1. Background
According to data from the International Diabetes Federation (IDF), the estimated number of people with diabetes aged 20–79 was 10.5% (536 million) in 2021. Projections for 2045 estimated an increase of this number to 12.2% [
1]. Diabetic foot syndrome (DFS) is one of the most serious long-term complications of diabetes mellitus. It is characterized by the presence of neuropathy, ischemia, and an increased susceptibility to infections [
2]. DFS is defined as an infection, ulceration, or destruction of the deep tissues of the foot, including bones, caused by damage to the peripheral nerves and/or blood vessels of the foot of varying severity [
3,
4]. A meta-analysis has shown that the overall prevalence of DFS was 9%, while the incidence was 4% [
5]. It was estimated that over half of diabetic foot ulcers (DFU) became infected, and about one-fifth of individuals with DFU might require lower limb amputation [
6]. The risk of death was found to be over two-fold in people with diabetes and DFU compared to those without ulcers [
7]. Global statistics have indicated that the risk of developing foot ulcers in the lifetime of a person with diabetes ranged from 10% to 25%. A quarter of the patients were found to experience foot complications [
2].
DFS prophylaxis is undoubtedly one of the most important aspects in the course of diabetes and includes the maintenance of metabolic balance by the patient and monitoring lipid parameters [
3,
8,
9]. Metabolic balance criteria are also determined by self-blood glucose monitoring (SBGM), glycated hemoglobin (HbA1c), or time in range in patients using continuous glucose monitoring (CGM) [
9]. The goals of balancing carbohydrate metabolism focus on adjusting HbA1c concentrations to the specific needs of different groups of patients. As regards the management of lipid metabolism, it is important to adjust the concentrations of LDL-C, non-HDL, HDL-C, and triglycerides to reduce cardiovascular risk, which is particularly high in people with diabetes [
3]. Good metabolic control of diabetes requires the patients to be consciously involved in the treatment process and to regularly monitor their own health [
3,
10].
In addition to biomedical factors, psychosocial aspects, behavioral skills, and traits play an important role in the course of DFS [
11,
12]. The occurrence of DFS significantly affects the mobility of the patients, limiting their ability to move and perform everyday activities. This may contribute to social isolation, depression, and a decrease in the quality of life [
13,
14,
15]. Ozyalcin and Sanlier found correlations between the acceptance of diabetes, depression, the quality of life, and emotional stress [
16]. It was confirmed that, with the decreasing acceptance of the disease, the number of manifestations on the feet increased in people with type 2 diabetes treated in primary care facilities [
17]. According to Akça Doğan et al., the degree of disease acceptance was related to the risk of developing the diabetic foot and the levels of fasting glucose and glycated hemoglobin in diabetic patients [
18]. A significant relationship was demonstrated between the acceptance of diabetes and health-related behaviors undertaken by the patients [
19].
Social support from the family, friends, or available support networks were indicated in the literature as the main factors facilitating the patients’ self-treatment for diabetes [
12,
20]. Social support may be understood to be the resources available to people in difficult or stressful circumstances. In medical and health sciences, it is seen to be the available support for the individual through social ties with other people, social groups, or the community. Social support is, quite broadly, any resource that flows between people. Social support may be understood to be any resource that flows through and from social relationships [
21]. It was confirmed that the lack of active participation of the family in the self-control of a patient’s glycemia was observed in as many as 75.2% of diabetics, which directly translated into the patients’ mental and physical condition [
22]. One study showed that only 1.8% of patients sought medical care in hospitals within 24 h of the onset of DFS-related symptoms. Patients with low social support and negative perceptions of the disease reported to medical facilities significantly later [
23]. Research results showed that insufficient support might lead to poorer glycemic control, which increased the risk of complications [
24,
25]. People with diabetes who experienced strong social support were more likely to have better mental health, lower levels of stress, depression, and anxiety, as well as greater life satisfaction [
26,
27]. In addition to family support, social support is also important, which may include access to support groups, psychological counseling, as well as health education that helps the patient understand the disease and learn to cope with it [
15,
28,
29]. The literature review has showed that the psychosocial aspects of the functioning of people with DFS and their relationship with the metabolic control of the disease were the subject of few studies for this group of patients. A qualitative study was conducted to examine the socio-cultural aspects of diabetic foot syndrome in a group of Italian patients hospitalized in a vascular surgery ward. Several key topics were identified that should be addressed when providing care to the patient, including the patient’s awareness of the diabetic foot, the life of the patient with the diabetic foot (the patient’s role in the family, body image, emotional state), the patient’s capacity of performing work, and the costs associated with the occurrence of the diabetic foot, barriers related to healthcare and ways of managing the diabetic foot at home, and implementing alternative medicine [
30].
5. Discussion
The assessment of the degree of the metabolic control of the disease based on the indices included in the 2006 PDA guidelines was performed in a group of 1045 patients with type 2 diabetes. It showed that the concentration of triglycerides was the most frequently met compensation criterion (50.4% of patients), while the HbA1c value was met the least frequently (12.1% of patients). The authors also analyzed the compliance of the results with the 2011 PDA guidelines, where 66.9% of the patients met the criterion for the diastolic blood pressure, and 18.7% achieved a satisfactory concentration of the LDL cholesterol fraction [
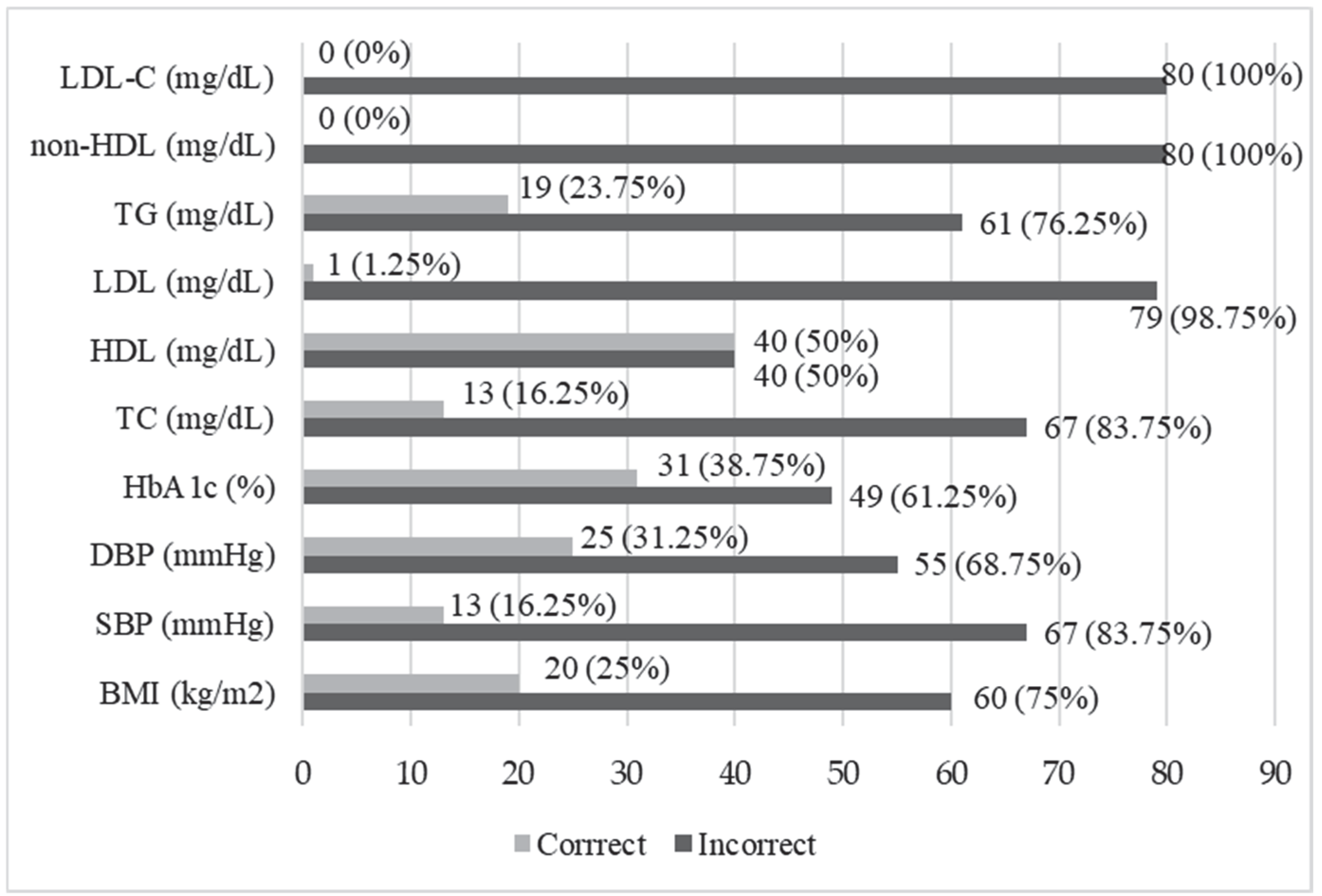
36]. The study showed that TG values were normal only in 23.75% of the patients with DFS, while no patients achieved normal non-HDL or LDL-C values. Abnormal LDL, DBP, and TC values occurred in about 90% of the subjects. The recommended HbA1c concentration was achieved by 38.75% of the patients. The results of this study indicated that there was significant room for improvement in the context of comprehensive metabolic control, and the results of systolic blood pressure and lipid level control showed much higher values than in patients with type 2 diabetes, taking account of the results published by Łagowska-Batyra et al. [
36]. Intensive glycemic control (HbA1c 6.0–7.5%) was associated with a significant reduction in the risk of amputation [
37]. Achieving such a goal requires continuous monitoring and the adaptation of treatment strategies in order to better adapt to the needs of the individual patients. This may significantly contribute to improving their quality of life and reducing the risk of developing and deteriorating the complications of diabetes, including DFS. In a 2018 study conducted in type 2 diabetics in a diabetes clinic, elevated levels of TC were confirmed in 60.7% of the patients, LDL fraction in 79.2%, DBP in 63.9%, and elevated HbA1c values in 44.8% [
10]. In a study conducted in China, significant differences in the ratios between total cholesterol/high-density lipoprotein cholesterol and low-density lipoprotein cholesterol/high-density lipoprotein cholesterol were observed in patients with type 2 diabetes compared to patients with type 2 diabetes and DFS. The study also showed that the patients with type 2 diabetes and DFS were characterized by poorer glycemic control than the patients with type 2 diabetes [
38]. A study by Ardelean et al. [
39] showed that the end-stage disease might be indicated by lower HDL-C levels and better lipid profile in the patients with type 2 diabetes and infected diabetic foot ulcers compared to those patients with type 2 diabetes. Notably, the study included patients hospitalized in the vascular surgery department, and a high percentage of them had already undergone amputations in the lower limb or had been hospitalized due to a poorly healing wound in the lower limb.
Some authors have indicated a relationship between psychosocial factors and diabetes control [
40]. Therefore, the aim of this study was to assess social support and acceptance of the disease in the patients with DFS and their relationship with metabolic control of the disease. This study showed poor acceptance of diabetes in patients with DFS. The average score obtained in this study (18.40) was lower than in the group of patients hospitalized with type 2 diabetes, excluding those with DFS, or patients with type 2 diabetes in primary care [
16,
17,
41,
42]. The average AIS score was 27.65 for hospitalized patients after lower limb amputation due to DFS, confirming low disease acceptance [
43]. In this study, 41.24% of the patients had their lower limb amputated. The AIS was used to assess disease acceptance in the patients with DFS after lower limb amputation in two studies in Poland. The first one confirmed that the level of acceptance of the disease was lower in individuals experiencing greater pain, and the second one confirmed that it was higher in those with a better quality of life [
43,
44].
The study did not confirm a significant association between disease acceptance and metabolic control scores achieved by the patients, except for higher TG values in the patients with lower acceptance levels. Also, a study by Rusin-Pawełek et al. [
41] did not confirm the relationship between diabetes control indices, such as BMI, HbA1c concentration and the degree of disease acceptance. Akça Doğan et al. reported that higher acceptance of the disease in patients with type 2 diabetes was correlated with lower fasting glucose and HbA1c concentrations, and a lower risk of DFS [
18].
The overall level of social support received in the studied group of patients with DFS was moderate and varied depending on the area of self-care for diabetes. The patients received the highest support in terms of cigarette smoking and glycemic self-control, while the lowest in the case of foot care. Although the S4-MAD questionnaire is a tool designed to measure social support in the self-care of middle-aged (30–60 years) people with type 2 diabetes, in this study it was first used to assess support in the group of people with type 1 and 2 diabetes, with diabetic foot syndrome and over 60 years of age. Therefore, the present results may only be applied to those obtained in patients with type 2 diabetes. Similar findings were published by Ciemińska and Kobos [
35] regarding a group of outpatients with type 2 diabetes. The data showed that diabetic foot self-care behaviors were at a moderate level [
29], and treatment failure might be associated with patient non-compliance. Effective treatment for diabetes depends on the ability and willingness of the patients to adhere to treatment and practice self-care behaviors, and this may be related to the level of acceptance of the disease [
17]. Taking account of the fact that this study included patients with already diagnosed DFS, and that many of them had undergone amputations of a lower limb, the social support received from relatives or medical staff may have been insufficient. This may confirm that those persons in the patients’ environment should be more involved than before by medical staff in diabetes education. Moreover, the awareness of the importance of supporting a close person in regular foot inspection and care should be increased. The availability of a social support network should be analyzed with the patients in clinical practice and developed with the use of various sources of support. In a study by Werfalli et al. [
44,
45], 75% of the people with type 2 diabetes over 60 years of age believed that their family supported them in complying with all aspects of self-care management. Low social support was recorded in 23.2% of the participants. Greater social support received by the patients was associated with greater self-care, as shown by Ampofo et al. [
46]. The mean S4-MAD score obtained in the study by Ampofo et al. was 54.27 ± 16.37. Notably, the research was carried out in culturally different conditions, where the direct involvement of family members in caring for the sick or elderly might be greater than in European countries.
The analysis of the results of this study showed that the level of social support received did not significantly correlate with the metabolic control indices of diabetes in the patients with DFS, which may indicate the lack of a direct relationship between social support and those indices. This study did not confirm the relationship between support regarding the smoking subscale and metabolic control of diabetes in people with DFS. However, according to the literature, cigarette smoking and glycemic control are important factors in the formation and progression of lesions in the course of DFS [
2,
5]. Mohebi et al. [
47] demonstrated an association between better social support and self-care behaviors in patients with type 2 diabetes. Patients with higher HbA1c levels experienced lower levels of social support. However, the finding was statistically insignificant.
The lack of psychosocial support was commonly indicated by diabetic patients [
48]. Scientific data confirmed that support was a social factor that affected glycemic control to varying degrees in people with diabetes [
20,
39,
49,
50,
51,
52,
53]. Lu et al. reported a tendency to higher levels of blood glucose and HbA1c, TG, TC, low- and high-density lipoproteins in middle-aged men with diabetes and experiencing greater social isolation [
54]. According to Ozturk et al., as the social support levels reported by the patients increased, self-care behaviors also increased as regards diet, physical activity, glucose level monitoring, foot care, and the total score of diabetes self-care activities scale [
55]. As regards the primary care of the patients with uncontrolled type 2 diabetes, higher HbA1c concentrations were associated with reduced social bonds [
56]. However, there are conflicting study results indicating no association between social support and HbA1c levels and high social support contributing to poor glycemic control [
40,
57]. Some authors have claimed that higher social support was associated with higher TC, LDL, and TG levels [
55]. Another study confirmed that social support was a predictor of physical activity. The chances of minimal physical activity in the patients increased as social support grew [
58].
Higher social support received by the DFS patients was associated with greater acceptance of the disease. One study, including people with type 2 diabetes (without the diagnosis of DFS), showed that family support had a positive effect on disease acceptance, metabolic control and adherence [
42].
Costa et al. emphasized the need to integrate scientific data from social sciences into all aspects of the care of patients with DFS (biomedical, behavioral, social, and cultural aspects). The integrated model proposed by the authors is based on the relationship between biomedical aspects and social sciences, in order to improve clinical outcomes and satisfaction in the patients with DFS. This requires the involvement of not only healthcare professionals but psychologists, sociologists, or anthropologists in the care of patients [
30].