Abstract
Background: Endoscopic treatment is one of the first-line treatments for bile leaks after hepatic surgery. However, detailed reports of endoscopic treatment for bile leaks after hepatic resection (HR) or liver transplantation (LT) are scarce. The outcomes of endoscopic treatment for bile leaks after hepatic surgery were examined, and factors related to successful treatment were identified. Methods: A total of 122 patients underwent endoscopic treatment for bile leaks after hepatic surgery. The diagnosis of a bile leak is based on the ISGLS criteria. The decision to perform endoscopic retrograde cholangiography (ERC) is made based on the amount of drainage output, laboratory data, clinical symptoms, and CT scan findings. In our study, the site of the bile leak was assessed using ERC. Endoscopic stents were placed to bridge across the bile leak site as much as possible. Otherwise, stents were placed near the leak site. Endoscopic stents were replaced every 2–3 months until an improvement in the bile leak was observed with or without biliary strictures. The outcomes of endoscopic treatment and the factors related to clinical success were evaluated. Results: Seventy-four patients with HR and forty-eight patients with LT were treated endoscopically. Technical and clinical success was achieved in 89% (109/122) and 82% (100/122) of patients, respectively. Three (2%) patients died from uncontrollable bile leaks. Bridging stent placement (p < 0.001), coexistent percutaneous drainage (p = 0.0025), and leak severity (p = 0.015) were identified as independent factors related to the clinical success of endoscopic treatment. During a median observation period of 1162 days after the achievement of clinical success, bile leak recurrence was observed in only three cases (3%). Conclusions: Endoscopic treatment is safe and effective for bile leaks after hepatic surgery. Bridging stent placement across the leak site is the most crucial factor for clinical success.
1. Introduction
The number of patients with biliary tract cancer (BTC) is increasing [1,2,3]. Hepatocellular carcinoma (HCC), despite its advances in antiviral therapy for hepatitis viruses, remains the 13th most diagnosed cancer worldwide and is the 7th leading cause of cancer-related deaths [4,5]. Hepatic resection (HR) is one of the curative treatments for these cancers, and about 10,000 patients undergo liver transplantation (LT) each year in the United States [6], aiming to obtain a complete cure for both benign and malignant liver diseases. A bile leak is one of the most common adverse events after hepatobiliary surgery, occurring in 4.5–18% of patients [7,8,9,10,11,12,13].
Infection with a bile leak is a well-known cause of sepsis and abscess formation, which can be fatal [14,15,16]. Reoperation is considered a highly invasive therapy; thus, percutaneous transhepatic biliary drainage (PTBD) is commonly performed to resolve bile leaks, but it has some limitations [17,18]. PTBD is challenging to perform under the continuous administration of anticoagulants or with uncontrolled ascites [19,20]. Moreover, quality of life (QOL) is impaired during the period when an external fistula tube is in place. Endoscopic treatment overcomes these disadvantages and allows stent placement within the body as an internal fistula [21].
Since the first initial report in the 1990s [22], clinical success rates of endoscopic retrograde cholangiography (ERC) for bile leaks after cholecystectomy have been frequently investigated, with reports ranging from 79 to 100% [22,23,24,25,26,27,28]. It is generally recommended to combine endoscopic sphincterotomy (EST) with stent placement in treatment and place the stent so that it bridges across the leak site for effective drainage [29,30,31,32]. Reports of endoscopic treatment for bile leaks after HR or LT are rare, with clinical success rates ranging from 64 to 89% [33,34,35,36]. Due to the anatomical complexity, ERC procedures after hepatic surgery for bile leaks are more challenging compared to after surgery for the common bile duct or cystic duct. Although the location of stents is an important factor related to the improvement of bile leaks [37], detailed investigations of this have not been conducted so far in patients after hepatic surgery.
In this study, the outcomes of ERC treatment for bile leaks after hepatic surgery were investigated, and the factors related to clinical success after endoscopic treatment were evaluated.
2. Materials and Methods
2.1. Patients
Between July 2004 and December 2022, 122 consecutive patients underwent initial endoscopic treatment for bile leaks after hepatobiliary surgery at Okayama University Hospital (Okayama, Japan). In all cases, external drain tubes were placed near the resection site during surgery, and the diagnosis of a bile leak was based on the ISGLS 2010 criteria [38]. The decision to perform ERCP was made based on the amount of drainage output, laboratory data, clinical symptoms, and CT scan findings. This study included the following patients: (1) those who had confirmed fluid retention on postoperative CT after liver transplantation or hepatic resection, accompanied by clinical symptoms such as fever, abdominal pain, and an abnormal blood test result; and (2) men and women aged 20 years or older. The following patients were excluded: (1) those who opted for conservative treatment only and (2) those with significant abnormalities in their vital signs, precluding endoscopic procedures. This study was approved by the Ethics Committee of Okayama University Hospital in accordance with the guidelines of the Declaration of Helsinki (approval number 2408–004, dated 31 May 2024).
2.2. Endoscopic Procedure
Endoscopic treatments were performed using duodenoscopes JF-260V, TJF-260V, and TJF-Q290V (Olympus, Tokyo, Japan). Patients were consciously sedated with intravenous sedative drugs. After biliary cannulation, cholangiography was performed. Bile leaks were recognized by the leakage of contrast medium from any part of the biliary tract, and endoscopic stent placement using a plastic stent (PS) was performed. Basically, a PS was placed to bridge the leak site. That is, the proximal end of the PS was placed in the biliary tract upstream of the leak site. If the proximal bile duct at the leak site was narrow or not identified, or the PS did not stabilize well due to the angle or bifurcation of the bile duct, the PS was placed distal to the leak site or in another nearby bile duct (Figure 1). If endoscopic stenting was not successful due to a severe bile leak or stricture, PTBD or re-operation was considered a form of salvage treatment.
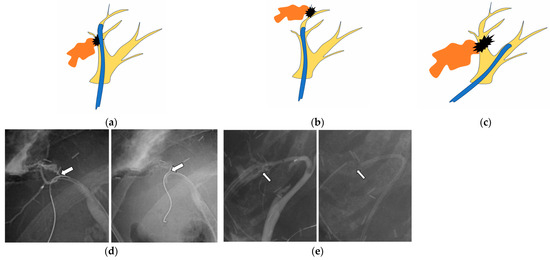
Figure 1.
The stent is placed (a) bridging across the leak site, (b) distal to the leak site, or (c) at another bile duct near the bile leak site. (a–c) Yellow: bile duct branch, black: leak site, orange: leaked bile, blue: plastic stent. (d) The bile leak (arrow) is found at B6, and a stent is placed bridging across the leak site. (e) The leak (arrow) is found at B5, and a stent is placed distal to the leak site.
Technical success was defined as successful biliary stent placement at the intended site. Clinical success was defined as an improvement in clinical symptoms (fever, abdominal pain, etc.) after treatment. An improvement in a bile leak was defined as the disappearance of the bile leak on ERC after stent placement. The severity of bile leaks was classified into two groups, according to the report by Sandha et al. [26]. In low-grade cases, a bile leak was observed after filling the intrahepatic bile duct with a contrast agent, and in high-grade cases, it was observed before filling the intrahepatic bile duct with a contrast agent (Supplementary Figure S1). Adverse events due to endoscopic procedures were evaluated according to the ASGE 2010 guideline [39].
2.3. Follow-Up After Stent Placement by ERC
After stent placement, ERC was performed 2–3 months later. If the bile leak was resolved on ERC, the PS was removed. If the bile leak did not improve or a bile duct stricture remained regardless of the improvement in the bile leak on ERC, PS placement was repeated every 2–3 months until an improvement in the bile leak or bile duct stricture was observed. For patients who achieved stent-free status, ERC was not performed unless there was a recurrence of clinical symptoms. Patients were followed up with blood tests and/or imaging examinations at least every six months. A typical case is presented in Supplementary Figure S2.
2.4. Statistical Analysis
Continuous variables were presented as median and range or interquartile range (IQR) values. Comparisons of continuous variables were made using the Wilcoxon signed-rank test. Comparisons of dichotomous variables were performed using Fisher’s exact test. Logistic regression analysis was performed to identify important factors affecting the outcome of endoscopic procedures for postoperative bile leaks. p < 0.05 was considered significant. JMP 16 statistical software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
3. Results
3.1. Patients’ Characteristics
The patients’ characteristics are shown in Table 1. The total number of cases was 122. Background diseases included hepatocellular carcinoma (n = 51), cholangiocarcinoma (n = 9), other cancers/tumors (including metastatic liver tumors) (n = 14), liver failure due to liver cirrhosis (n = 40), transplant donors (n = 5), and three other cases. The median interval from surgery to the first ERCP was 41 days (IQR 22–85 days), with surgical procedures including left or right lobectomy (including extended resection) (n = 38), segmental resection (n = 36), and LT (n = 48). LT consisted of living donor liver transplantation (LDLT) (n = 47) and deceased donor liver transplantation (DDLT) (n = 1). At the time of the initial ERCP, 89 patients (72%) had an existing external drainage tube in place. Of these, 68 patients retained the surgical drain placed during surgery, whereas 21 patients underwent additional percutaneous transhepatic biliary drainage under abdominal ultrasound guidance.

Table 1.
Patients’ characteristics.
3.2. Results of ERCP
Table 2 shows the results of the ERCP. Bile leak sites in HR cases included a common hepatic duct in 2 cases, a hilar bile duct in 54 cases, and a peripheral bile duct in 18 cases. In LT cases, bile leaks were observed in 44 cases at the bile duct anastomosis and in 4 cases at the residual cystic duct. EST was performed in 65 cases (53%), including those performed previously. A PS was placed to bridge the leak site in 82 cases (67%), at the distal site of the bile leak in 12 cases, and at another nearby bile duct in 15 cases. Technical success was achieved in 109 cases (89%). In the 13 cases (11%) with technical failure, the guide wire or stent could not be delivered proximally to the bile leak site (Supplemental Table S1). Of the cases with technical success, 100 (90%) had clinical success. A total of 22 cases (with 13 technical and 9 clinical failures) included 15 cases of PTBD and 7 cases of reoperation as forms of salvage treatment. The improvement in bile leak symptoms was achieved in 19 cases, which was considered clinical success with salvage treatment. Three cases died due to uncontrolled bile leaks (Figure 2).

Table 2.
ERCP results.
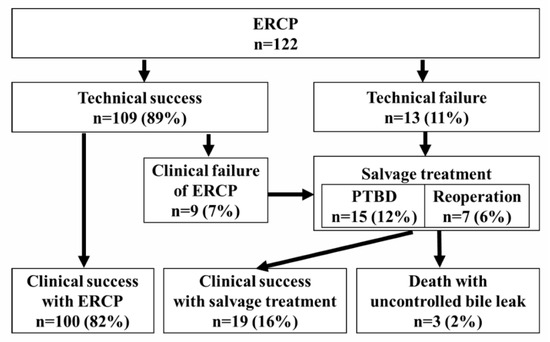
Figure 2.
A flow diagram of bile leak management from the first ERCP to clinical success. Technical success: successful biliary stent placement at the intended site. Clinical success: improvement in clinical symptoms.
Adverse events during the initial ERCP procedure for bile duct leaks were observed in nine cases (7%). There were seven cases of post-ERCP pancreatitis (6%), two cases of cholangitis (2%), and one case of cholangitis with a liver abscess (1%); all cases improved with conservative treatment.
3.3. Long-Term Outcomes
Of the 100 cases that achieved clinical success with ERCP, 90 cases (90%) had improved bile leaks. The median day until the improvement of the bile leak was 122 days (IQR 82–200 days). Ten patients died from liver failure after the bile leak was controlled during the follow-up period. Of the 90 patients with an improvement in bile leaks, 46 had no bile duct strictures and became stent-free, and 44 patients had remaining bile duct strictures that required repeat ERCP. Of them, 33 patients had improved bile duct strictures at a median of 189 days (IQR 91–367 days), whereas 11 patients still had significant strictures and required stent replacement (with a median observation period of 527 days (IQR 150–1134 days). After the achievement of stent-free status, bile leaks recurred in three patients, and repeat ERCP was performed (112, 180, and 1352 days after stent removal). The remaining 76 stent-free patients had no recurrent bile leaks during the median observation period of 1162 days (IQR 350–1781 days) (Figure 3).
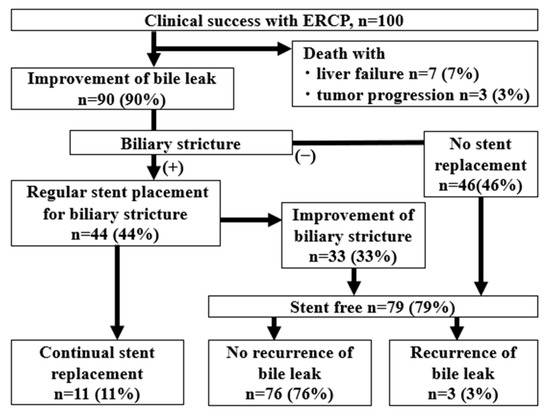
Figure 3.
A flow diagram showing the outcomes from clinical success. After clinical success, the bile leak disappeared in 90% of cases, of which 79% of cases were at least temporarily stent-free, and 11% had persistent stenosis requiring regular stent replacement.
3.4. Factors Related to Clinical Success of Endoscopic Treatments
Factors related to the clinical success of endoscopy were evaluated. Conducting univariate analysis showed that leak severity (p = 0.019), a leak-bridging stent (p = 0.001), and coexisting external fistulas (p = 0.010) were significant. For multivariate analysis, a bridging stent (OR, 12.2; 95% CI, 3.49–42.5; p < 0.001), coexisting external fistulas (OR, 7.10; 95%CI, 1.99–25.3; p = 0.0025), and leak severity (OR, 0.21; 95% CI, 0.06–0.74; p = 0.015) were also found to be independent factors related to clinical success (Table 3).

Table 3.
Factors related to clinical success of endoscopic treatments.
4. Discussion
In this study, endoscopic treatment outcomes for bile leaks were evaluated in 122 patients after hepatic surgery. The technical and clinical success rates were 89% and 82%, respectively. Three patients died due to uncontrollable bile leaks. After the achievement of clinical success, only three patients had recurrent bile leaks with a median follow-up of 1162 days. Bridging stent placement was related to clinical success.
PTBD has traditionally been used to treat bile leaks after hepatic surgery [17,18,19,20]. Whereas external fistulas offer the advantage of allowing the visual assessment of drainage, the performance of contrast studies involving flushing the lumen shows that prolonged placement significantly reduces patients’ QOL. Recently, the effectiveness of endoscopic stenting for bile leaks has been recognized [26,28,40,41]. However, only a few reports have examined in detail how to place stents and how to intervene in the bile ducts. In the present study, bridging stent placement across the leak site was positively related to clinical success. Regarding the placement of stents, positioning them distally or in nearby branches supports this pressure gradient. In contrast, promoting the closure of the fistula is also considered crucial in treating bile leaks. Bridging stent placement not only reduces bile duct pressure and decreases bile leaks, but it also facilitates fistula closure by reducing bile contact with the fistula site [29,30]. Thus, in ERC for bile leaks after cholecystectomy, it is considered most effective to place a stent bridging the leak site [24,29], and the present study suggests that this is also true for bile leaks after hepatic surgery.
EST is believed to promote the healing of bile leaks by reducing the pressure gradient across the sphincter of Oddi through an incision, thereby increasing bile flow into the duodenum [42,43,44,45]. In the present study, including cases that had already undergone previous examinations, EST was performed in 65 of 122 cases (53%). The clinical success rates were 80% (52/65) in the group with EST and 82% (41/50) in the group without EST, showing no significant difference. There were two reasons for this result. First, all stents were placed across the papilla, so the pressure in the biliary duct decreased with or without EST. Second, there may have been differences in the background factors that determined whether EST was performed. In cases of hepatic resection (HR), when there was concern about the recurrence of hepatocellular carcinoma, EST tended to be avoided (HR: 36/73; LT: 29/49; p = 0.35).
Regarding the size and number of stents placed, previous reports did not find differences between large and small-diameter stents [29,46]. In the present study, stent diameter ≥7 Fr and multiple stents were not significantly associated with clinical success. We previously reported that the severity of bile leaks was negatively related to the improvement of bile leaks [47]. The severity here was defined as mild if the leak was seen after peripheral cholangiography was performed and severe if the leak was seen from the early stages of cholangiography. The present results were the same in that less clinical improvement and bile leak improvement were seen in the severe bile leak group.
In the endoscopic treatment of bile leaks, while demonstrating the effectiveness of stent placement primarily focuses on bridging, there has been a renewed recognition of the efficacy of external fistula creation. In the present study, a surgical drain and/or PTBD was used in 89 cases (72%) during endoscopic treatment, and ERCP was performed in cases that did not show improvement. PTBD enabled biliary pressure reduction, infection control, irrigation, and the confirmation of biliary tract anatomy through contrast imaging. PTBD placement affected technical success, clinical success, and improvements in bile leaks. In refractory cases, approaching both sides is considered necessary.
This study had limitations. This was a retrospective, single-center analysis. However, reports of endoscopic treatment for bile leaks after hepatic surgery have been scarce. This report included a large number of cases and provided long-term follow-up after treatment. Also, although some reports have described the effectiveness of self-expanding metallic stents (SEMSs) for patients with bile leaks after pancreaticobiliary surgery [45,48], Japanese health insurance does not allow the use of SEMSs for benign diseases. Nevertheless, placing SEMSs in a narrow peripheral bile duct is controversial because of the possible obstruction and excessive dilatation of bile duct branches. Finally, this study does not clarify the total number of patients who developed a bile leak among those who underwent hepatic surgery during the study period, nor the proportion of those who received endoscopic treatment.
5. Conclusions
Endoscopic treatment is safe and effective for bile leaks after hepatic surgery. Bridging stent placement across the leak site should be attempted first.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14103381/s1. Figure S1: Severity of bile leaks; Figure S2: Case presentation. Table S1. Patients’ characteristics and technical and clinical failure.
Author Contributions
T.O. and K.M. (Kazuyuki Matsumoto): conception and design of the research and writing the paper. K.H., N.H., R.S., A.M., K.M. (Kazuya Miyamoto), H.T., Y.F., D.U., S.H. and K.T.: reviewed the paper. M.O.: review and final approval of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 24K11111 (to K.M.) and Grant Number 24K18948 (to Y.F.).
Institutional Review Board Statement
This study was approved by the Ethics Committee of Okayama University Hospital in accordance with the guidelines of the Declaration of Helsinki (approval number 2408–004, date 31 May 2024).
Informed Consent Statement
This study is a retrospective observational study that uses only existing anonymized data. Therefore, the ethics committee approved the use of an opt-out method, allowing the omission of individual informed consent from patients.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASGE | American Society for Gastrointestinal Endoscopy |
| BTC | Biliary tract cancer |
| CT | Computed tomography |
| DDLT | Deceased donor living transplantation |
| ERC | Endoscopic retrograde cholangiography |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| EST | Endoscopic sphincterotomy |
| HCC | Hepatocellular carcinoma |
| HR | Hepatic resection |
| IQR | Interquartile range |
| LDLT | Living donor liver transplantation |
| LT | Liver transplantation |
| PS | Plastic stent |
| PTBD | Percutaneous transhepatic biliary drainage |
| QOL | Quality of life |
| SEMS | Self-expanding metallic stents |
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemel, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Zaun, D.A.; Martinez, A.; Schaffhausen, C.R.; Lozano, C.; McKinney, W.T.; Miller, J.M.; Snyder, J.J. OPTN/SRTR 2023 Annual Data Report: Deceased Organ Donation. Am. J. Transplant. 2025, 25, S490–S517. [Google Scholar] [CrossRef]
- Yildirim, A.C., IV; Zeren, S.; Ekici, M.F.; Yaylak, F.; Algin, M.C.; Arik, O. Comparison of Fenestrating and Reconstituting Subtotal Cholecystectomy Techniques in Difficult Cholecystectomy. Cureus 2022, 14, e22441. [Google Scholar] [CrossRef]
- Kokas, B.; Ulmann, L.; Rozman, P.; Farkas, N.; Szijártó, A.; Szücs, Á. Postoperative bile leak after hepa-to-pancreato-biliary surgery in malignant biliary obstruction: Rates, treatments, and outcomes in a high-volume tertiary referral center. BMC Surg. 2024, 24, 410. [Google Scholar] [CrossRef]
- van Dijk, A.H.; Donkervoort, S.C.; Lameris, W.; de Vries, E.; Eijsbouts, Q.A.J.; Vrouenraets, B.C.; Busch, O.R.; Boermeester, M.A.; de Reuver, P.R. Short- and Long-Term Outcomes after a Reconstituting and Fenestrating Subtotal Chol-ecystectomy. J. Am. Coll. Surg. 2017, 225, 371–379. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Puleston, J.; Davidson, B.R.; Dooley, J.S. Outcome of early endoscopic biliary drainage in the management of bile leaks after hepatic resection. Gastrointest. Endosc. 2003, 57, 526–530. [Google Scholar] [CrossRef]
- Rerknimitr, R.; Sherman, S.; Fogel, E.L.; Kalayci, C.; Lumeng, L.; Chalasani, N.; Kwo, P.; Lehman, G.A. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: Endoscopic findings and results of therapy. Gastrointest. Endosc. 2002, 55, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Elmahi, E.; Fairclough, S.; Knifton, H. The Rate of Postoperative Bile Leak in Minimally Invasive Liver Resection in Comparison with Open Surgery: A Systematic Review. Cureus 2024, 16, e74313. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.J.; Atassi, T.; Lee, J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003, 23, 156–162. [Google Scholar] [CrossRef]
- Kubo, N.; Shirabe, K. Treatment strategy for isolated bile leakage after hepatectomy: Literature review. Ann. Gastroenterol. Surg. 2019, 4, 47–55. [Google Scholar] [CrossRef]
- Eurich, D.; Henze, S.; Boas-Knoop, S.; Pratschke, J.; Seehofer, D. T-drain reduces the incidence of biliary leakage after liver resection. Updates Surg. 2016, 68, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.F.; Blenkharn, J.I.; Edwards, W.J.; Orloff, M.; Blumgart, L.H.; Benjamin, I.S. Intra-abdominal sepsis after hepatic resection. Ann. Surg. 1989, 209, 302–306. [Google Scholar] [CrossRef]
- Mosconi, C.; Calandri, M.; Mirarchi, M.; Vara, G.; Breatta, A.D.; Cappelli, A.; Brandi, N.; Paccapelo, A.; De Benedittis, C.; Ricci, C.; et al. Percutaneous management of postoperative Bile leak after hepato-pancreato-biliary surgery: A multi-center experience. HPB 2021, 23, 1518–1524. [Google Scholar] [CrossRef]
- Pedicini, V.; Poretti, D.; Mauri, G.; Trimboli, M.; Brambilla, G.; Sconfienza, L.M.; Cornalba, G.; Sardanelli, F. Management of post-surgical biliary leakage with percutaneous transhepatic biliary drainage (PTBD) and occlusion balloon (OB) in patients without dilatation of the biliary tree: Preliminary results. Eur. Radiol. 2010, 20, 1061–1068. [Google Scholar] [CrossRef]
- Patel, I.J.; Rahim, S.; Davidson, J.C.; Hanks, S.E.; Tam, A.L.; Walker, T.G.; Wilkins, L.R.; Sarode, R.; Weinberg, I. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-Part II: Recommendations: Endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J. Vasc. Interv. Radiol. 2019, 30, 1168.e1–1184.e1. [Google Scholar]
- Houghton, E.J.; Uribe, A.K.; De Battista, J.M.; Finger, C.; Acquafresca, P.; Palermo, M.; Giménez, M.E. Risk Factors for Hemorrhagic Adverse Events in Percutaneous Transhepatic Biliary Drainage: A Prospective Multicenter Study. J. Vasc. Interv. Radiol. 2022, 33, 919.e2–925.e2. [Google Scholar] [CrossRef]
- Veitch, A.M.; Radaelli, F.; Alikhan, R.; Dumonceau, J.M.; Eaton, D.; Jerrome, J.; Lester, W.; Nylander, D.; Thoufeeq, M.; Vanbiervliet, G.; et al. Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update. Gut 2021, 70, 1611–1628. [Google Scholar] [CrossRef]
- Binmoeller, K.F.; Katon, R.M.; Shneidman, R. Endoscopic management of postoperative biliary leaks: Review of 77 cases and report of two cases with biloma formation. Am. J. Gastroenterol. 1991, 86, 227–231. [Google Scholar] [PubMed]
- Carannante, F.; Mazzotta, E.; Miacci, V.; Bianco, G.; Mascianà, G.; D’Agostino, F.; Caricato, M.; Capolupo, G.T. Identification and management of subvesical bile duct leakage after laparoscopic cholecystectomy: A systematic review. Asian J. Surg. 2023, 46, 4161–4168. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Murali, A.R.; Masadeh, M.; Silverman, W.B.; Johlin, F.C. Comparison of Biliary Stent versus Biliary Sphincterotomy Alone in the Treatment of Bile Leak. Dig. Dis. 2020, 38, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Rio-Tintoa, R.; Canena, J. Endoscopic Treatment of Post Cholecystectomy Biliary Leaks. GE Port. J. Gastroenterol. 2021, 28, 265–273. [Google Scholar] [CrossRef]
- Sandha, G.S.; Bourke, M.J.; Haber, G.B.; Kortan, P.P. Endoscopic therapy for bile leak based on a new classification: Results in 207 patients. Gastrointest. Endosc. 2004, 60, 567–574. [Google Scholar] [CrossRef]
- Desai, A.; Twohig, P.; Trujillo, S.; Dalal, S.; Kochhar, G.S.; Sandhu, D.S. Clinical efficacy, timing, and outcomes of ERCP for management of bile duct leaks: A nationwide cohort study. Endosc. Int. Open. 2021, 9, E247–E252. [Google Scholar]
- Gawlik, C.; Carneval, M. A Review of the Management of Bile Leaks. Cureus 2021, 13, e14937. [Google Scholar] [CrossRef]
- Vlaemynck, K.; Lahousse, L.; Vanlander, A.; Piessevaux, H.; Hindryckx, P. Endoscopic management of biliary leaks: A systematic review with meta-analysis. Endoscopy 2019, 51, 1074–1081. [Google Scholar] [CrossRef]
- Schaible, A.; Schemmer, P.; Hackert, T.; Rupp, C.; Schleithoff, A.E.S.; Gotthardt, D.N.; Büchler, M.W.; Sauer, P. Location of a biliary leak after liver resection determines success of endoscopic treatment. Surg. Endosc. 2017, 31, 1814–1820. [Google Scholar] [CrossRef]
- Moy, B.T.; Birk, J.W. A review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Nagra, N.; Klair, J.S.; Jayaraj, M.; Murali, A.R.; Singh, D.; Law, J.; Larsen, M.; Irani, S.; Kozarek, R.; Ross, A.; et al. Biliary Sphincterotomy Alone versus Biliary Stent with or without Biliary Sphincterotomy for the Management of Post-Cholecystectomy Bile Leak: A Systematic Review and Meta-Analysis. Dig. Dis. 2022, 40, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-W.; Lee, S.K.; Song, T.J.; Park, D.H.; Lee, S.S.; Seo, D.-W.; Kim, M.-H. Endoscopic Management of Bile Leakage after Liver Transplantation. Gut Liver 2015, 9, 417–423. [Google Scholar] [CrossRef]
- Sendino, O.; Fernández-Simon, A.; Law, R.; Dayyeh, B.A.; Leise, M.; Chavez-Rivera, K.; Cordova, H.; Colmenero, J.; Crespo, G.; de Miguel, C.R.; et al. Endoscopic management of bile leaks after liver transplantation: An analysis of two high-volume transplant centers. United Eur. Gastroenterol. J. 2018, 6, 89–96. [Google Scholar] [CrossRef]
- Wu, G.; Li, W.-Y.; Gong, Y.-X.; Lin, F.; Sun, C. Impact of open hepatectomy on postoperative bile leakage in patients with biliary tract cancer. World J. Gastrointest. Surg. 2024, 16, 67–75. [Google Scholar] [CrossRef]
- Çelik, M.; Yilmaz, H.; Akbudak, İ.H.; Kılıç, M.C.; Ozban, M.; Yılmaz, M.; Soykan, M. Efficacy and safety of endoscopic retrograde cholangiopancreatography with endoscopic sphincterotomy and biliary stenting in post-operative bile leaks. Ulus. Travma. Acil. Cerrahi. Derg. 2023, 29, 904–908. [Google Scholar] [CrossRef]
- Tewani, S.K.; Turner, B.G.; Chuttani, R.; Pleskow, D.K.; Sawhney, M.S. Location of bile leak predicts the success of ERCP performed for postoperative bile leaks. Gastrointest. Endosc. 2013, 77, 601–608. [Google Scholar] [CrossRef]
- Koch, M.; Garden, O.J.; Padbury, R.; Rahbari, N.N.; Adam, R.; Capussotti, L.; Fan, S.T.; Yokoyama, Y.; Crawford, M.; Makuuchi, M.; et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011, 149, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Cotton, P.B.; Eisen, G.M.; Aabakken, L.; Baron, T.H.; Hutter, M.M.; Jacobson, B.C.; Mergener, K.; Nemcek, A., Jr.; Petersen, B.T.; Petrini, J.L.; et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010, 71, 446–454. [Google Scholar] [CrossRef]
- Canena, J.; Horta, D.; Coimbra, J.; Meireles, L.; Russo, P.; Marques, I.; Ricardo, L.; Rodrigues, C.; Capela, T.; Carvalho, D.; et al. Outcomes of endoscopic management of primary and refractory postcholecystectomy biliary leaks in a multicentre review of 178 patients. BMC Gastroenterol. 2015, 15, 105. [Google Scholar] [CrossRef]
- Haidar, H.; Manasa, E.; Yassin, K.; Suissa, A.; Kluger, Y.; Khamaysi, I. Endoscopic treatment of post-cholecystectomy bile leaks: A tertiary center experience. Surg. Endosc. 2021, 35, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Dechêne, A.; Jochum, C.; Fingas, C.; Paul, A.; Heider, D.; Syn, W.-K.; Gerken, G.; Canbay, A.; Zöpf, T. Endoscopic management is the treatment of choice for bile leaks after liver resection. Gastrointest. Endosc. 2014, 80, 626–633.e1. [Google Scholar] [CrossRef]
- Bjorkman, D.J.; Carr-Locke, D.L.; Lichtenstein, D.R.; Ferrari, A.P.; Slivka, A.; Van Dam, J.; Brooks, D.C. Postsurgical bile leaks: Endoscopic obliteration of the transpapillary pressure gradient is enough. Am. J. Gastroenterol. 1995, 90, 2128–2133. [Google Scholar]
- Baron, T.H.; Poterucha, J.J. Insertion and Removal of Covered Expandable Metal Stents for Closure of Complex Biliary Leaks. Clin. Gastroenterol. Hepatol. 2006, 4, 381–386. [Google Scholar]
- Macías-Gómez, C.; Dumonceau, J.-M. Endoscopic management of biliary complications after liver transplantation: An evidence-based review. World J. Gastrointest. Endosc. 2015, 7, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Mavrogiannis, C.; Liatsos, C.; Papanikolaou, I.S.; Karagiannis, S.; Galanis, P.; Romanos, A. Biliary stenting alone versus biliary stenting plus sphincterotomy for the treatment of post-laparoscopic cholecystectomy biliary leaks: A prospective randomized study. Eur. J. Gastroenterol. Hepatol. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.; Kato, H.; Mizukawa, S.; Akimoto, Y.; Uchida, D.; Seki, H.; Tomoda, T.; Matsumoto, K.; Yamamoto, N.; Horiguchi, S.; et al. Predictive factors for outcomes of patients undergoing endoscopic therapy for bile leak after hepatobiliary surgery. Dig. Endosc. 2017, 29, 353–361. [Google Scholar] [CrossRef]
- Canena, J.; Liberato, M.; Horta, D.; Romão, C.; Coutinho, A. Short-term stenting using fully covered self-expandable metal stents for treatment of refractory biliary leaks, postsphincterotomy bleeding, and perforations. Surg. Endosc. 2013, 27, 313–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).