Abstract
Cardiac tumors, though rare, present significant diagnostic and therapeutic challenges due to their heterogeneous nature and anatomical complexity. This narrative review synthesizes current evidence on prevalence, diagnostic modalities, and management strategies for primary and metastatic cardiac tumors. Echocardiography, cardiac MRI, and CT remain cornerstone imaging tools for differentiating tumors from non-neoplastic masses, while advances in PET/CT and tissue characterization techniques refine staging and treatment planning. Surgical resection with clear margins (R0) is critical for resectable tumors, particularly benign myxomas, though malignant tumors like sarcomas require multimodal approaches combining surgery, radiotherapy, and systemic therapies. Emerging strategies such as heart autotransplantation and staged resections offer promise for complex cases, while oligometastatic disease management highlights the role of stereotactic radiotherapy and immunotherapy. Key challenges include standardizing resection margins, optimizing neoadjuvant therapies, and addressing high recurrence rates in malignancies. Future directions emphasize integrating AI-driven imaging analysis, molecular biomarkers, and genomic profiling to personalize therapies, alongside global registries to enhance data on rare tumors. Equitable access to advanced diagnostics and multidisciplinary collaboration are essential to improve outcomes. This review underscores the need for standardized guidelines, technological innovation, and patient-centered research to address gaps in cardiac oncology.
1. Introduction
Cardiac tumors are rare, with an incidence of approximately 0.03%, making the available studies about their management relatively limited, particularly for malignant tumors [1]. Cardiac masses encompass a broad spectrum of lesions, including neoplastic masses (primary tumors, both benign and malignant, and metastatic tumors) and non-neoplastic masses (such as thrombi, vegetations, hamartomas, and calcified amorphous tumors) [2]. According to the 2021 WHO classification of cardiac tumors, cardiac masses should be carefully distinguished into pseudotumors (non-neoplastic) and true neoplasms (benign or malignant) [3].
Among primary cardiac tumors, 75% are benign and 25% are malignant [1,4]. Atrial myxoma remains the most common primary cardiac tumor in adults, whereas rhabdomyosarcoma is the most frequent in pediatric populations [1,2]. It is important to emphasize that cardiac metastases are significantly more prevalent than primary cardiac tumors, occurring in up to 10% of patients with advanced cancers [3]. The most common cancer to metastasize to the heart is lung (37%), hematological malignancies (20%), breast (7%), and esophageal cancer (6%) [2].
Despite the rarity of cardiac tumors, several meta-analyses have attempted to synthesize available evidence on their prevalence and outcomes. A recent comprehensive meta-analysis including data from 74 studies and 8849 patients provided updated insights into tumor types and mortality trends [5]. Earlier meta-analyses focused on narrower topics, such as coronary disease in myxoma patients [6] and pediatric tumor mortality [7], but showed wide variation due to differences in study populations and histologic distributions. Comparative details are presented in Supplementary Table S1 and illustrated in Figure 1.
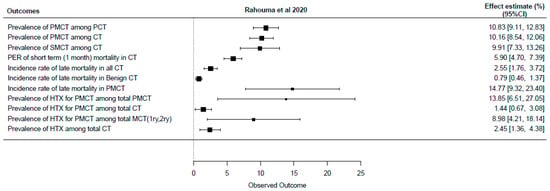
Figure 1.
Forest plot of most recently published meta-analysis (CT: cardiac tumor, HTX: heart transplantation, PER: pooled event rate, PMCT: primary malignant cardiac tumor, SMCT: secondary malignant cardiac tumor) [5].
This narrative review aims to synthesize the current evidence on the prevalence, diagnosis, management strategies, and future directions for both primary and secondary cardiac tumors. The review is organized into sections on diagnostic approaches (clinical, imaging, and biopsy), management of benign and malignant tumors, prognosis, and future innovations in the field.
2. Diagnosis
The diagnosis of cardiac tumors relies on a combination of clinical evaluation, imaging techniques, and, when necessary, histological confirmation through biopsy.
2.1. Clinical Manifestations and Laboratory Correlations
The clinical presentation of cardiac tumors varies significantly between benign and malignant lesions. Benign tumors often remain asymptomatic or are discovered incidentally, while malignant tumors are more likely to present with significant symptoms such as dyspnea, chest pain, pulmonary embolism, cardiac tamponade, or systemic embolization [8].
Electrocardiographic (ECG) findings can provide additional diagnostic clues. A normal ECG is more commonly associated with benign tumors. In contrast, red flags such as tachycardia, low voltages, bradyarrhythmias, right axis deviation, or ischemic-like repolarization changes are more suggestive of malignant cardiac tumors [9].
Laboratory testing may support diagnosis in specific contexts. For example, elevated inflammatory markers and bacteremia in patients with prosthetic valves suggest infective endocarditis, while certain cardiac tumors in children, such as rhabdomyomas, may be associated with genetic syndromes like tuberous sclerosis complex (manifested by ash-leaf spots, facial angiofibromas, and cortical tubers) [10].
In patients with known primary cancers and widespread metastatic disease, a cardiac mass is often presumed to be metastatic, especially when consistent with systemic disease burden [3,11,12].
2.2. Non-Invasive Imaging Assessment
Transthoracic echocardiography (TTE) is typically the first-line imaging modality due to its wide availability, cost-effectiveness, and utility in hemodynamic assessment [8]. Transesophageal echocardiography (TEE) provides superior resolution, especially for characterizing valvular lesions and left atrial tumors [8]. In addition to anatomic localization, echocardiography may offer valuable clues regarding the likelihood of malignancy. Certain echocardiographic parameters—such as mass size, mobility, infiltration of surrounding structures, and irregular margins—can serve as markers of malignancy [12].
One recently validated tool, the Diagnostic Echocardiographic Mass (DEM) score, combines several of these echocardiographic features into a practical scoring algorithm to help differentiate benign from malignant cardiac tumors. A DEM score > 6 points has demonstrated strong diagnostic performance in suggesting malignancy and can help guide further imaging and treatment strategies [12,13]. The individual components and scoring criteria of the DEM score are summarized in Supplementary Table S2.
Cardiac magnetic resonance imaging (CMR) is considered the gold standard for tissue characterization of cardiac masses. CMR offers multiparametric assessment, including T1 and T2 signal characteristics, perfusion, and late gadolinium enhancement, allowing distinction between benign and malignant lesions with high diagnostic accuracy. Paolisso et al. reported near-perfect performance (AUC 0.976) using a multiparametric CMR approach [8,12,13].
Computed tomography (CT) is a valuable alternative for patients unable to undergo MRI and provides excellent spatial resolution, especially for evaluating the extent of mass infiltration and the presence of calcification [14]. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is particularly useful for detecting metabolically active lesions and for staging, especially in cases of suspected metastases [10,15].
A multimodal imaging strategy, tailored to clinical presentation, patient-specific contraindications, and institutional resources, is often essential for accurate diagnosis [10,15,16]. Recent expert guidelines advocate for an integrated approach combining echocardiography, CMR, CT, and PET to optimize diagnostic confidence and clinical decision-making [16,17,18,19] (Table 1).

Table 1.
Different characteristics of some cardiac tumors [3,20,21].
2.3. Role of Biopsy in Cardiac Tumors
While non-invasive imaging modalities such as echocardiography, cardiac MRI, and CT often provide sufficient information for diagnosis and treatment planning, biopsy remains the definitive method for histologic confirmation in cases where malignancy is suspected or when systemic therapy depends on accurate tumor classification. The decision to pursue biopsy must balance the potential diagnostic value against the procedural risks, particularly in anatomically complex or high-risk cardiac locations [22].
Biopsy may be omitted when there is a high clinical suspicion of non-neoplastic etiologies such as thrombus or infective endocarditis, especially when the modified Duke criteria are fulfilled [23]. Similarly, benign tumors like myxomas can often be diagnosed confidently based on imaging and clinical features alone. Conversely, when malignancy is suspected, tissue diagnosis becomes essential to guide oncologic planning, including the use of neoadjuvant chemotherapy or the decision to avoid unnecessary surgery in cases such as cardiac lymphoma.
When biopsy is indicated, the choice of technique depends on tumor location and patient factors. For right-sided intracavitary masses, endomyocardial biopsy is commonly performed via a percutaneous transvenous approach through the internal jugular or femoral vein [24]. Left-sided or extracavitary lesions that are inaccessible via percutaneous routes may require a surgical biopsy, typically performed through a mini-thoracotomy or median sternotomy, and often under cardiopulmonary bypass to ensure adequate exposure and safety [25,26]. In many cases, especially when resection is already planned, biopsy is performed intraoperatively at the time of tumor excision, either as a frozen section to guide surgical margins or for definitive histopathology [27].
The risks of cardiac biopsy include bleeding, cardiac tamponade, arrhythmias, and embolization, with higher risk associated with tumors located near coronary vessels, the left atrium, or other structurally sensitive areas. Multidisciplinary evaluation is essential to determine whether biopsy is warranted and, if so, to select the safest and most effective approach for obtaining diagnostic tissue [22]. Guidelines for diagnosis of cardiac masses and different characteristics are shown in Figure 2, Table 2, and Supplementary Figures S1–S3.
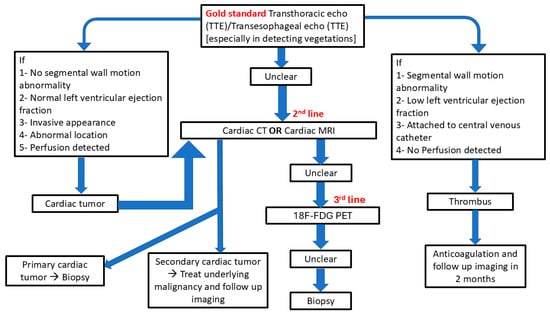
Figure 2.
Guidelines for diagnosis of cardiac masses.

Table 2.
Resection and Reconstruction in cardiac tumors.
3. Management of Benign Cardiac Tumors
Surgical excision remains the cornerstone of treatment for most benign cardiac tumors. Complete resection is usually curative and associated with excellent long-term outcomes [4,28]. Serial echocardiographic follow-up is recommended postoperatively to monitor for recurrence [4,28].
3.1. Surgical Techniques
The approach to tumor excision depends largely on the tumor location. Left atrial tumors are typically excised through a left atriotomy or superior transseptal approach, whereas right atrial tumors are accessed via right atriotomy [29]. In cases involving cardiac valves, primary repair or replacement may be necessary.
3.2. Safety Margins
Achieving clear surgical margins (R0 resection) is important to reduce recurrence risk, even in benign tumors like myxomas [28]. While the ideal margin remains debated, a 2–5 mm tissue buffer is commonly targeted [30,31].
Li et al. found that R0 resection significantly improved survival (58 vs. 11 months) and recurrence-free interval (36 vs. 6 months) compared to R1 (p < 0.001), with no survival difference between R1 and R2. They recommended aggressive attempts to achieve R0 and multimodal therapy after R1/R2 resections [32]. In contrast, Chan et al. observed no survival difference across R0–R2 resections in 122 patients, possibly due to universal chemotherapy use regardless of margin status [33].
3.3. Special Considerations in Pediatric Populations
In children, certain benign tumors, such as rhabdomyomas, may regress spontaneously and may not require surgical intervention unless they cause significant symptoms [22]. Close imaging surveillance is typically sufficient in asymptomatic pediatric patients.
3.4. Outcomes
Postoperative complications are infrequent; arrhythmias are the most common, typically managed medically [29]. Long-term survival rates following benign tumor resection are excellent, approximating survival rates of the general population in matched cohorts [30].
4. Management of Malignant Cardiac Tumors
Malignant cardiac tumors, primarily sarcomas and lymphomas, require aggressive multimodal therapy due to their poor prognosis [11,32,34].
4.1. Resectable Malignant Tumors
Whenever possible, complete surgical resection (R0) remains the primary goal, significantly improving survival compared to incomplete resections (R1 or R2) [32].
Complex resections may involve heart autotransplantation, an advanced surgical technique in which the heart is explanted, the tumor resected ex vivo, and the heart subsequently reimplanted [35]. This approach is particularly beneficial for tumors located in anatomically difficult areas such as the left atrium, mitral valve, or pulmonary veins, where in situ resection would risk incomplete excision or structural damage.
Studies have shown promising outcomes for autotransplantation, particularly when pneumonectomy is not required. However, combined autotransplantation and pneumonectomy carry high surgical mortality and are now considered contraindicated by some authors. In such cases, a two-stage approach may be employed to reduce perioperative risk [33]. Compared to orthotopic heart transplantation, autotransplantation avoids the need for immunosuppression, making it a favorable option for patients with aggressive histologies like angiosarcoma [32]. Detailed surgical steps, outcomes, and case series are summarized in Figure 3, Supplementary Tables S3 and S4.
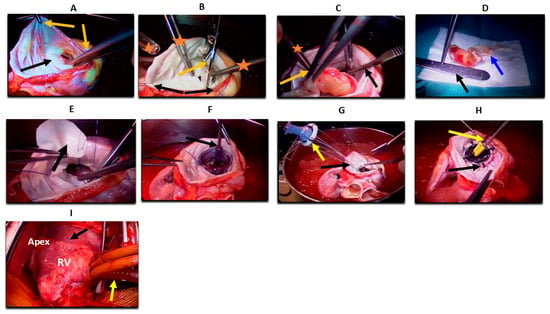
Figure 3.
Heart autotransplantation for primary cardiac malignancy (A) Heart ex vivo placed in ice; the left atrium is opened (black arrow). Two forceps (orange arrows) are retracting the left atrium for proper exposure, preparing for stay sutures to maximize exposure and facilitate stability of the heart. (B) Stay sutures are seen peripherally (black arrows). Scissors are used to excise the anterior mitral leaflet (orange arrow). Two forceps of the first assistant are visible inside the field (orange asterisk), and the head of a sucker is seen on the side of the image (triangle). (C) A number 15 blade is used to excise the tumor from the papillary muscle (black arrow). One forceps is holding the tumor (orange arrow), and the other is retracting the atrial wall (asterisk). (D) The tumor is completely excised and placed on a white sterile dressing, measuring ~4 cm in size (black arrow). Intimate attachment to the anterior mitral leaflet is visible (blue arrow). (E) Forceps holding a pericardial patch (black arrow) and sewing it with running Prolene sutures in a circumferential fashion into a left ventricular defect left after complete tumor resection. (F) A mitral valve sizer is shown in the annulus to assess the exact size of the needed valve (black arrow). (G) A prosthetic valve is held with Ethibond sutures (yellow arrow) sewn through and to the annulus (black arrow). (H) The mitral valve prosthesis is shown in place (black arrow), with a valve tester (yellow arrow). (I) The heart is reimplanted into the pericardial space (black arrow). The left atrium, vena cavae, aorta, pulmonary artery, and bypass cannulas are reanastomosed. Bicaval venous cannulas are visible (yellow arrow). RV: right ventricle.
Neoadjuvant chemotherapy or radiotherapy may improve resectability and survival in selected patients by downstaging tumors before surgery [36,37]. Following incomplete resection (R1 or R2), multimodal adjuvant therapy is recommended to address residual disease [32].
4.2. Non-Resectable Malignant Tumors
Tumors deemed non-resectable due to extensive local invasion, metastasis, or poor patient condition require alternative strategies:
- Palliative Chemotherapy and Radiotherapy: Systemic therapy aims to prolong survival and control symptoms [11,38].
- Heart Transplantation: Considered in select cases without metastasis; however, outcomes remain controversial due to high recurrence and the effects of immunosuppression [37,38,39,40,41].
- Heart Autotransplantation: It may also be considered in borderline resectable cases where standard approaches are not feasible, and there is no evidence of systemic spread. (See Section 4.1 and Supplementary Table S4 for detailed criteria and outcomes) [35].
Decision-making must be multidisciplinary, weighing surgical feasibility, systemic disease burden, and patient functional status.
4.3. Management of Oligometastatic Cardiac Tumors
Oligometastatic disease represents an intermediate state between localized and widely metastatic cancer, typically defined by the presence of five or fewer metastatic lesions. Although rare, oligometastatic cardiac tumors may offer opportunities for aggressive, potentially life-prolonging treatment [10]. A multimodal approach involving surgical resection, stereotactic body radiotherapy (SBRT), and systemic therapies—such as chemotherapy, targeted agents, or immunotherapy—is often warranted depending on tumor location, burden, and patient fitness [38,42,43,44,45,46,47].
Prognostic factors that favor aggressive management include a well-controlled primary tumor, low nodal stage (N0 or N1), and a disease-free interval (DFI) greater than 6 to 12 months [48]. A longer DFI is generally associated with more indolent tumor biology and improved survival outcomes following local treatment. These parameters may help guide patient selection for surgery or SBRT [34,48].
Individual case reports of oligometastatic cardiac tumors, treatment strategies, and outcomes are summarized in Supplementary Table S5.
4.4. Management of Cardiac Metastases
Cardiac metastases are significantly more common than primary cardiac tumors and are often detected incidentally or during advanced cancer staging. The most frequent primary tumors associated with cardiac metastases include lung carcinoma (particularly adenocarcinoma and small cell lung cancer), breast carcinoma, hematologic malignancies (especially lymphomas and leukemias), melanoma, renal cell carcinoma, and esophageal cancer [10,16].
These malignancies may reach the heart through one of three main routes: direct extension (e.g., lung or breast tumors infiltrating the pericardium), lymphatic spread (e.g., from breast or mediastinal cancers into the pericardium), and hematogenous dissemination (common with melanoma, renal cell carcinoma, and sarcomas) [6,7,16]. The pericardium is the most commonly involved site, followed by the myocardium and endocardium.
Clinical manifestations of cardiac metastases vary by location and burden of disease but may include pericardial effusion with tamponade, arrhythmias, obstruction of inflow or outflow tracts, or nonspecific symptoms such as fatigue or dyspnea. Diagnosis typically relies on multimodal imaging, including echocardiography, cardiac MRI, and 18F-FDG PET-CT, the latter of which is particularly helpful in detecting metabolically active metastatic lesions [10,15].
Management is generally palliative and tailored to symptom control. Pericardiocentesis or surgical pericardial window is indicated in cases of tamponade, and intrapericardial administration of chemotherapeutic agents or radioisotopes may help prevent recurrence [49]. For patients with life-threatening arrhythmias due to metastatic infiltration, radiofrequency ablation may offer symptomatic relief [50,51]. Antiarrhythmic medications are often required for rhythm stabilization. Rarely, surgical debulking may be considered for isolated, symptomatic cardiac metastases in otherwise stable patients [50,52,53]. In select cases of limited metastasis (oligometastatic disease), a more aggressive approach combining local and systemic therapies may be justified (see Section 4.3).
5. Prognosis
Prognosis in cardiac tumors depends heavily on tumor type, resectability, and completeness of surgical excision. Benign tumors generally have excellent outcomes, while malignant tumors—particularly sarcomas—are associated with poor survival despite aggressive treatment.
5.1. Benign Tumors
Most benign tumors, such as myxomas and lipomas, have favorable long-term outcomes following complete surgical resection (R0). Recurrence is rare with adequate excision, and postoperative survival approximates age-matched general population rates [30,54,55,56]. Pediatric benign tumors such as rhabdomyomas often regress spontaneously, further contributing to an excellent prognosis in this subgroup [22].
5.2. Malignant Tumors
Primary malignant cardiac tumors, such as angiosarcomas and undifferentiated sarcomas, carry a dismal prognosis. Median survival typically ranges from 6 to 12 months for patients with unresected or incompletely resected tumors. However, complete surgical resection (R0) has been associated with significantly improved outcomes. In a study by Li et al., patients who underwent R0 resections had a median survival of 58 months compared to only 11 months for those with R1 or R2 resections (p < 0.001) [32]. Conversely, Chan et al. reported no significant difference in survival between R0, R1, and R2 resections in a cohort where all patients received chemotherapy, suggesting that systemic therapy may play a crucial role in mitigating the negative impact of incomplete resection [33].
5.3. Prognosis in Oligometastatic Disease
In selected patients with oligometastatic cardiac involvement, prognosis is more favorable when the primary tumor is well controlled, nodal disease is limited (N0 or N1), and the disease-free interval (DFI) exceeds 6–12 months [48]. A longer DFI often reflects less aggressive tumor biology and a better response to localized therapies such as surgical resection or stereotactic body radiotherapy (SBRT). For instance, patients with brain metastases and a DFI greater than 360 days, or with adrenal metastases and a DFI exceeding 6 months, demonstrated improved survival outcomes [48]. In one reported case of cardiac metastasis from lung adenocarcinoma, treatment with SBRT resulted in a complete metabolic response on PET imaging and sustained tumor control lasting over 18 months [38].
5.4. Metastatic Cardiac Tumors
Cardiac metastases generally indicate advanced-stage malignancy with a poor prognosis. Survival is typically determined by the behavior of the primary tumor rather than the cardiac involvement itself. Palliative care remains the mainstay of treatment in such cases, and survival is often measured in weeks to months [2,50].
6. Future Directions
Advances in cardiac tumor management increasingly depend on the integration of artificial intelligence (AI), precision diagnostics, and collaborative global data sharing. AI-enhanced imaging, including machine learning algorithms applied to echocardiography and cardiac MRI, holds significant promise in improving diagnostic accuracy and enabling earlier tumor detection. One such tool is the echocardiographic DEM score, a multiparametric algorithm that can help differentiate benign from malignant cardiac tumors and guide further diagnostic steps [12].
Genomic profiling and molecular diagnostics are expected to refine treatment planning, particularly for aggressive histologies such as sarcomas. As more molecular targets are identified, the use of precision medicine—including targeted therapies and immunotherapies—may help individualize treatment regimens and improve survival.
Innovations in surgical techniques, such as 3D modeling and minimally invasive tumor resections, could enhance operative precision and reduce complications. Refinement of criteria for advanced interventions like heart autotransplantation will also be critical for selecting suitable candidates and improving outcomes.
Furthermore, international registries and prospective databases are urgently needed to improve the understanding of these rare tumors. Pediatric-focused studies should address the unique tumor biology and clinical course of cardiac tumors in children, especially those associated with syndromic conditions like tuberous sclerosis.
Addressing global disparities in access to diagnostic imaging and specialized care remains a key priority. Equitable distribution of resources such as cardiac MRI and PET-CT, particularly in low- and middle-income settings, will be essential to improve diagnostic capabilities and reduce outcome gaps.
Finally, future clinical trials and treatment strategies must prioritize patient-centered outcomes, including quality of life, functional recovery, and long-term surveillance. Public health efforts should promote awareness of early cardiac tumor symptoms—such as unexplained arrhythmias or embolic events—to support earlier diagnosis and referral to specialized centers.
7. Conclusions
Cardiac tumors, though rare, present substantial diagnostic and therapeutic challenges due to their diverse pathology and the critical structures they involve. Differentiating benign from malignant lesions is essential, as management strategies and prognostic implications vary significantly. While echocardiography remains the first-line diagnostic tool, advanced imaging modalities such as cardiac MRI and PET-CT have greatly enhanced diagnostic accuracy and risk stratification.
For resectable tumors, particularly benign lesions like myxomas, surgical excision is usually curative with excellent long-term outcomes. Malignant tumors, however, often require a multimodal approach involving surgery, chemotherapy, radiotherapy, or innovative techniques like heart autotransplantation. Prognosis in malignant cases remains guarded, although survival improves with complete resection and carefully selected aggressive interventions in oligometastatic settings.
Progress in cardiac oncology will depend on standardizing treatment algorithms, integrating novel technologies such as artificial intelligence and genomic profiling, and expanding global registries to better understand rare tumor types. Special emphasis is needed on pediatric populations and on bridging diagnostic and therapeutic disparities across regions.
Ultimately, a multidisciplinary, patient-centered approach—supported by technological innovation and collaborative research—is essential to improve outcomes and reduce recurrence in patients with primary or metastatic cardiac tumors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14103392/s1. Ref. [57] is cited in the Supplementary Table S1.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoffmeier, A.; Sindermann, J.R.; Scheld, H.H.; Martens, S. Cardiac Tumors—Diagnosis and Surgical Treatment. Dtsch. Ärztebl. Int. 2014, 111, 205–211. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Kochav, J.; O’Connor, D.S.; Rosner, G.F. Cardiac Tumors: Clinical Presentation, Diagnosis, and Management. Curr. Treat. Options Oncol. 2019, 20, 66. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Basso, C.; Bois, M.C.; Glass, C.; Klarich, K.W.; Leduc, C.; Padera, R.F.; Tavora, F. The 2021 WHO Classification of Tumors of the Heart. J. Thorac. Oncol. 2022, 17, 510–518. [Google Scholar] [CrossRef]
- Yin, L.; He, D.; Shen, H.; Ling, X.; Li, W.; Xue, Q.; Wang, Z. Surgical treatment of cardiac tumors: A 5-year experience from a single cardiac center. J. Thorac. Dis. 2016, 8, 911–919. [Google Scholar] [CrossRef]
- Rahouma, M.; Arisha, M.J.; Elmously, A.; El-Sayed Ahmed, M.M.; Spadaccio, C.; Mehta, K.; Baudo, M.; Kamel, M.; Mansor, E.; Ruan, Y.; et al. Cardiac tumors prevalence and mortality: A systematic review and meta-analysis. Int. J. Surg. 2020, 76, 178–189. [Google Scholar] [CrossRef]
- Silva, M.; Carneiro, M.; Nunes, J.; da Silva, A.; de Sousa, M. Systematic Review and Meta-analysis of Prevalence of Coronary Artery Disease in Adult Patients with Cardiac Myxomas. F1000Research 2015, 4, 194. [Google Scholar] [CrossRef]
- Tzani, A.; Doulamis, I.P.; Mylonas, K.S.; Avgerinos, D.V.; Nasioudis, D. Cardiac Tumors in Pediatric Patients: A Systematic Review. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 624–632. [Google Scholar] [CrossRef]
- Palaskas, N.; Thompson, K.; Gladish, G.; Agha, A.M.; Hassan, S.; Iliescu, C.; Kim, P.; Durand, J.B.; Lopez-Mattei, J.C. Evaluation and Management of Cardiac Tumors. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 29. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.-M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Frudit, P.; Vitturi, B.K.; Navarro, F.C.; Rondelli, I.; Pozzan, G. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: Case report and review of the literature. Autops. Case Rep. 2019, 9, e2019125. [Google Scholar] [CrossRef]
- Raiker, N.K.; Jensen, E.; Wodzinski, B.; Maganti, K. Metastatic renal cell carcinoma presenting as a cardiac tumour. BMJ Case Rep. 2018, 11, e227336. [Google Scholar] [CrossRef]
- Paolisso, P.; Foà, A.; Bergamaschi, L.; Graziosi, M.; Rinaldi, A.; Magnani, I.; Angeli, F.; Stefanizzi, A.; Armillotta, M.; Sansonetti, A. Echocardiographic markers in the diagnosis of cardiac masses. J. Am. Soc. Echocardiogr. 2023, 36, 464–473. [Google Scholar] [CrossRef]
- Paolisso, P.; Foà, A.; Magnani, I.; Bergamaschi, L.; Graziosi, M.; Angeli, F.; Chiti, C.; Fabrizio, M.; Rinaldi, A.; Stefanizzi, A.; et al. Development and Validation of a Diagnostic Echocardiographic Mass Score in the Approach to Cardiac Masses. JACC Cardiovasc. Imaging 2022, 15, 2010–2012. [Google Scholar] [CrossRef]
- Kassop, D.; Donovan, M.S.; Cheezum, M.K.; Nguyen, B.T.; Gambill, N.B.; Blankstein, R.; Villines, T.C. Cardiac Masses on Cardiac CT: A Review. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9281. [Google Scholar] [CrossRef]
- Tamura, Y.; Kawaoka, T.; Aikata, H.; Namba, M.; Fujii, Y.; Morio, K.; Murakami, E.; Yamauchi, M.; Hiramatsu, A.; Nakahara, T.; et al. Isolated cardiac metastases of hepatocellular carcinoma after resection: A case report. Clin. J. Gastroenterol. 2020, 13, 421–427. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, W.; Gao, L.; Ji, M.; Xie, M.; Li, Y. Multimodality Imaging of Benign Primary Cardiac Tumor. Diagnostics 2022, 12, 2543. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar]
- Angeli, F.; Bodega, F.; Bergamaschi, L.; Armillotta, M.; Amicone, S.; Canton, L.; Fedele, D.; Suma, N.; Cavallo, D.; Foà, A.; et al. Multimodality Imaging in the Diagnostic Work-Up of Patients with Cardiac Masses: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2024, 6, 847–862. [Google Scholar] [CrossRef]
- Campia, U.; Nohria, A. Anthracycline Cardiotoxicity. JACC CardioOncology 2020, 2, 220–222. [Google Scholar] [CrossRef]
- Corradi, D.; Moreno, P.R.; Rahouma, M.; Abascal, V.M.; Guareschi, D.; Tafuni, A.; Grazioli, V.; Palumbo, A.; Niccoli, G.; Lorusso, R. Cardiac tumors: Updated classifications and main clinico-pathologic findings. Trends Cardiovasc. Med. 2025; in press. [Google Scholar] [CrossRef]
- Bussani, R.; Castrichini, M.; Restivo, L.; Fabris, E.; Porcari, A.; Ferro, F.; Pivetta, A.; Korcova, R.; Cappelletto, C.; Manca, P.; et al. Cardiac Tumors: Diagnosis, Prognosis, and Treatment. Curr. Cardiol. Rep. 2020, 22, 169. [Google Scholar] [CrossRef]
- Cooper, L.T.; Baughman, K.L.; Feldman, A.M.; Frustaci, A.; Jessup, M.; Kuhl, U.; Levine, G.N.; Narula, J.; Starling, R.C.; Towbin, J.; et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease. Circulation 2007, 116, 2216–2233. [Google Scholar] [CrossRef]
- ESC Scientific Document Group. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current Status of Endomyocardial Biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef]
- Sano, M.; Okada, T.; Yamashita, D.; Koyama, T.; Furukawa, Y. Left-sided primary cardiac lymphoma diagnosed by needle biopsy with total endoscopic anterolateral mini-thoracotomy: A case report. Eur. Heart J.-Case Rep. 2023, 7, ytad600. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, N.; Ma, Q.; Jin, L.; Pan, X. Intracardiac biopsy of cardiac tumors with echocardiographic guidance: Case report. Front. Cardiovasc. Med. 2023, 10, 1103918. [Google Scholar] [CrossRef]
- Li, Z.; Li, G.; Jiang, X.; Fu, X. Intraoperative frozen pathological diagnosis of cystic tumor of the atrioventricular node: A case report and review of the literature. Int. J. Clin. Exp. Pathol. 2018, 11, 2165–2169. [Google Scholar]
- Rao, A. Cardiac Tumors—Cardiovascular Disorders—Merck Manuals Professional Edition. Available online: https://www.merckmanuals.com/professional/cardiovascular-disorders/cardiac-tumors/cardiac-tumors (accessed on 26 February 2020).
- Barnes, H.; Conaglen, P.; Russell, P.; Newcomb, A. Clinicopathological and surgical experience with primary cardiac tumors. Asian Cardiovasc. Thorac. Ann. 2014, 22, 1054–1058. [Google Scholar] [CrossRef]
- Rahmanian, P.B.; Castillo, J.G.; Sanz, J.; Adams, D.H.; Filsoufi, F. Cardiac myxoma: Preoperative diagnosis using a multimodal imaging approach and surgical outcome in a large contemporary series. Interact. Cardiovasc. Thorac. Surg. 2007, 6, 479–483. [Google Scholar] [CrossRef]
- Raicea, V.C.; Suciu, H.; Raicea, A.D.; Macarie, G.C.; Mezei, T.; Maier, M.S. Giant left atrial myxoma—Literature review and case presentation. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2021, 62, 361–368. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.; Chen, Z.; Ding, W.; Hong, T.; Chen, H.; Shao, M.; Lai, H.; Hou, Y.; Wang, C. Prognostic Analysis for Survival After Resections of Localized Primary Cardiac Sarcomas: A Single-Institution Experience. Ann. Thorac. Surg. 2014, 97, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Ali, A.; Zubair, M.M.; Nguyen, D.T.; Ibarra-Cortez, S.H.; Graviss, E.A.; Shapira, O.M.; Ravi, V.; MacGillivray, T.E.; Reardon, M.J. Primary cardiac sarcomas: Treatment strategies. J. Thorac. Cardiovasc. Surg. 2023, 166, 828–838.e2. [Google Scholar] [CrossRef] [PubMed]
- Masci, G.; Magagnoli, M.; Grimaldi, A.; Covini, G.; Carnaghi, C.; Rimassa, L.; Santoro, A. Metastasis of Hepatocellular Carcinoma to the Heart: A Case Report and Review of the Literature. Tumori J. 2004, 90, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Ramlawi, B.; Al-jabbari, O.; Blau, L.N.; Davies, M.G.; Bruckner, B.A.; Blackmon, S.H.; Ravi, V.; Benjamin, R.; Rodriguez, L.; Shapira, O.M.; et al. Autotransplantation for the Resection of Complex Left Heart Tumors. Ann. Thorac. Surg. 2014, 98, 863–868. [Google Scholar] [CrossRef]
- Abu Saleh, W.K.; Ramlawi, B.; Shapira, O.M.; Al Jabbari, O.; Ravi, V.; Benjamin, R.; Durand, J.-B.; Leja, M.J.; Blackmon, S.H.; Bruckner, B.A.; et al. Improved Outcomes with the Evolution of a Neoadjuvant Chemotherapy Approach to Right Heart Sarcoma. Ann. Thorac. Surg. 2017, 104, 90–96. [Google Scholar] [CrossRef]
- Lau, C.; Leonard, J.R.; Schwann, A.N.; Soletti, G.; Abouarab, A.A.; Munjal, M.; Gaudino, M.; Girardi, L.N. A 20-Year Experience With Resection of Primary Cardiac Tumors and Metastatic Tumors of the Heart. Ann. Thorac. Surg. 2019, 107, 1126–1131. [Google Scholar] [CrossRef]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors: JACC CardioOncology State-of-the-Art Review. JACC CardioOncology 2020, 2, 293–311. [Google Scholar] [CrossRef]
- Andrushchuk, U.U.; Ostrovsky, Y.P.; Valentsiukevich, A.V.; Shestakova, L.G.; Amelchanka, S.G.; Krutau, V.G.; Yudina, O.A.; Chernoglaz, P.F.; Grinchuk, I.I.; Smalenski, A. Heart transplantation in the treatment of primary non-operable cardiac tumors. Kardiochirurgia Torakochirurgia Pol. Pol. J. Cardio-Thorac. Surg. 2017, 14, 271–279. [Google Scholar] [CrossRef]
- Jiménez Mazuecos, J.M.; Fuentes Manso, R.; Segovia Cubero, J.; Toquero Ramos, J.; Oteo Domínguez, J.F.; Alonso-Pulpón Rivera, L. Is heart transplantation for primary cardiac sarcoma a useful therapeutic option? Rev. Esp. Cardiol. 2003, 56, 408–411. [Google Scholar] [CrossRef]
- Gowdamarajan, A.; Michler, R.E. Therapy for primary cardiac tumors: Is there a role for heart transplantation? Curr. Opin. Cardiol. 2000, 15, 121–125. [Google Scholar] [CrossRef]
- Padalino, M.A.; Basso, C.; Milanesi, O.; Vida, V.L.; Moreolo, G.S.; Thiene, G.; Stellin, G. Surgically treated primary cardiac tumors in early infancy and childhood. J. Thorac. Cardiovasc. Surg. 2005, 129, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Shapira, O.M.; Korach, A.; Izhar, U.; Koler, T.; Wald, O.; Ayman, M.; Erez, E.; Blackmon, S.H.; Reardon, M.J. Radical multidisciplinary approach to primary cardiac sarcomas. Eur. J. Cardiothorac. Surg. 2013, 44, 330–336. [Google Scholar] [CrossRef]
- Bakaeen, F.G.; Jaroszewski, D.E.; Rice, D.C.; Walsh, G.L.; Vaporciyan, A.A.; Swisher, S.S.; Benjamin, R.; Blackmon, S.; Reardon, M.J. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J. Thorac. Cardiovasc. Surg. 2009, 137, 1454–1460. [Google Scholar] [CrossRef]
- Bonomo, P.; Cipressi, S.; Desideri, I.; Masi, L.; Doro, R.; Iermano, C.; Greto, D.; Simontacchi, G.; Mangoni, M.; Paiar, F.; et al. Stereotactic Body Radiotherapy with Cyberknife for Cardiac Malignancies. Tumori J. 2015, 101, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Jumeau, R.; Vincenti, M.G.; Pruvot, E.; Schwitter, J.; Vallet, V.; Zeverino, M.; Moeckli, R.; Bouchaab, H.; Bourhis, J.; Ozsahin, M. Curative management of a cardiac metastasis from lung cancer revealed by an electrical storm. Clin. Transl. Radiat. Oncol. 2020, 21, 62–65. [Google Scholar] [CrossRef]
- Linfeng, Q.; Xingjie, X.; Henry, D.; Zhedong, W.; Hongfei, X.; Haige, Z. Cardiac angiosarcoma: A case report and review of current treatment. Medicine 2019, 98, e18193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-T.; Li, Y.; Ren, S.-H.; Ren, W.-D.; Song, G.; Xiao, Y.-J.; Sun, F.-F.; Sun, L.; Yang, X.-H.; Tan, X.-Y. Isolated metastasis of hepatocellular carcinoma in the right ventricle. BMC Cardiovasc. Disord. 2019, 19, 287. [Google Scholar] [CrossRef]
- Burazor, I.; Aviel-Ronen, S.; Imazio, M.; Goitein, O.; Perelman, M.; Shelestovich, N.; Radovanovic, N.; Kanjuh, V.; Barshack, I.; Adler, Y. Metastatic cardiac tumors: From clinical presentation through diagnosis to treatment. BMC Cancer 2018, 18, 202. [Google Scholar] [CrossRef]
- Reynen, K.; Köckeritz, U.; Strasser, R.H. Metastases to the heart. Ann. Oncol. 2004, 15, 375–381. [Google Scholar] [CrossRef]
- Huang, J.; Lei, C.; Hsi, D.H.; Zheng, M.; Ma, H.; Ta, S.; Hu, R.; Han, C.; Li, W.; Li, J.; et al. Echocardiography-Guided Radiofrequency Ablation for Cardiac Tumors. JACC CardioOncology 2024, 6, 560–571. [Google Scholar] [CrossRef]
- Ravikumar, T.S.; Topulos, G.P.; Anderson, R.W.; Grage, T.B. Surgical Resection for Isolated Cardiac Metastases. Arch. Surg. 1983, 118, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Blauth, C.; Peckham, M.; Hendry, W.; Barrett, A.; Goldstraw, P. Intracardiac metastases from malignant teratoma of the testis. J. Thorac. Cardiovasc. Surg. 1986, 92, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, G.S.; Jung, Y.; Jeong, I.S.; Na, K.J.; Oh, B.S.; Ahn, B.H.; Oh, S.G. Surgical resection of cardiac myxoma—A 30-year single institutional experience. J. Cardiothorac. Surg. 2017, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Ju, M.H.; Lee, C.-H.; Lim, M.H.; Je, H.G. Surgical Outcomes of Cardiac Myxoma Resection Through Right Mini-Thoracotomy. J. Chest Surg. 2023, 56, 42–48. [Google Scholar] [CrossRef]
- Hill, M.; Cherry, C.; Maloney, M.; Midyette, P. Surgical Resection of Atrial Myxomas. AORN J. 2010, 92, 393–409. [Google Scholar] [CrossRef]
- He, S.; Cao, Y.; Qin, W.; Chen, W.; Yin, L.; Chai, H.; Tao, Z.; Tang, S.; Qiu, Z.; Chen, X. Prevalence of primary cardiac tumor malignancies in retrospective studies over six decades: A systematic review and meta-analysis. Oncotarget 2017, 8, 43284. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).