. 2025, 14, 3419. https://doi.org/10.3390/jcm14103419
Zengin O, Göre B, Öztürk O, Cengiz AM, Güler Kadıoğlu S, Asfuroğlu Kalkan E, Ateş İ. Evaluation of Acute Pancreatitis Severity and Prognosis Using the Aggregate Systemic Inflammation Index (AISI) as a New Marker: A Comparison with Other Inflammatory Indices. Journal of Clinical Medicine. 2025; 14(10):3419. https://doi.org/10.3390/jcm14103419
Chicago/Turabian StyleZengin, Oğuzhan, Burak Göre, Oğuz Öztürk, Arap Merve Cengiz, Senanur Güler Kadıoğlu, Emra Asfuroğlu Kalkan, and İhsan Ateş. 2025. "Evaluation of Acute Pancreatitis Severity and Prognosis Using the Aggregate Systemic Inflammation Index (AISI) as a New Marker: A Comparison with Other Inflammatory Indices" Journal of Clinical Medicine 14, no. 10: 3419. https://doi.org/10.3390/jcm14103419
APA StyleZengin, O., Göre, B., Öztürk, O., Cengiz, A. M., Güler Kadıoğlu, S., Asfuroğlu Kalkan, E., & Ateş, İ. (2025). Evaluation of Acute Pancreatitis Severity and Prognosis Using the Aggregate Systemic Inflammation Index (AISI) as a New Marker: A Comparison with Other Inflammatory Indices. Journal of Clinical Medicine, 14(10), 3419. https://doi.org/10.3390/jcm14103419





