Secondary Prevention After Acute Coronary Syndromes in Women: Tailored Management and Cardiac Rehabilitation
Abstract
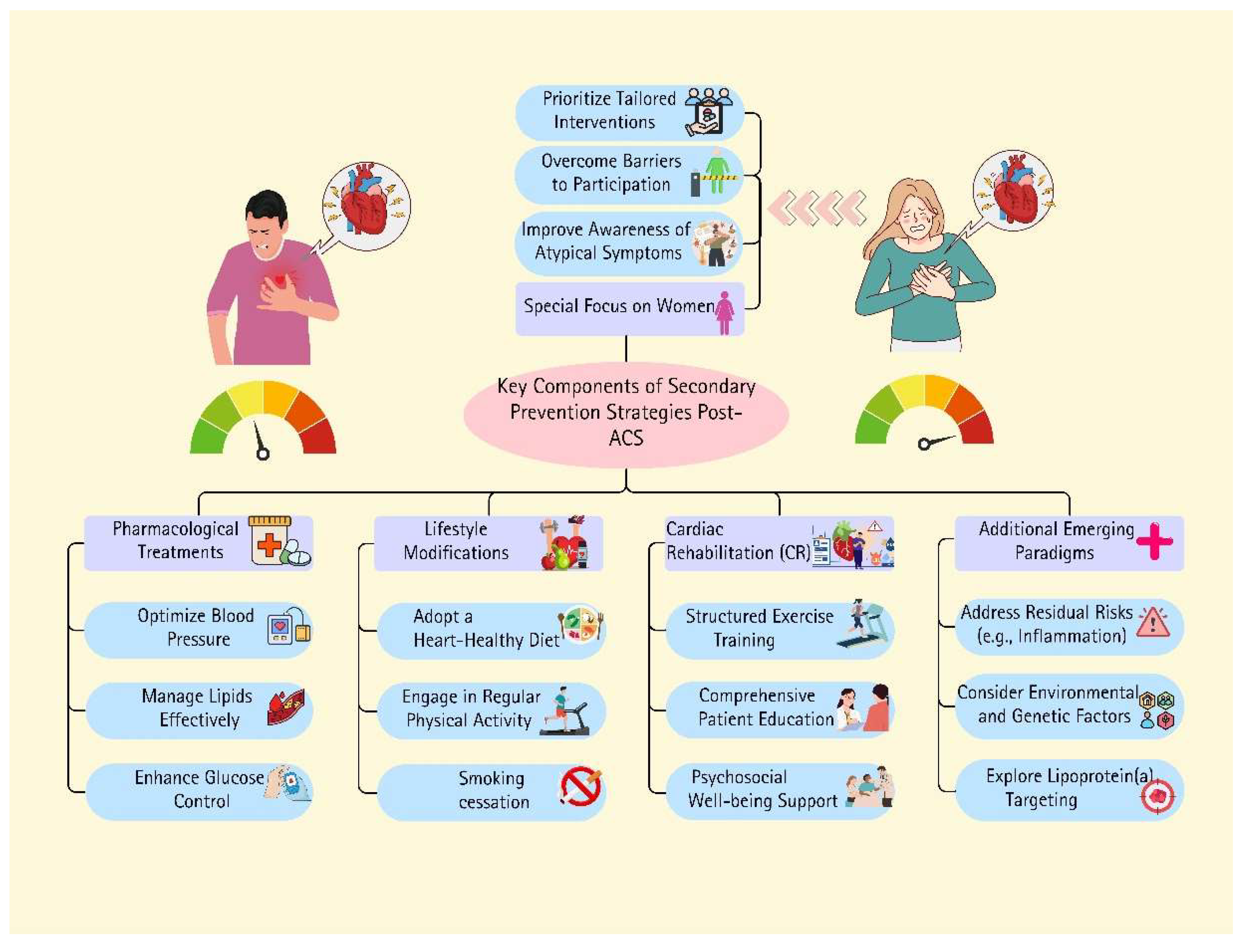
1. Introduction
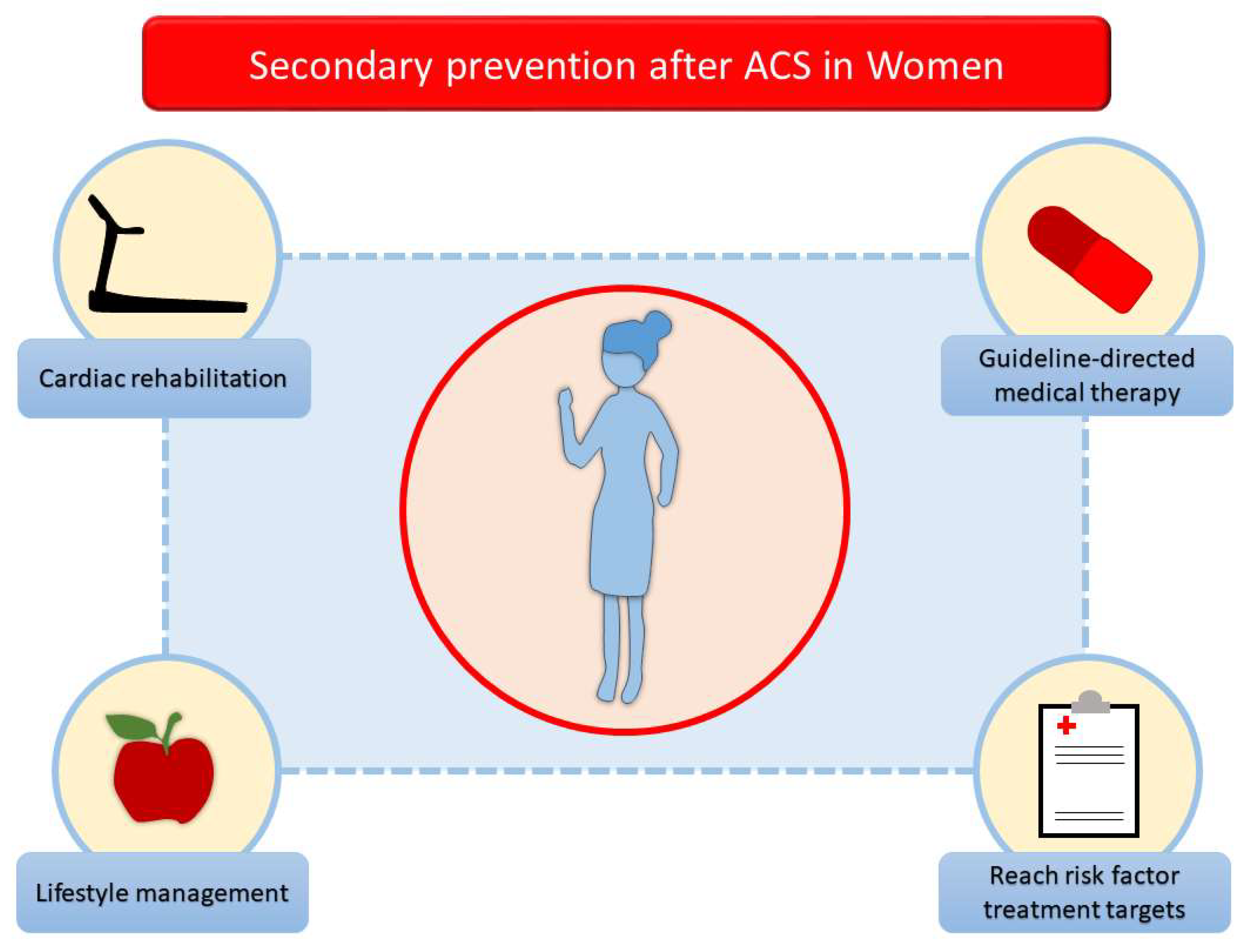
2. Lifestyle Management
2.1. Tobacco Cessation
2.2. Nutrition and Alcohol Intake
2.3. Physical Activity
2.4. Psychosocial Factors
3. Risk Factor Management
3.1. Blood Pressure
3.2. LDL-Cholesterol
3.3. Glycemia
4. Pharmacological Therapy
| Study | Study Population | Findings |
|---|---|---|
| Anand et al. [56] 2005 | Patients with ACS, 1998–2000 | Women were less likely to be treated with beta-blockers |
| Jneid et al. [57] 2008 | Patients with AMI in 420 US hospitals, 2001–2006 | Women were less likely to receive early aspirin treatment and early beta-blocker treatment |
| Akhter et al. [58] 2009 | Patients with ACS, 2004–2006 | Women were less likely to receive aspirin or glycoprotein IIb/IIIa inhibitors Women were less often discharged on aspirin or statin |
| Arora et al. [45] 2019 | AMI in four US communities, young patients aged from 35 to 54 years, 1995–2014 | Young women were less likely to be prescribed nonaspirin antiplatelet therapy (p < 0.0001), lipid-lowering medications (p < 0.0001), beta-blockers (p = 0.04), and ACEi/ARBs (p = 0.02) |
| Hao et al. [59] 2019 | Patients with ACS, 2014–2018 | Women were less likely to receive DAPT, renin-angiotensin system inhibitors, and statins at discharge |
| Vynckier et al. [60] 2020 | Patients with coronary heart disease | No gender differences in the prescription of aspirin, beta-blockers, and ACE-I/ARBs Women were less likely on statins at follow-up Women were more likely to receive calcium channel blockers |
| Dagan et al. [42] 2022 | Patients with ACS within the multicentre Melbourne Interventional Group registry, 2005–2017 | Women were less likely to receive a second anti-platelet agent (p = 0.03), a statin (p < 0.001), an ACEi/ARB (p < 0.001), and a beta-blocker (p < 0.001) compared to men Women were more likely to be prescribed clopidogrel (p < 0.001) and less likely to be prescribed ticagrelor (p = 0.001) |
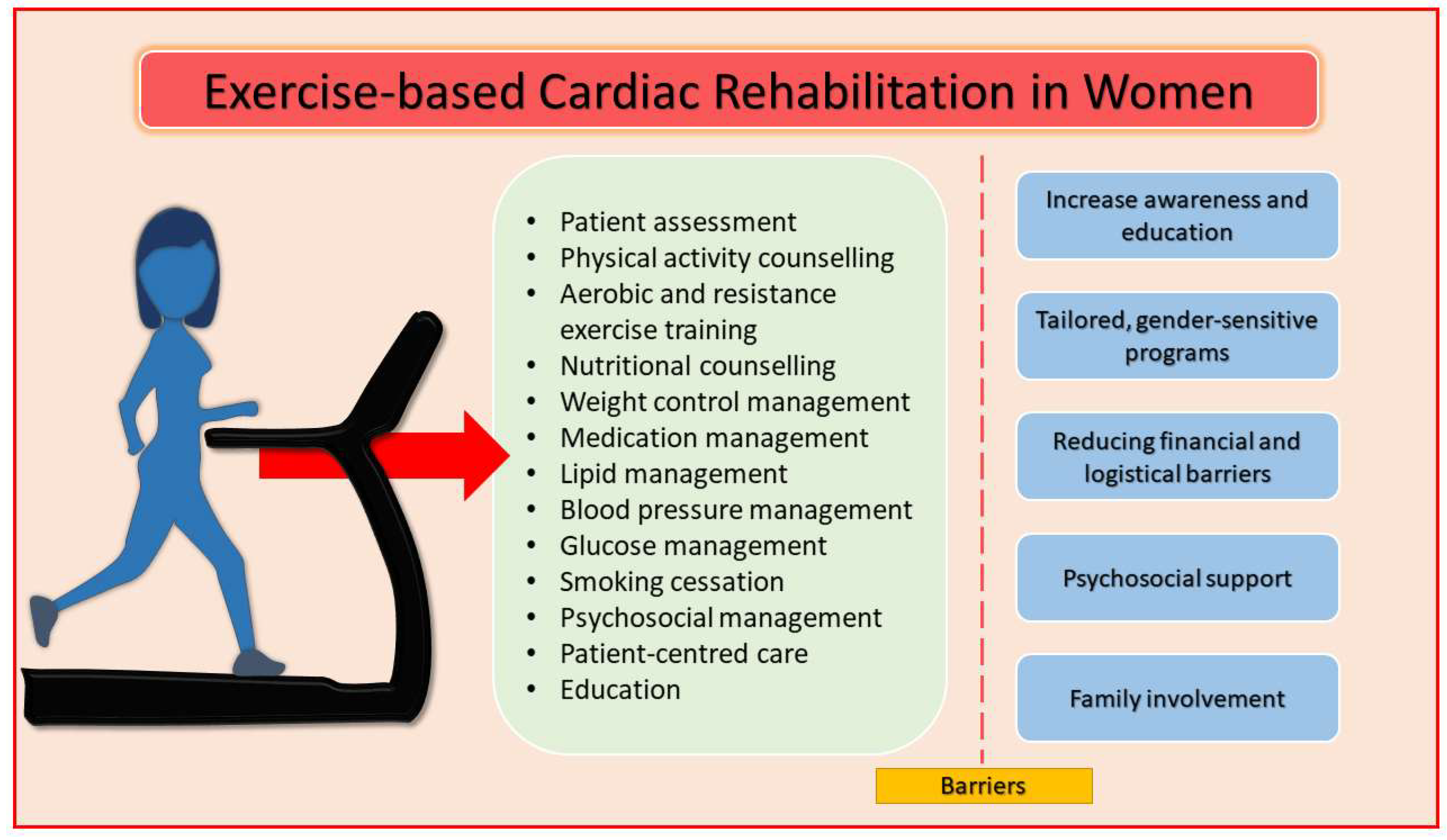
5. Cardiac Rehabilitation
- Increase awareness and education: healthcare providers should clearly communicate the benefits of CR, emphasizing how exercise, education, and psychosocial support can reduce the risk of recurrent cardiovascular events and improve long-term quality of life [1].
- Tailored, gender-sensitive programs: CR programs should account for the unique challenges women face, such as balancing family responsibilities, lower physical fitness, and higher rates of mental health issues. Flexible scheduling, home-based programs, and social support can increase adherence [72,74].
- Reducing financial and logistical barriers: Programs should offer financial assistance, lower fees, and virtual participation options. Providing transportation support or offering community-based programs can improve accessibility for women [72].
- Psychosocial support: Addressing psychological concerns is critical. Integrating mental health support within CR programs can help manage stress, anxiety, and depression, thereby improving participation and long-term adherence [72].
- Family involvement: encouraging family members to participate in CR can provide additional emotional support and increase motivation for women to stick with the program [69].
6. Tailored Management for Women After Acute Coronary Syndrome: A Secondary Prevention Strategy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESCGuidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Panattoni, G.; Monzo, L.; Gugliotta, M.; Proietti, G.; Tatangelo, M.; Jacomelli, I.; Zimbardo, G.; Meringolo, F.; Fedele, E.; Calò, L. Optimal management of patients after acute coronary syndrome. Eur. Heart J. Suppl. 2023, 25, C84–C89. [Google Scholar] [CrossRef] [PubMed]
- Silverio, A.; Cancro, F.P.; Esposito, L.; Bellino, M.; D’Elia, D.; Verdoia, M.; Vassallo, M.G.; Ciccarelli, M.; Vecchione, C.; Galasso, G.; et al. Secondary Cardiovascular Prevention after Acute Coronary Syndrome: Emerging Risk Factors and Novel Therapeutic Targets. J. Clin. Med. 2023, 12, 2161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bentzel, S.; Ljungman, C.; Hjerpe, P.; Schiöler, L.; Manhem, K.; Boström, K.B.; Kahan, T.; Mourtzinis, G. Long-term secondary prevention and outcome following acute coronary syndrome: Real-world results from the Swedish Primary Care Cardiovascular Database. Eur. J. Prev. Cardiol. 2024, 31, 812–821. [Google Scholar] [CrossRef]
- Kotseva, K.; De Backer, G.; De Bacquer, D.; Rydén, L.; Hoes, A.; Grobbee, D.; Maggioni, A.; Marques-Vidal, P.; Jennings, C.; Abreu, A.; et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: Results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur. J. Prev. Cardiol. 2019, 26, 824–835. [Google Scholar] [CrossRef]
- Ray, K.K.; Molemans, B.; Schoonen, W.M.; Giovas, P.; Bray, S.; Kiru, G.; Murphy, J.; Banach, M.; De Servi, S.; Gaita, D.; et al. EU-Wide Cross-Sectional Observational Study of Lipid-Modifying Therapy Use in Secondary and Primary Care: The DA VINCI study. Eur. J. Prev. Cardiol. 2021, 28, 1279–1289. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESCGuidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]
- Al-Kindi, S.; Paneni, F.; Brook, R.D.; Rajagopalan, S. Residual environmental risk in patients with cardiovascular disease: An overlooked paradigm. Eur. Heart J. 2023, 44, 4612–4614. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Raya-Cruz, M.; Katsiki, N.; Delgado-Lista, J.; Perez-Martinez, P. Residual cardiovascular risk: When should we treat it? Eur. J. Intern. Med. 2024, 120, 17–24. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Arca, M.; Scicchitano, P.; Alonzo, A.; Perone, F.; Gulizia, M.M.; Gabrielli, D.; Oliva, F.; Imperoli, G.; Colivicchi, F. Lipoprotein(a): A risk factor for atherosclerosis and an emerging therapeutic target. Heart 2022, 109, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, L.D.; Rosenson, R.S.; Deng, L.; Monda, K.L.; Dai, Y.; Farkouh, M.E.; Safford, M.M.; Philip, K.; Mues, K.E.; Muntner, P. Adherence to Statin Therapy Among US Adults Between 2007 and 2014. J. Am. Heart Assoc. 2019, 8, e010376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheikhy, A.; Fallahzadeh, A.; Jameie, M.; Aein, A.; Masoudkabir, F.; Maghsoudi, M.; Tajdini, M.; Salarifar, M.; Jenab, Y.; Pourhosseini, H.; et al. In-hospital and 1-year outcomes of patients without modifiable risk factors presenting with acute coronary syndrome undergoing PCI: A Sex-stratified analysis. Front. Cardiovasc. Med. 2023, 10, 1235667. [Google Scholar] [CrossRef]
- Elia, E.; Bruno, F.; Crimi, G.; Wańha, W.; Leonardi, S.; Mauro, M.; Raposeiras Roubin, S.; Fabris, E.; Giannino, G.; Mancone, M.; et al. Gender differences in the development of heart failure after acute coronary syndrome: Insight from the CORALYS registry. Int. J. Cardiol. 2024, 397, 131622. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, J.N.; Kaur, G.; Hansen, B.; Bushana, N.; Gulati, M. Sex differences in the management of atherosclerotic cardiovascular disease. Atherosclerosis 2023, 384, 117268. [Google Scholar] [CrossRef]
- Sucato, V.; Comparato, F.; Ortello, A.; Galassi, A.R. Myocardical Infarction with Non-Obstructive Coronary Arteries (MINOCA): Pathogenesis, diagnosis and treatment. Curr. Probl. Cardiol. 2024, 49, 102583. [Google Scholar] [CrossRef]
- Dutta, D.; Mahajan, K.; Verma, L.; Gupta, G.; Sharma, M. Gender differences in the management and outcomes of acute coronary syndrome in indians: A systematic review and meta-analysis. Indian Heart J. 2024, 76, 333–341. [Google Scholar] [CrossRef]
- Cader, F.A.; Sareen, N.; Press, M.C. Acute Coronary Syndrome in Women. Interv. Cardiol. Clin. 2025, 14, 9–19. [Google Scholar] [CrossRef]
- Blacher, J.; Olié, V.; Gabet, A.; Cinaud, A.; Tuppin, P.; Iliou, M.C.; Grave, C. Two-year prognosis and cardiovascular disease prevention after acute coronary syndrome: The role of cardiac rehabilitation-a French nationwide study. Eur. J. Prev. Cardiol. 2024, 31, 1939–1947. [Google Scholar] [CrossRef]
- Norekvål, T.M.; Bale, M.; Bedane, H.K.; Hole, T.; Ingul, C.B.; Munkhaugen, J. Cardiac rehabilitation participation within 6 months of discharge in 37 136 myocardial infarction survivors: A nationwide registry study. Eur. J. Prev. Cardiol. 2024, 31, 1977–1980. [Google Scholar] [CrossRef]
- Haider, A.; Bengs, S.; Luu, J.; Osto, E.; Siller-Matula, J.M.; Muka, T.; Gebhard, C. Sex and gender in cardiovascular medicine: Presentation and outcomes of acute coronary syndrome. Eur. Heart J. 2020, 41, 1328–1336. [Google Scholar] [CrossRef]
- Liblik, K.; Mulvagh, S.L.; Hindmarch, C.C.T.; Alavi, N.; Johri, A.M. Depression and anxiety following acute myocardial infarction in women. Trends Cardiovasc. Med. 2022, 32, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Derout, J.; Shao, X.M.; Lao, C.J.; Hasan, K.M.; Rivera, J.C.; Jordan, M.C.; Echeverria, V.; Roos, K.P.; Sinha-Hikim, A.P.; Friedman, T.C. Electronic Cigarette Use and the Risk of Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 879726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kavousi, M.; Pisinger, C.; Barthelemy, J.C.; De Smedt, D.; Koskinas, K.; Marques-Vidal, P.; Panagiotakos, D.; Prescott, E.B.; Tiberi, M.; Vassiliou, V.S.; et al. Electronic cigarettes and health with special focus on cardiovascular effects: Position paper of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 2021, 28, 1552–1566. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESCGuidelines on sports cardiology exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Tsugawa, Y.; Mayeda, E.R.; Ritz, B. Association of Daily Step Patterns with Mortality in US Adults. JAMA Netw. Open 2023, 6, e235174, Erratum in JAMA Netw. Open 2023, 6, e2311413. https://doi.org/10.1001/jamanetworkopen.2023.11413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winzer, E.B.; Woitek, F.; Linke, A. Physical Activity in the Prevention and Treatment of Coronary Artery Disease. J. Am. Heart Assoc. 2018, 7, e007725. [Google Scholar] [CrossRef]
- Flygare, O.; Boberg, J.; Rück, C.; Hofmann, R.; Leosdottir, M.; Mataix-Cols, D.; de la Cruz, L.F.; Richman, P.; Wallert, J. Association of anxiety or depression with risk of recurrent cardiovascular events and death after myocardial infarction: A nationwide registry study. Int. J. Cardiol. 2023, 381, 120–127. [Google Scholar] [CrossRef]
- López Ferreruela, I.; Obón Azuara, B.; Malo Fumanal, S.; Rabanaque Hernández, M.J.; Aguilar-Palacio, I. Gender inequalities in secondary prevention of cardiovascular disease: A scoping review. Int. J. Equity Health 2024, 23, 146. [Google Scholar] [CrossRef]
- Rizza, V.; Tondi, L.; Patti, A.M.; Cecchi, D.; Lombardi, M.; Perone, F.; Ambrosetti, M.; Rizzo, M.; Cianflone, D.; Maranta, F. Diabetic cardiomyopathy: Pathophysiology, imaging assessment and therapeutical strategies. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 23, 200338. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Agarwala, A.; Michos, E.D. Secondary Prevention of Cardiovascular Disease in Women: Closing the Gap. Eur. Cardiol. 2021, 16, e41. [Google Scholar] [CrossRef] [PubMed]
- Ramezankhani, A.; Azizi, F.; Hadaegh, F. Sex differences in risk factors for coronary heart disease events: A prospective cohort study in Iran. Sci. Rep. 2023, 13, 22398. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Perrone, V.; Veronesi, C.; Gambera, M.; Nati, G.; Perone, F.; Tagliabue, P.F.; Buda, S.; Borghi, C. Modifications in drug adherence after switch to fixed-dose combination of perindopril/amlodipine in clinical practice. Results of a large-scale Italian experience. The amlodipine-perindopril in real settings (AMPERES) study. Curr. Med. Res. Opin. 2018, 34, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Perrone, V.; Veronesi, C.; Gambera, M.; Nati, G.; Perone, F.; Tagliabue, P.F.; Degli Esposti, L.; Volpe, M. Treatment with Free Triple Combination Therapy of Atorvastatin, Perindopril, Amlodipine in Hypertensive Patients: A Real-World Population Study in Italy. High. Blood Press. Cardiovasc. Prev. 2019, 26, 399–404. [Google Scholar] [CrossRef]
- Alanezi, M.; Yan, A.T.; Tan, M.K.; Bourgeois, R.; Malek-Marzban, P.; Beharry, R.; Alkurtass, S.; Gyenes, G.T.; Nadeau, P.L.; Nwadiaro, N.; et al. Optimizing Post-Acute Coronary Syndrome Dyslipidemia Management: Insights from the North American Acute Coronary Syndrome Reflective III. Cardiology 2024, 149, 266–274. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Maggioni, A.P.; Bernelli, C.; Perone, F.; De Marzo, V.; Conte, E.; Musella, F.; Uccello, G.; Luca, L.; Gabrielli, D.; et al. Inclisiran: A New Pharmacological Approach for Hypercholesterolemia. Rev. Cardiovasc. Med. 2022, 23, 375. [Google Scholar] [CrossRef]
- Lin, Z.; He, J.; Yuan, S.; Song, C.; Bian, X.; Yang, M.; Dou, K. Glycemic control and cardiovascular outcomes in patients with diabetes and coronary artery disease according to triglyceride-glucose index: A large-scale cohort study. Cardiovasc. Diabetol. 2024, 23, 11. [Google Scholar] [CrossRef]
- Qaseem, A.; Obley, A.J.; Shamliyan, T.; Hicks, L.A.; Harrod, C.S.; Clinical Guidelines Committee of the American College of Physicians; Balk, E.M.; Cooney, T.G.; Cross, J.T., Jr.; Fitterman, N.; et al. Newer Pharmacologic Treatments in Adults with Type 2 Diabetes: A Clinical Guideline From the American College of Physicians. Ann. Intern. Med. 2024, 177, 658–666. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S179–S218. [Google Scholar] [CrossRef]
- Gasecka, A.; Zimodro, J.M.; Appelman, Y. Sex differences in antiplatelet therapy: State-of-the art. Platelets 2023, 34, 2176173. [Google Scholar] [CrossRef] [PubMed]
- Dagan, M.; Dinh, D.T.; Stehli, J.; Tan, C.; Brennan, A.; Warren, J.; Ajani, A.E.; Freeman, M.; Murphy, A.; Reid, C.M.; et al. Sex disparity in secondary prevention pharmacotherapy and clinical outcomes following acute coronary syndrome. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 420–428. [Google Scholar] [CrossRef]
- Sarma, A.A.; Braunwald, E.; Cannon, C.P.; Guo, J.; Im, K.; Antman, E.M.; Gibson, C.M.; Newby, L.K.; Giugliano, R.P.; Morrow, D.A.; et al. Outcomes of Women Compared with Men After Non-ST-Segment Elevation Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2019, 74, 3013–3022. [Google Scholar] [CrossRef]
- Smolina, K.; Ball, L.; Humphries, K.H.; Khan, N.; Morgan, S.G. Sex Disparities in Post-Acute Myocardial Infarction Pharmacologic Treatment Initiation and Adherence: Problem for Young Women. Circ. Cardiovasc. Qual. Outcomes 2015, 8, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Stouffer, G.A.; Kucharska-Newton, A.M.; Qamar, A.; Vaduganathan, M.; Pandey, A.; Porterfield, D.; Blankstein, R.; Rosamond, W.D.; Bhatt, D.L.; et al. Twenty Year Trends and Sex Differences in Young Adults Hospitalized with Acute Myocardial Infarction. Circulation 2019, 139, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Madonis, S.M.; Skelding, K.A.; Roberts, M. Management of acute coronary syndromes: Special considerations in women. Heart 2017, 103, 1638–1646. [Google Scholar] [CrossRef]
- Mauri, L.; Smith, S.C., Jr. Focused Update on Duration of Dual Antiplatelet Therapy for Patients with Coronary Artery Disease. JAMA Cardiol. 2016, 1, 733–734. [Google Scholar] [CrossRef]
- Husted, S.; James, S.K.; Bach, R.G.; Becker, R.C.; Budaj, A.; Heras, M.; Himmelmann, A.; Horrow, J.; Katus, H.A.; Lassila, R.; et al. The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective randomized PLATelet inhibition patient Outcomes (PLATO) trial. Eur. Heart J. 2014, 35, 1541–1550. [Google Scholar] [CrossRef]
- Bots, S.H.; Inia, J.A.; Peters, S.A.E. Medication Adherence After Acute Coronary Syndrome in Women Compared with Men: A Systematic Review and Meta-Analysis. Front. Glob. Womens Health 2021, 2, 637398. [Google Scholar] [CrossRef]
- Weizman, O.; Hauguel-Moreau, M.; Tea, V.; Albert, F.; Barragan, P.; Georges, J.L.; Delarche, N.; Kerneis, M.; Bataille, V.; Drouet, E.; et al. Prognostic impact of high-intensity lipid-lowering therapy under-prescription after acute myocardial infarction in women. Eur. J. Prev. Cardiol. 2024, 31, 1850–1860. [Google Scholar] [CrossRef]
- Ang, S.P.; Chia, J.E.; Krittanawong, C.; Lee, K.; Iglesias, J.; Misra, K.; Mukherjee, D. Sex Differences and Clinical Outcomes in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries: A Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e035329. [Google Scholar] [CrossRef] [PubMed]
- Lawless, M.; Appelman, Y.; Beltrame, J.F.; Navarese, E.P.; Ratcovich, H.; Wilkinson, C.; Kunadian, V. Sex differences in treatment and outcomes amongst myocardial infarction patients presenting with and without obstructive coronary arteries: A prospective multicentre study. Eur. Heart J. Open 2023, 3, oead033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciliberti, G.; Compagnucci, P.; Urbinati, A.; Bianco, F.; Stronati, G.; Lattanzi, S.; Dello Russo, A.; Guerra, F. Myocardial Infarction Without Obstructive Coronary Artery Disease (MINOCA): A Practical Guide for Clinicians. Curr. Probl. Cardiol. 2021, 46, 100761. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.H.; Chang, K.; Lee, K.Y.; Lee, D.; Kim, J.G.; Ahn, Y.; Kim, Y.J.; Chae, S.C.; Cho, M.C.; Kim, C.J.; et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction with Non-Obstructive Coronary Arteries. J. Am. Heart Assoc. 2019, 8, e011990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nordenskjöld, A.M.; Agewall, S.; Atar, D.; Baron, T.; Beltrame, J.; Bergström, O.; Erlinge, D.; Gale, C.P.; López-Pais, J.; Jernberg, T.; et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am. Heart J. 2021, 231, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Xie, C.C.; Mehta, S.; Franzosi, M.G.; Joyner, C.; Chrolavicius, S.; Fox, K.A.; Yusuf, S.; CURE Investigators. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J. Am. Coll. Cardiol. 2005, 46, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Jneid, H.; Fonarow, G.C.; Cannon, C.P.; Hernandez, A.F.; Palacios, I.F.; Maree, A.O.; Wells, Q.; Bozkurt, B.; Labresh, K.A.; Liang, L.; et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation 2008, 118, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Milford-Beland, S.; Roe, M.T.; Piana, R.N.; Kao, J.; Shroff, A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am. Heart J. 2009, 157, 141–148. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Liu, J.; Yang, N.; Smith SCJr Huo, Y.; Fonarow, G.C.; Ge, J.; Taubert, K.A.; Morgan, L.; Zhou, M.; et al. Sex Differences in In-Hospital Management and Outcomes of Patients with Acute Coronary Syndrome. Circulation 2019, 139, 1776–1785. [Google Scholar] [CrossRef]
- Vynckier, P.; Ferrannini, G.; Rydén, L.; Tokgözoğlu, L.; Bruthans, J.; Kotseva, K.; Wood, D.; De Backer, T.; Gevaert, S.; De Bacquer, D.; et al. Medical Treatment in Coronary Patients: Is there Still a Gender Gap? Results from European Society of Cardiology EUROASPIRE V Registry. Cardiovasc. Drugs Ther. 2021, 35, 801–808. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016, 2016, CD001800. [Google Scholar] [CrossRef] [PubMed]
- Ambrosetti, M.; Abreu, A.; Corrà, U.; Davos, C.H.; Hansen, D.; Frederix, I.; Iliou, M.C.; Pedretti, R.F.E.; Schmid, J.P.; Vigorito, C.; et al. Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 460–495. [Google Scholar] [CrossRef]
- Perone, F.; Spadafora, L.; Pratesi, A.; Nicolaio, G.; Pala, B.; Franco, G.; Ruzzolini, M.; Ambrosetti, M. Obesity and cardiovascular disease: Risk assessment, physical activity, and management of complications. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 23, 200331. [Google Scholar] [CrossRef]
- Clark, A.M.; Hartling, L.; Vandermeer, B.; McAlister, F.A. Meta-analysis: Secondary prevention programs for patients with coronary artery disease. Ann. Intern. Med. 2005, 143, 659–672. [Google Scholar] [CrossRef]
- Supervia Pola, M.; Medina-Inojosa, J.; Yeung, C.; Lopez-Jimenez, F.; Squires, R.; Pérez-Terzic, C.; Brewer, L.; Leth, S.; Thomas, R. Cardiac rehabilitation for women: A systematic review of barriers and solutions. Mayo Clin. Proc. 2017, 92, 761–768. [Google Scholar] [CrossRef]
- De Feo, S.; Tramarin, R.; Ambrosetti, M.; Riccio, C.; Temporelli, P.L.; Favretto, G.; Furgi, G.; Griffo, R. Gender differences in cardiac rehabilitation programs from the Italian survey on cardiac rehabilitation (ISYDE-2008). Int. J. Cardiol. 2012, 160, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rengo, J.L.; Khadanga, S.; Savage, P.D.; Ades, P.A. Response to exercise training during cardiac rehabilitation differs by sex. J. Cardiopulm. Rehabil. Prev. 2020, 40, 319–324. [Google Scholar] [CrossRef]
- Savage, P.D.; Antkowiak, M.; Ades, P.A. Failure to improve cardiopulmonary fitness in cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2009, 29, 284–291. [Google Scholar] [CrossRef]
- De Schutter, A.; Kachur, S.; Lavie, C.J.; Menezes, A.; Shum, K.K.; Bangalore, S.; Arena, R.; Milani, R.V. Cardiac rehabilitation fitness changes and subsequent survival. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 173–179. [Google Scholar] [CrossRef]
- Saeidifard, F.; Medina-Inojosa, J.R.; West, C.P.; Olson, T.P.; Somers, V.K.; Bonikowske, A.R.; Prokop, L.J.; Vinciguerra, M.; Lopez-Jimenez, F. The association of resistance training with mortality: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 1647–1665. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Cavero-Redondo, I.; Ramírez-Vélez, R.; Ruiz, J.R.; Ortega, F.B.; Lee, D.C.; Martínez-Vizcaíno, V. Muscular strength as a predictor of all-cause mortality in an apparently healthy population: A systematic review and meta-analysis of data from approximately 2 million men and women. Arch. Phys. Med. Rehabil. 2018, 99, 2100–2113.e5. [Google Scholar] [CrossRef] [PubMed]
- Price, J.; Landry, M.; Rolfe, D.; Delos-Reyes, F.; Groff, L.; Sternberg, L. Women’s cardiac rehabilitation: Improving access using principles of women’s health. Can. J. Cardiovasc. Nurs. 2005, 15, 32–41. [Google Scholar] [PubMed]
- Heran, B.S.; Chen, J.M.; Ebrahim, S.; Moxham, T.; Oldridge, N.; Rees, K.; Thompson, D.R.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2011, CD001800. [Google Scholar] [CrossRef]
- Angeli, F.; Ricci, F.; Moscucci, F.; Sciomer, S.; Bucciarelli, V.; Bianco, F.; Mattioli, A.V.; Pizzi, C.; Gallina, S. Sex- and gender-related disparities in chest pain syndromes: The feminine mystique of chest pain. Curr. Probl. Cardiol. 2024, 49, 102457. [Google Scholar] [CrossRef] [PubMed]
| Barrier | Solution |
|---|---|
| Gender bias in referral | Raise awareness among healthcare providers about the benefits of CR for women |
| Socioeconomic barriers | Provide financial assistance, sliding scale fees, and transportation support |
| Psychosocial barriers | Integrate mental health support, counseling, and stress management |
| Lack of awareness | Educate women about the importance of CR and its long-term benefits |
| Family responsibilities | Offer flexible schedules and home-based CR options to accommodate caregiving roles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorescu, L.-V.; Prisacariu, I.; Aboueddahab, C.; Taheri, M.; Jaiswal, V.; Avagimyan, A.; Ghram, A.; Dumitrescu, S.I.; Banach, M.; Perone, F. Secondary Prevention After Acute Coronary Syndromes in Women: Tailored Management and Cardiac Rehabilitation. J. Clin. Med. 2025, 14, 3357. https://doi.org/10.3390/jcm14103357
Iorescu L-V, Prisacariu I, Aboueddahab C, Taheri M, Jaiswal V, Avagimyan A, Ghram A, Dumitrescu SI, Banach M, Perone F. Secondary Prevention After Acute Coronary Syndromes in Women: Tailored Management and Cardiac Rehabilitation. Journal of Clinical Medicine. 2025; 14(10):3357. https://doi.org/10.3390/jcm14103357
Chicago/Turabian StyleIorescu, Luana-Viviana, Irina Prisacariu, Chaimae Aboueddahab, Maryam Taheri, Vikash Jaiswal, Ashot Avagimyan, Amine Ghram, Silviu Ionel Dumitrescu, Maciej Banach, and Francesco Perone. 2025. "Secondary Prevention After Acute Coronary Syndromes in Women: Tailored Management and Cardiac Rehabilitation" Journal of Clinical Medicine 14, no. 10: 3357. https://doi.org/10.3390/jcm14103357
APA StyleIorescu, L.-V., Prisacariu, I., Aboueddahab, C., Taheri, M., Jaiswal, V., Avagimyan, A., Ghram, A., Dumitrescu, S. I., Banach, M., & Perone, F. (2025). Secondary Prevention After Acute Coronary Syndromes in Women: Tailored Management and Cardiac Rehabilitation. Journal of Clinical Medicine, 14(10), 3357. https://doi.org/10.3390/jcm14103357









