Partial Breast Reirradiation for Breast Cancer Recurrences After Repeat Breast-Conserving Surgery with Proton Beam Therapy: The Prospective BREAST Trial (NCT06954623)
Abstract
1. Introduction
1.1. Mastectomy Versus BCS
1.2. Common re-RT Options and Toxicity: Brachytherapy, Intraoperative (Electron) RT, and External Beam RT
1.3. Oncological Outcome After Repeat BCS Plus re-RT
1.4. Patient Selection
- -
- -
- -
- -
- -
1.5. Proton Beam Therapy
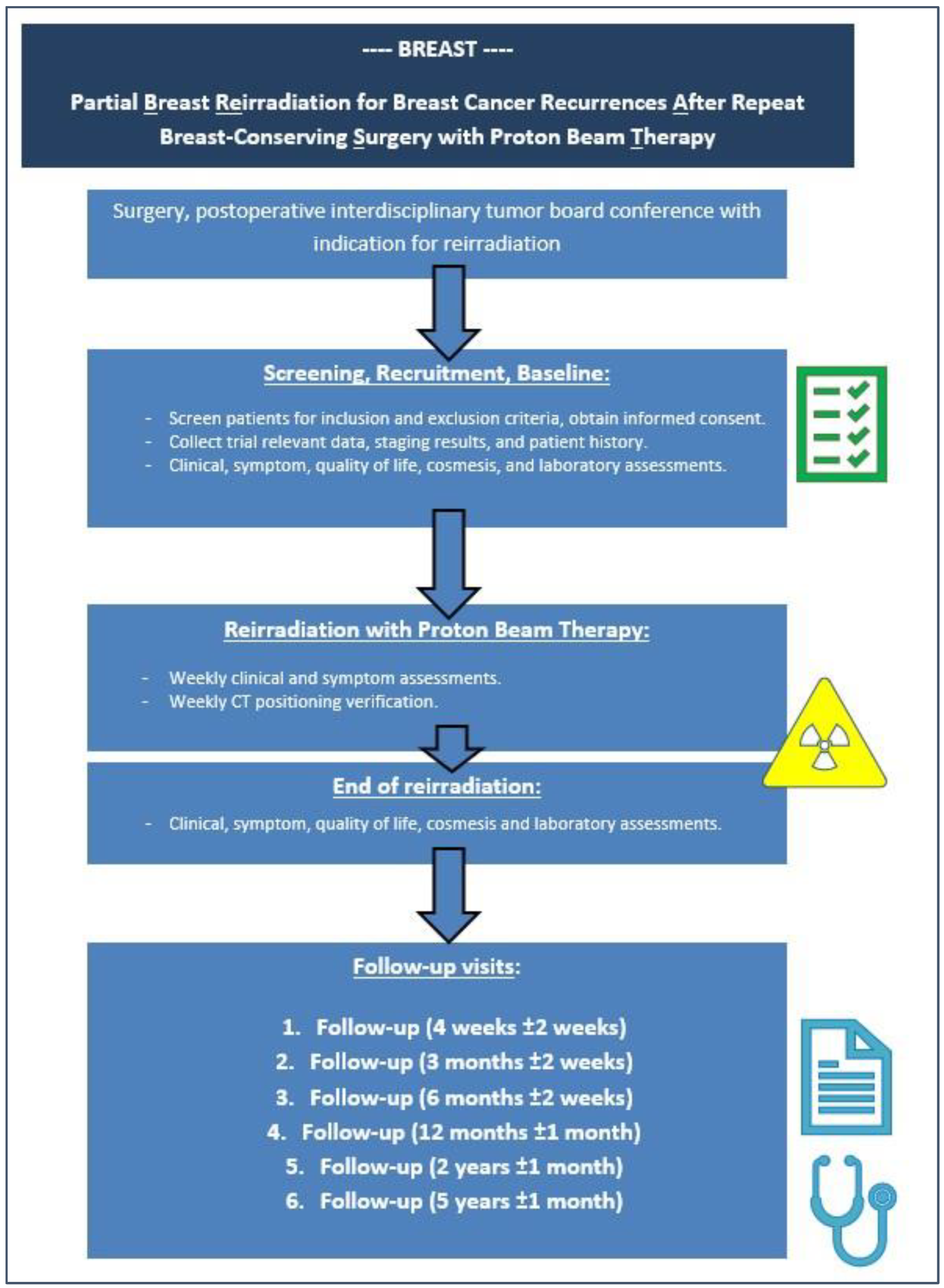
2. Materials and Methods
2.1. Study Design
2.2. Trial Objectives and Assessment
2.2.1. Primary Objective
2.2.2. Secondary Objectives
2.2.3. Explorative and Translational Objectives
2.3. Treatment Planning and Target Volume Definition
- -
- Detailed knowledge of preoperative imaging (e.g., mammogram, MRI, CT scans, etc.), the surgical procedure (including oncoplastic approaches, tumor bed clips, etc.), and the pathological report.
- -
- The recommended total safety margin around the surgical bed is 2 cm, considering the size of the surgical resection margins in all directions.
- -
- The identification and delineation of the skin scar, surgical clips, and whole visible surgical scar tissue inside the breast.
- -
- The identification of the estimated tumor bed, considering and related to the tumor’s localization and size in pre- and postoperative imaging.
- -
- The delineation of the CTV (clinical target volume), which is defined as the estimated tumor bed plus the above-mentioned 2 cm margin in the corresponding direction.
- -
- The thoracic wall/rib plane and the skin are not part of the CTV.
- -
- An additional margin to the CTV will be generated to account for positioning and planning uncertainties of 5–10 mm, depending on individual patient-specific factors or beam angles, resulting in the planning target volume (PTV).
- -
- An additional PTV_evaluate (the PTV minus a skin subtraction of 3 mm) for the meaningful analysis of a dose–volume histogram will be generated.
2.4. Statistical Considerations and Sample Size Calculation
2.5. Ethical Aspects
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APBI | accelerated partial-breast irradiation |
| BCS | breast-conserving surgery |
| BRA | breast retraction assessment |
| BT | brachytherapy |
| CT | computed tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTV | clinical target volume |
| DCIS | ductal carcinoma in situ |
| DMFS | distant-metastases-free survival |
| EBRT | external beam RT |
| ECOG | Eastern Cooperative Oncology Group Performance Score |
| Gy | gray |
| IO(E)RT | intraoperative (electron) radiotherapy |
| LAD | left anterior descending artery |
| LRFS | locoregional free survival |
| OAR | organs-at-risk |
| OS | overall survival |
| PBI | partial-breast irradiation |
| PBT | proton beam therapy |
| PTV | planning target volume |
| RBE | relative biological effectiveness |
| Re-RT | reirradiation |
| RT | radiotherapy |
| WBI | whole-breast irradiation |
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- RKI (Robert-Koch-Institut), Epidemiologischen Maßzahlen für Deutschland, ICD-10 C50. 2020. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2023/kid_2023_c50_brust.pdf?__blob=publicationFile (accessed on 1 March 2025).
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. Available online: http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom (accessed on 1 March 2025).
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [PubMed]
- Salvadori, B.; Marubini, E.; Miceli, R.; Conti, A.R.; Cusumano, F.; Andreola, S.; Zucali, R.; Veronesi, U. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br. J. Surg. 1999, 86, 84–87. [Google Scholar] [CrossRef]
- Krug, D.; Banys-Paluchowski, M.; Brucker, S.Y.; Denkert, C.; Ditsch, N.; Fasching, P.A.; Haidinger, R.; Harbeck, N.; Heil, J.; Huober, J.; et al. Radiotherapy statements of the 18th St. Gallen International Breast Cancer Consensus Conference-a German expert perspective. Strahlenther. Onkol. 2024, 200, 461–467. [Google Scholar] [CrossRef]
- Kolben, T.; Schwarz, T.M.; Goess, C.; Blume, C.; Degenhardt, T.; Engel, J.; Wuerstlein, R.; Ditsch, N.; Harbeck, N.; Kahlert, S. Surgical management of ipsilateral breast tumor recurrence. Int. J. Surg. 2015, 23 Pt A, 141–146. [Google Scholar] [CrossRef]
- Gentilini, O.; Botteri, E.; Veronesi, P.; Sangalli, C.; Del Castillo, A.; Ballardini, B.; Galimberti, V.; Rietjens, M.; Colleoni, M.; Luini, A.; et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: Criteria for selecting the best candidates. Ann. Surg. Oncol. 2012, 19, 3771–3776. [Google Scholar] [CrossRef]
- Alpert, T.E.; Kuerer, H.M.; Arthur, D.W.; Lannin, D.R.; Haffty, B.G. Ipsilateral breast tumor recurrence after breast conservation therapy: Outcomes of salvage mastectomy vs. salvage breast-conserving surgery and prognostic factors for salvage breast preservation. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 845–851. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.M.; Gal, J.; Van Limbergen, E.; Chand, M.E.; Schiappa, R.; Smanyko, V.; Kauer-Domer, D.; Pasquier, D.; Lemanski, C.; Racadot, S.; et al. Salvage Mastectomy Versus Second Conservative Treatment for Second Ipsilateral Breast Tumor Event: A Propensity Score-Matched Cohort Analysis of the GEC-ESTRO Breast Cancer Working Group Database. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 452–461. [Google Scholar] [CrossRef]
- Abdul-Latif, M.; Gal, J.; Schiappa, R.; Rizzi, Y.; Gautier, M.; Hannoun-Levi, J.M. Salvage brachytherapy for second ipsilateral breast tumor event: Relating dosimetric analysis to late side effects. Brachytherapy. 2024, 23, 335–341. [Google Scholar] [CrossRef]
- Thangarajah, F.; Heilmann, J.; Malter, W.; Kunze, S.; Marnitz, S.; Mallmann, P.; Wenz, F.; Sperk, E. Breast conserving surgery in combination with intraoperative radiotherapy after previous external beam therapy: An option to avoid mastectomy. Breast Cancer Res. Treat. 2018, 168, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.; Jadeja, P.; Taback, B.; Horowitz, D.P.; Feldman, S.M.; Ha, R.; Connolly, E.P. Evaluation of Partial Breast Reirradiation with Intraoperative Radiotherapy after Prior Thoracic Radiation: A Single-Institution Report of Outcomes and Toxicity. Front. Oncol. 2017, 7, 75. [Google Scholar] [CrossRef]
- Kraus-Tiefenbacher, U.; Bauer, L.; Scheda, A.; Schoeber, C.; Schaefer, J.; Steil, V.; Wenz, F. Intraoperative radiotherapy (IORT) is an option for patients with localized breast recurrences after previous external-beam radiotherapy. BMC Cancer 2007, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Guenzi, M.; Belgioia, L.; Bonzano, E.; Configliacco, E.; Tornari, E.; Cavagnetto, F.; Bosetti, D.; Fozza, A.; Friedman, D.; et al. Adjuvant intraoperative radiotherapy for selected breast cancers in previously irradiated women: Evidence for excellent feasibility and favorable outcomes. Rep. Pract. Oncol. Radiother. 2017, 22, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.E.; Deutsch, M.; Bloomer, W.D. Salvage radiotherapy for local failures of lumpectomy and breast irradiation. Radiother. Oncol. 1997, 42, 25–29. [Google Scholar] [CrossRef]
- Deutsch, M. Repeat high-dose external beam irradiation for in-breast tumor recurrence after previous lumpectomy and whole breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 687–691. [Google Scholar] [CrossRef]
- Janssen, S.; Rades, D.; Meyer, A.; Fahlbusch, F.B.; Wildfang, I.; Meier, A.; Schild, S.; Christiansen, H.; Henkenberens, C. Local recurrence of breast cancer: Conventionally fractionated partial external beam re-irradiation with curative intention. Strahlenther. Onkol. 2018, 194, 806–814. [Google Scholar] [CrossRef]
- Bazan, J.G.; Wobb, J.L.; Dicostanzo, D.J.; Healy, E.; White, J.R. Re-irradiation of local-regional disease in breast cancer using modern radiation techniques: Preliminary results of tolerability and efficacy. Int. J. Radiat. Oncol. Biol. 2018, 102, 597–598. [Google Scholar] [CrossRef]
- Arthur, D.W.; Winter, K.A.; Kuerer, H.M.; Haffty, B.; Cuttino, L.; Todor, D.A.; Anne, P.R.; Anderson, P.; Woodward, W.A.; McCormick, B.; et al. Effectiveness of Breast-Conserving Surgery and 3-Dimensional Conformal Partial Breast Reirradiation for Recurrence of Breast Cancer in the Ipsilateral Breast: The NRG Oncology/RTOG 1014 Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 75–82. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.M.; Resch, A.; Gal, J.; Kauer-Dorner, D.; Strnad, V.; Niehoff, P.; Loessl, K.; Kovács, G.; Van Limbergen, E.; Polgár, C.; et al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: Multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother. Oncol. 2013, 108, 226–231. [Google Scholar] [CrossRef]
- Veronesi, U.; Orecchia, R.; Maisonneuve, P.; Viale, G.; Rotmensz, N.; Sangalli, C.; Luini, A.; Veronesi, P.; Galimberti, V.; Zurrida, S.; et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013, 14, 1269–1277. [Google Scholar] [CrossRef]
- Vaidya, J.S.; Bulsara, M.; Baum, M.; Wenz, F.; Massarut, S.; Pigorsch, S.; Alvarado, M.; Douek, M.; Saunders, C.; Flyger, H.L.; et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ 2020, 370, m2836. [Google Scholar] [CrossRef]
- Orecchia, R.; Veronesi, U.; Maisonneuve, P.; Galimberti, V.E.; Lazzari, R.; Veronesi, P.; Jereczek-Fossa, B.A.; Cattani, F.; Sangalli, C.; Luini, A.; et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021, 22, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.M.; Jacquemier, J.; Amalric, R.; Brandone, H.; Ayme, Y.; Hans, D.; Bressac, C.; Spitalier, J.M. Is breast conservation after local recurrence feasible? Eur. J. Cancer 1991, 27, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Hannoun-Levi, J.M.; Gal, J.; Polgar, C.; Strnad, V.; Loessl, K.; Polat, B.; Kauer-Domer, D.; Schiappa, R.; Gutierrez, C. Second Conservative Treatment for Local Recurrence Breast Cancer: A GEC-ESTRO Oncological Outcome and Prognostic Factor Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Kauer-Dorner, D.; Pötter, R.; Resch, A.; Handl-Zeller, L.; Kirchheiner, K.; Meyer-Schell, K.; Dörr, W. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: Alternative to mastectomy? Results from a prospective trial. Radiother. Oncol. 2012, 102, 96–101. [Google Scholar] [CrossRef]
- Harms, W.; Krempien, R.; Grehn, C.; Hensley, F.; Berns, C.; Wannenmacher, M.; Debus, J. Rebestrahlung der Thoraxwand bei lokal rezidivierenden Mammakarzinomen [Reirradiation of chest wall local recurrences from breast cancer]. Zentralbl. Gynakol. 2004, 126, 19–23. [Google Scholar] [CrossRef]
- Merino, T.; Tran, W.T.; Czarnota, G.J. Re-irradiation for locally recurrent refractory breast cancer. Oncotarget 2015, 6, 35051–35062. [Google Scholar] [CrossRef]
- Oldenborg, S.; Griesdoorn, V.; van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Zum Vörde Sive Vörding, P.J.; Heymans, M.W.; Kolff, M.W.; Rasch, C.R.; et al. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: Size matters. Radiother. Oncol. 2015, 117, 223–228. [Google Scholar] [CrossRef]
- Wahl, A.O.; Rademaker, A.; Kiel, K.D.; Jones, E.L.; Marks, L.B.; Croog, V.; McCormick, B.M.; Hirsch, A.; Karkar, A.; Motwani, S.B.; et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 477–484. [Google Scholar] [CrossRef]
- Fattahi, S.; Ahmed, S.K.; Park, S.S.; Petersen, I.A.; Shumway, D.A.; Stish, B.J.; Yan, E.S.; Remmes, N.B.; Mutter, R.W.; Corbin, K.S. Reirradiation for Locoregional Recurrent Breast Cancer. Adv. Radiat. Oncol. 2020, 6, 100640. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.C.; Arculeo, S.; Frassoni, S.; Zerella, M.A.; Gerardi, M.A.; Fodor, C.; Veronesi, P.; Galimberti, V.E.; Magnoni, F.; Milovanova, E.; et al. Hypofractionated partial breast re-irradiation in the conservative retreatment of breast cancer local recurrence. Pract. Radiat. Oncol. 2025, 15, 31–47. [Google Scholar] [CrossRef]
- Meixner, E.; Hoeltgen, L.; Sandrini, E.; Erdem, S.; Schlüter, F.; Lau, H.H.; Schramm, O.; Neugebauer, D.; Hoegen-Saßmannshausen, P.; Liermann, J.; et al. External beam photon re-irradiation with curative intent for recurrent or new primary breast cancer: Analysis of dosimetric factors to predict acute and late toxicity. 2025; Under review. [Google Scholar]
- Hannoun-Lévi, J.M.; Savignoni, A.; Féron, J.G.; Malhaire, C.; Ezzili, C.; Brédart, A.; Loap, P.; Kirova, Y. Management of second ipsilateral breast tumor event: An advocacy for a randomized trial. Cancer Radiother. 2024, 28, 188–194. [Google Scholar] [CrossRef]
- Hannoun-Levi, J.M.; Gal, J.; Schiappa, R.; Chand, M.E. 10-Year oncological outcome report after second conservative treatment for ipsilateral breast tumor event. Clin. Transl. Radiat. Oncol. 2022, 38, 71–76. [Google Scholar] [CrossRef]
- Montagne, L.; Gal, J.; Chand, M.E.; Schiappa, R.; Falk, A.T.; Kinj, R.; Gauthier, M.; Hannoun-Levi, J.M. GEC-ESTRO APBI classification as a decision-making tool for the management of 2nd ipsilateral breast tumor event. Breast Cancer Res. Treat. 2019, 176, 149–157. [Google Scholar] [CrossRef]
- Shaitelman, S.F.; Anderson, B.M.; Arthur, D.W.; Bazan, J.G.; Bellon, J.R.; Bradfield, L.; Coles, C.E.; Gerber, N.K.; Kathpal, M.; Kim, L.; et al. Partial Breast Irradiation for Patients with Early-Stage Invasive Breast Cancer or Ductal Carcinoma In Situ: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2024, 14, 112–132. [Google Scholar] [CrossRef] [PubMed]
- Polgár, C.; Van Limbergen, E.; Pötter, R.; Kovács, G.; Polo, A.; Lyczek, J.; Hildebrandt, G.; Niehoff, P.; Guinot, J.L.; Guedea, F.; et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother. Oncol. 2010, 94, 264–273. [Google Scholar] [PubMed]
- Strnad, V.; Krug, D.; Sedlmayer, F.; Piroth, M.D.; Budach, W.; Baumann, R.; Feyer, P.; Duma, M.N.; Haase, W.; Harms, W.; et al. DEGRO practical guideline for partial-breast irradiation. Strahlenther. Onkol. 2020, 196, 749–763. [Google Scholar] [CrossRef]
- Massaccesi, M.; Fontana, A.; Palumbo, I.; Argenone, A.; De Santis, M.C.; Masiello, V.; Pontoriero, A.; Ciabattoni, A. Pattern of practice of re-irradiation for ipsilateral breast tumor recurrence in Italy: A survey by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Clin. Transl. Oncol. 2024, 26, 644–652. [Google Scholar] [CrossRef]
- Hardy-Abeloos, C.; Xiao, J.; Oh, C.; Barbee, D.; Perez, C.A.; Oratz, R.; Schnabel, F.; Axelrod, D.; Guth, A.; Braunstein, L.Z.; et al. Early effectiveness and toxicity outcomes of reirradiation after breast conserving surgery for recurrent or new primary breast cancer. Breast Cancer Res. Treat. 2023, 198, 43–51. [Google Scholar] [CrossRef]
- Freedman, G.M.; Li, T.; Garver, E.; Shillington, K.; Shinkle, B.; Tchou, J.C.; Fayanju, O.M.; Lin, L.; Taunk, N.K. Five-Year Outcomes of a Phase 1/2 Trial of Accelerated Partial Breast Irradiation Using Proton Therapy for Women with Stage 0-IIA Breast Cancer. Adv. Radiat. Oncol. 2023, 9, 101334. [Google Scholar] [CrossRef]
- Buchholz, T.A.; Ali, S.; Hunt, K.K. Multidisciplinary Management of Locoregional Recurrent Breast Cancer. J. Clin. Oncol. 2020, 38, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Mutter, R.W.; Choi, J.I.; Jimenez, R.B.; Kirova, Y.M.; Fagundes, M.; Haffty, B.G.; Amos, R.A.; Bradley, J.A.; Chen, P.Y.; Ding, X.; et al. Proton Therapy for Breast Cancer: A Consensus Statement From the Particle Therapy Cooperative Group Breast Cancer Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Plastaras, J.P.; Berman, A.T.; Freedman, G.M. Special cases for proton beam radiotherapy: Re-irradiation, lymphoma, and breast cancer. Semin. Oncol. 2014, 41, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Vitti, E.T.; Parsons, J.L. The Radiobiological Effects of Proton Beam Therapy: Impact on DNA Damage and Repair. Cancers 2019, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.B.; Hickey, S.; DePauw, N.; Yeap, B.Y.; Batin, E.; Gadd, M.A.; Specht, M.; Isakoff, S.J.; Smith, B.L.; Liao, E.C.; et al. Phase II Study of Proton Beam Radiation Therapy for Patients with Breast Cancer Requiring Regional Nodal Irradiation. J. Clin. Oncol. 2019, 37, 2778–2785. [Google Scholar] [CrossRef]
- Vrieling, C.; Collette, L.; Bartelink, E.; Borger, J.H.; Brenninkmeyer, S.J.; Horiot, J.C.; Pierart, M.; Poortmans, P.M.; Struikmans, H.; Van der Schueren, E.; et al. Validation of the methods of cosmetic assessment after breast-conserving therapy in the EORTC “boost versus no boost” trial. EORTC Radiotherapy and Breast Cancer Cooperative Groups. European Organization for Research and Treatment of Cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 667–676. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Ravdin, P.; Hayes, D.F.; Bates, S.; Fritsche, H., Jr.; Jessup, J.M.; Kemeny, N.; Locker, G.Y.; Mennel, R.G.; Somerfield, M.R.; et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J. Clin. Oncol. 2001, 19, 1865–1878, Erratum in J. Clin. Oncol. 2001, 19, 4185–4188; Erratum in J. Clin. Oncol. 2002, 20, 2213. [Google Scholar] [CrossRef]
- Major, T.; Gutierrez, C.; Guix, B.; Van Limbergen, E.; Strnad, V.; Polgár, C. Recommendations from GEC ESTRO breast cancer working group (II): Target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving open cavity surgery. Radiother. Oncol. 2016, 118, 199–204. [Google Scholar] [CrossRef]
- Strnad, V.; Hannoun-Levi, J.M.; Guinot, J.L.; Lössl, K.; Kauer-Dorner, D.; Resch, A.; Kovács, G.; Major, T.; Van Limbergen, E.; Working Group Breast Cancer of GEC-ESTRO. Recommendations from GEC ESTRO Breast Cancer Working Group (I): Target definition and target delineation for accelerated or boost Partial Breast Irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother. Oncol. 2015, 115, 342–348. [Google Scholar] [CrossRef]
- Choi, J.I.; Khan, A.J.; Powell, S.N.; McCormick, B.; Lozano, A.J.; Del Rosario, G.; Mamary, J.; Liu, H.; Fox, P.; Gillespie, E.; et al. Proton reirradiation for recurrent or new primary breast cancer in the setting of prior breast irradiation. Radiother. Oncol. 2021, 165, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Gabani, P.; Patel, H.; Thomas, M.A.; Bottani, B.; Goddu, S.M.; Straube, W.; Margenthaler, J.A.; Ochoa, L.; Bradley, J.D.; Zoberi, I. Clinical outcomes and toxicity of proton beam radiation therapy for re-irradiation of locally recurrent breast cancer. Clin. Transl. Radiat. Oncol. 2019, 19, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.S.; Niska, J.R.; Girardo, M.E.; Kosiorek, H.E.; McGee, L.A.; Hartsell, W.F.; Larson, G.L.; Tsai, H.K.; Rossi, C.J.; Rosen, L.R.; et al. Proton beam therapy reirradiation for breast cancer: Multi-institutional prospective PCG registry analysis. Breast J. 2019, 25, 1160–1170. [Google Scholar] [CrossRef]
- Sayan, M.; Vergalasova, I.; Jan, I.; Kumar, S.; Chan, N.; Haffty, B.G.; Ohri, N. Toxicities and Locoregional Control After External Beam Chest Wall and/or Regional Lymph Node Re-irradiation for Recurrent Breast Cancer. Anticancer Res. 2022, 42, 93–96. [Google Scholar] [CrossRef]
- LaRiviere, M.J.; Dreyfuss, A.; Taunk, N.K.; Freedman, G.M. Proton Reirradiation for Locoregionally Recurrent Breast Cancer. Adv. Radiat. Oncol. 2021, 6, 100710. [Google Scholar] [CrossRef] [PubMed]
- McGee, L.A.; Iftekaruddin, Z.; Chang, J.H.C.; Gondi, V.; Schmidt, S.; Kaplan, D.; Gans, S.; Pankuch, M.; Hartsell, W.F. Postmastectomy chest wall reirradiation with proton therapy for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E34–E35. [Google Scholar] [CrossRef]
- Choi, J.I.; McCormick, B.; Park, P.; Millar, M.; Walker, K.; Tung, C.C.; Huang, S.; Florio, P.; Chen, C.C.; Lozano, A.; et al. Comparative Evaluation of Proton Therapy and Volumetric Modulated Arc Therapy for Brachial Plexus Sparing in the Comprehensive Reirradiation of High-Risk Recurrent Breast Cancer. Adv. Radiat. Oncol. 2023, 9, 101355. [Google Scholar] [CrossRef] [PubMed]
- Hooning, M.J.; Botma, A.; Aleman, B.M.; Baaijens, M.H.; Bartelink, H.; Klijn, J.G.; Taylor, C.W.; van Leeuwen, F.E. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef]
- Prosnitz, R.G.; Hubbs, J.L.; Evans, E.S.; Zhou, S.M.; Yu, X.; Blazing, M.A.; Hollis, D.R.; Tisch, A.; Wong, T.Z.; Borges-Neto, S.; et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: Analysis of data 3 to 6 years after treatment. Cancer 2007, 110, 1840–1850. [Google Scholar] [CrossRef]
- Paszat, L.F.; Vallis, K.A.; Benk, V.M.; Groome, P.A.; Mackillop, W.J.; Wielgosz, A. A population-based case-cohort study of the risk of myocardial infarction following radiation therapy for breast cancer. Radiother. Oncol. 2007, 82, 294–300. [Google Scholar] [CrossRef]
- Harris, E.E.; Correa, C.; Hwang, W.T.; Liao, J.; Litt, H.I.; Ferrari, V.A.; Solin, L.J. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J. Clin. Oncol. 2006, 24, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Correa, C.R.; Litt, H.I.; Hwang, W.T.; Ferrari, V.A.; Solin, L.J.; Harris, E.E. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J. Clin. Oncol. 2007, 25, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther. Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef]
- Gagliardi, G.; Constine, L.S.; Moiseenko, V.; Correa, C.; Pierce, L.J.; Allen, A.M.; Marks, L.B. Radiation dose-volume effects in the heart. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76 (Suppl. S3), S77–S85. [Google Scholar] [CrossRef]
- Doyle, J.J.; Neugut, A.I.; Jacobson, J.S.; Grann, V.R.; Hershman, D.L. Chemotherapy and cardiotoxicity in older breast cancer patients: A population-based study. J. Clin. Oncol. 2005, 23, 8597–8605. [Google Scholar] [CrossRef]
- Gyenes, G.; Fornander, T.; Carlens, P.; Rutqvist, L.E. Morbidity of ischemic heart disease in early breast cancer 15–20 years after adjuvant radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 1235–1241. [Google Scholar] [CrossRef]
- Palumbo, B.; Siepi, D.; Lupattelli, G.; Sinzinger, H.; Fiorucci, G.; Anniboletti, P.F.; Latini, R.A.; Mannarino, E.; Palumbo, R. Usefulness of brain natriuretic peptide levels to discriminate patients with stable angina pectoris without and with electrocardiographic myocardial ischemia and patients with healed myocardial infarction. Am. J. Cardiol. 2004, 94, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Tang, W.H.; Francis, G.S.; Morrow, D.A.; Newby, L.K.; Cannon, C.P.; Jesse, R.L.; Storrow, A.B.; Christenson, R.H.; Apple, F.S.; Ravkilde, J.; et al. National Academy of Clinical Biochemistry Laboratory Medicine practice guidelines: Clinical utilization of cardiac biomarker testing in heart failure. Circulation 2007, 116, e99–e109. [Google Scholar] [CrossRef]
- Palumbo, I.; Palumbo, B.; Fravolini, M.L.; Marcantonini, M.; Perrucci, E.; Latini, M.E.; Falcinelli, L.; Sabalich, I.; Tranfaglia, C.; Schillaci, G.; et al. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: A prospective study. Breast 2016, 25, 45–50. [Google Scholar] [CrossRef]
- D’Errico, M.P.; Petruzzelli, M.F.; Gianicolo, E.A.; Grimaldi, L.; Loliva, F.; Tramacere, F.; Andreassi, M.G.; Pili, G.; Picano, E.; Portaluri, M. Kinetics of B-type natriuretic peptide plasma levels in patients with left-sided breast cancer treated with radiation therapy: Results after one-year follow-up. Int. J. Radiat. Biol. 2015, 91, 804–809. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, M.P.; Grimaldi, L.; Petruzzelli, M.F.; Gianicolo, E.A.; Tramacere, F.; Monetti, A.; Placella, R.; Pili, G.; Andreassi, M.G.; Sicari, R.; et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int. J. Radiat Oncol. Biol. Phys. 2012, 82, e239–e246. [Google Scholar] [CrossRef] [PubMed]

| Inclusion criteria |
|---|
|
|
|
|
|
|
|
|
|
|
| Exclusion criteria |
|
| Organ at Risk/Target | Dose Constraints and Prescription |
|---|---|
| PTV_evaluate | V95% ≥ 95% (38.05 Gy(RBE)) |
| Heart | Dmean < 2 Gy(RBE), D0.01 cm3 < 3 Gy(RBE) |
| LAD | Dmean < 2 Gy(RBE), D0.03 cm3 < 8 Gy(RBE) |
| Ipsilateral lung | V20 Gy < 20%, Dmean < 10 Gy(RBE) |
| Skin (3 mm beneath the body surface) | D1 cm3~90–95% |
| Ribs plane/chest wall | D0.01 cm3 ≤ 95% |
| Spinal cord | D0.01 cm3 < 5 Gy(RBE) |
| Contralateral breast, contralateral lung, stomach, liver | ALARA * |
| Author/Citation | Title | Trial Design/ re-RT Target | Time Interval/ Prior RT Dose | Median Dose Proton re-RT/ Cumulative Dose | Toxicity | Oncological Outcome | |
|---|---|---|---|---|---|---|---|
| 1 | Fattahi et al. [33] | Reirradiation for Locoregional Recurrent Breast Cancer | Retrospective, n = 20 PBT (n = 52 photons + electrons). Breast/chest wall/nodes/± regional nodes. | 6.1 years (73 months). Initial RT: median 60 Gy (range: 50–60.4 Gy). | median 50 Gy(RBE) (1.8–2 Gy) + n = 5 additional boost (10 Gy(RBE)) + n = 2 integrated boost of 5.6/6.25 Gy(RBE) or hypofractionation 40.05 Gy(RBE) + n = 1 additional boost (10 Gy(RBE)) cumulative median EQD2: 103.54 Gy | Overall grade 3 toxicity: 13%. Skin grade 3: 13% acute/3% late. | Median follow-up: 22 months. 2-years LRFS (curative): 93.1%. OS: 76.8%. Distant-metastases-free survival: 59.0%. |
| 2 | Choi et al. [54] | Proton reirradiation for recurrent or new primary breast cancer in the setting of prior breast irradiation | Retrospective, n = 46. Breast/partial breast/chest wall ± implant/nodes ± regional nodes. | 7.1 years (85.5 months) (range: 5–360 months). Initial RT: median 60 Gy (range: 45–66 Gy). | median 50.4 Gy(RBE) (range: 40–66.6 Gy(RBE)) cumulative median 110 Gy(RBE) (96.6–169.4) | Skin grade 2/3: 56.5%/30.4%. Esophagitis grade 2: 8.7%. Late implant contracture 6.7%. Pain grade 3: 7.7%. | Median follow-up: 21 months. No local recurrences. Distant recurrence: 17%. 2-/3-year DMFS: 92.0% and 60.0%. 2-/3-year OS: 93.6%/88.1%. |
| 3 | Gabani et al. [55] | Clinical outcomes and toxicity of proton beam radiation therapy for reirradiation of locally recurrent breast cancer | Retrospective, n = 16. Chest wall (± implant) ± regional nodes. Concurrent hyperthermia (62.5%). | 10.2 years (range: 0.7–20.2). Initial RT: median. 50 Gy (range: 45.0–50.4) + Boost 10 Gy. | median 50.4 Gy(RBE) (range: 41.4–50.4 Gy(RBE)) + n = 3 boost 10 Gy(RBE) (range: 10–16 Gy(RBE)) | Skin grade 2/3/4: 37.5%/25.0%/6.2%. Fibrosis grade 2/3/4: 12.5%/12.5%/6.2%. Pneumonitis 12.5%. Teleangiectasia 25.0%. Rib fracture 6.2%. Brachial plexopathy 6.2%. Lymphedema 6.2%. | Median follow-up: 18.7 months. No local failures. |
| 4 | Sayan et al. [57] | Toxicities and Locoregional Control After External Beam Chest Wall and/or Regional Lymph Node Reirradiation for Recurrent Breast Cancer | Retrospective, n = 12 (n = 15, but only 80% PBT). Breast/chest wall ± regional nodes. | 5.7 years (68.3 months) (range: 7.8–245 months). Initial RT: median 50 Gy (range: 33.5–50.4 Gy). | median 45 Gy(RBE) (range: 42.3–50.4 Gy(RBE)) | Skin grade 2/3: 80%/7%. Pain grade 2/3: 20%/13%. Lymphedema 13%. Fatigue grade 2: 40%. Brachial plexopathy: 0%. | Median follow-up: 14 months (range: 1.0–90.5). Locoregional recurrence: 13%. Distant metastases 33%. |
| 5. | Thorpe et al. [56] | Proton beam therapy reirradiation for breast cancer: Multi-institutional prospective PCG registry analysis | Prospective registry, n = 50. Breast/chest wall ± regional nodes. | 8.7 years (103.8 months) (range: 5.5–430.8 months). Initial RT: median 60 Gy (range: 10–96.7) (only n = 43 (86%) had prior RT indication for breast cancer). | median 55.1 Gy(RBE) (45.1–76.3 Gy(RBE)) cumulative median 110.6 Gy(RBE) (range: 70.6–156.8 Gy(RBE)) | Overall grade 3: 16%. Acute/late pain grade 3: 10%/4%. Acute/late skin grade 3: 2%/4%. Acute lymphedema grade 3: 2%. Late wound infection: 2%. | Median follow-up: 12.7 months. 1-year LRFS: 93%. 1-year OS: 97%. |
| 6. | LaRiviere et al. [58] | Proton Reirradiation for Locoregionally Recurrent Breast Cancer | Retrospective, n = 27. Whole breast/chest wall ± regional nodes. | 9.7 years (range: 0.9–37.6). Initial RT: median 46.8 Gy (20–61 Gy). | 51 Gy(RBE) (1.5 Gy twice daily) proton double scattering/pencil beam scanning | Acute skin grade 3: 7.4%. Acute pain grade 3: 7.4%. Late skin grade 3/4: 3.7%/3.7%. Late grade 2 rip fractures: 22.2%. Late grade 2 brachial plexopathy: 3.7%. | Median follow-up: 16.6 months. 1-year LRFS: 78.5%. 1-year OS: 78.5%. |
| 7. | McGee et al. [59] | Postmastectomy Chest Wall Reirradiation With Proton Therapy for Breast Cancer | Abstract only, n = 22. Chest wall + regional nodes. | 12 years (range: 3–36 years). Initial RT: median 60 Gy (10–70 Gy). | median 50.51 Gy(RBE) (range: 45.1–76.3 Gy(RBE)) uniform scanning (n = 19) or pencil beam scanning (n = 3) | Acute skin grade 2/3: 68.1%/9.1%. Acute grade 2 esophagitis: 31.8%. Acute grade 2 lymphedema: 9.1%. Acute grade 2/3 pain: 9.1%/4.5%. Grade 3 pneumonitis: 4.5%. Rip fracture: 13.6%. Fibrosis grade 3: 9.1%. | Median follow-up: 15 months. 0% local recurrences. 17 months: 1/22 patients with distant metastases. |
| 8. | Choi et al. [60] | Comparative Evaluation of Proton Therapy and Volumetric Modulated Arc Therapy for Brachial Plexus Sparing in the Comprehensive Reirradiation of High-Risk Recurrent Breast Cancer | Retrospective, n = 10. Chest wall ± regional nodes. | 48 months (range: 12–276). Initial RT: median 50.4 Gy (range, 42.6–60.0). | 50.4 Gy(RBE) (45.0–64.4), pencil beam scanning cumulative 102.4 Gy(RBE) (range: 95.0–120.0 Gy(RBE)) | brachial plexopathy grade 1: 20%. | Median follow-up: 15 months: 70% alive. 18 months: 2 local recurrences. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meixner, E.; Harrabi, S.; Seidensaal, K.; Koczur, B.; Tessonnier, T.; Lentz-Hommertgen, A.; Hoeltgen, L.; Hoegen-Saßmannshausen, P.; Weykamp, F.; Liermann, J.; et al. Partial Breast Reirradiation for Breast Cancer Recurrences After Repeat Breast-Conserving Surgery with Proton Beam Therapy: The Prospective BREAST Trial (NCT06954623). J. Clin. Med. 2025, 14, 3416. https://doi.org/10.3390/jcm14103416
Meixner E, Harrabi S, Seidensaal K, Koczur B, Tessonnier T, Lentz-Hommertgen A, Hoeltgen L, Hoegen-Saßmannshausen P, Weykamp F, Liermann J, et al. Partial Breast Reirradiation for Breast Cancer Recurrences After Repeat Breast-Conserving Surgery with Proton Beam Therapy: The Prospective BREAST Trial (NCT06954623). Journal of Clinical Medicine. 2025; 14(10):3416. https://doi.org/10.3390/jcm14103416
Chicago/Turabian StyleMeixner, Eva, Semi Harrabi, Katharina Seidensaal, Beata Koczur, Thomas Tessonnier, Adriane Lentz-Hommertgen, Line Hoeltgen, Philipp Hoegen-Saßmannshausen, Fabian Weykamp, Jakob Liermann, and et al. 2025. "Partial Breast Reirradiation for Breast Cancer Recurrences After Repeat Breast-Conserving Surgery with Proton Beam Therapy: The Prospective BREAST Trial (NCT06954623)" Journal of Clinical Medicine 14, no. 10: 3416. https://doi.org/10.3390/jcm14103416
APA StyleMeixner, E., Harrabi, S., Seidensaal, K., Koczur, B., Tessonnier, T., Lentz-Hommertgen, A., Hoeltgen, L., Hoegen-Saßmannshausen, P., Weykamp, F., Liermann, J., Hörner-Rieber, J., & Debus, J. (2025). Partial Breast Reirradiation for Breast Cancer Recurrences After Repeat Breast-Conserving Surgery with Proton Beam Therapy: The Prospective BREAST Trial (NCT06954623). Journal of Clinical Medicine, 14(10), 3416. https://doi.org/10.3390/jcm14103416







