Nasal Rinsing with Probiotics—Microbiome Evaluation in Patients with Inflammatory Diseases of the Nasal Mucosa
Abstract
1. Introduction
2. Materials and Methods
- -
- Lactobacillus plantarum AMT4 1 × 1010 cfu/g;
- -
- Lactobacillus plantarum AMT14 1 × 1010 cfu/g;
- -
- Bifidobacterium animalis AMT30 1 × 1010 cfu/g.
- -
- Lactobacillus plantarum AMT4 2 × 108 cfu/mL;
- -
- Lactobacillus plantarum AMT14 2 × 108 cfu/mL;
- -
- Bifidobacterium animalis AMT30 2 × 108 cfu/mL;
- -
- A 0.9% sodium chloride solution;
- -
- A 0.2% xylitol solution.
2.1. Metagenomic Analysis
- Automatic sample demultiplexing;
- Generating FASTQ files containing raw reads.
- 1.
- Quality assessment of reads:
- Analysis of the error profile for individual samples and dynamic generation of quality control parameters using the FIGARO tool;
- Quality control based on the coefficient of the maximum expected errors of the sample.
- 2.
- Preprocessing data using the Cutadapt tool:
- Removal of adapter sequences;
- Rejection reads shorter than 30 nucleotides.
- 3.
- Selection of unique ASV sequences (using the DADA2 package) by the following:
- Filtering out the sequences containing errors created during the sequencing process (denoising);
- Merging of paired-end reads to enhance accuracy—the corresponding forward and reverse readings must be combined at later stages of the analysis;
- Dereplication—merging of identical sequences while preserving the number of springs and quality profiles;
- Chimera filtering to remove artifacts resulting from incorrect sequence assembly during PCR.
- 4.
- Assigning a taxonomy to the ASV sequence based on the Silva reference database using a hybrid approach:
- Comparison of ASV sequences against the reference database using vsearch for identical reference sequences;
- Classification of atypical sequences remaining after the previous step using machine learning (sklearn).
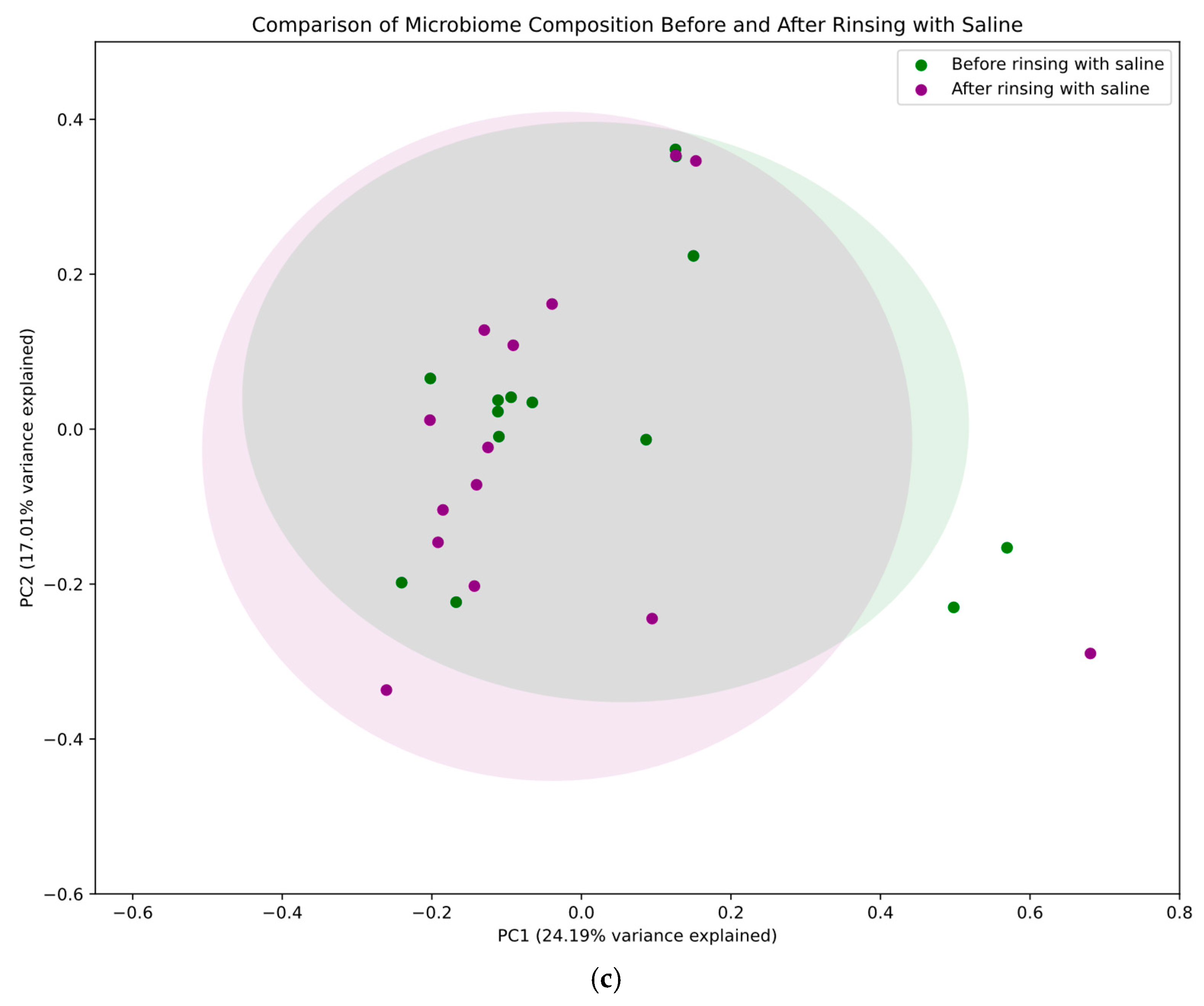
2.2. Principal Coordinates Analysis
- -
- Overall comparison: a comprehensive visualization comparing all four groups (before/after probiotic rinsing in the study group and before/after saline rinsing in the control group).
- -
- Probiotic treatment effect: a focused comparison of microbiome composition before and after rinsing with probiotics in the study group.
- -
- Saline treatment effect: a targeted analysis of microbiome composition before and after rinsing with saline in the control group.
2.3. Statistical Analysis
3. Results
3.1. Microbiome Analysis
3.2. Clinical Outcome and Microbiome Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 16S rRNA | 16S ribosomal RNA |
| ASV | Amplicon sequence variants |
| CRS | Chronic rhinosinusitis |
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| CRSsNP | Chronic rhinosinusitis without nasal polyps |
| EPOS2020 | European Position Paper on Rhinosinusitis and Nasal Polyps 2020 |
| ESS | Endoscopic sinus surgery |
| GPA | Granulomatosis with polyangiitis |
| NGS | Next-generation sequencing |
| NSP | Nasal septum perforation |
| PE | Paired-end |
| PC | Principal coordinate |
| PCoA | Principal coordinates analysis |
| PCR | Polymerase chain reaction |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Alammar, Y.; Rousseau, S.; Desrosiers, M.; Tewfik, M.A. The Effect of Corticosteroids on Sinus Microbiota in Chronic Rhinosinusitis Patients with Nasal Polyposis. Am. J. Rhinol. Allergy 2023, 37, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, A.; Paramasivan, S.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.S.; et al. Microbiotyping the Sinonasal Microbiome. Front. Cell. Infect. Microbiol. 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.; Moreno-Jimenez, E.; Morgado, N.; García-Sánchez, A.; Gil-Melcón, M.; Pérez-Pazos, J.; Estravís, M.; Isidoro-García, M.; Dávila, I.; Sanz, C. The Role of the Gut and Airway Microbiota in Chronic Rhinosinusitis with Nasal Polyps: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 8223. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, R.; Xiong, X.; Hu, Z.; Mao, X.; Wang, X.; Zhang, J.; Sun, P.; Yue, Z.; Wang, W.; et al. Alterations of nasal microbiome in eosinophilic chronic rhinosinusitis. J. Allergy Clin. Immunol. 2023, 151, 1286–1295.e2. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Feazel, L.M.; Gitomer, S.A.; Ir, D.; Robertson, C.E.; Frank, D.N. The Microbiome of the Middle Meatus in Healthy Adults. PLoS ONE 2013, 8, e85507. [Google Scholar] [CrossRef]
- Cho, D.Y.; Hunter, R.C.; Ramakrishnan, V.R. The Microbiome and Chronic Rhinosinusitis. Immunol. Allergy Clin. N. Am. 2020, 40, 251–263. [Google Scholar] [CrossRef]
- Psaltis, A.J.; Wormald, P.J. Therapy of Sinonasal Microbiome in CRS: A Critical Approach. Curr. Allergy Asthma Rep. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Park, I.-H.; Lee, J.S.; Park, S.M.; Kang, S.H.; Hong, S.-M.; Byun, S.-H.; Jung, Y.G.; Hong, S.J. Microbiome of Unilateral Chronic Rhinosinusitis: A Controlled Paired Analysis. Int. J. Environ. Res. Public Health 2021, 18, 9878. [Google Scholar] [CrossRef]
- Ziuzia-Januszewska, L.; Zygało, J.; Brożek-Mądry, E. Oral and Oropharyngeal Microbiome—Characteristics, Significance, and Potential Therapeutic Interventions. Pol. Otorhino Rev. 2024, 13, 19–30. [Google Scholar] [CrossRef]
- Bartosik, T.J.; Campion, N.J.; Freisl, K.; Liu, D.T.; Gangl, K.; Stanek, V.; Tu, A.; Pjevac, P.; Hausmann, B.; Eckl-Dorna, J.; et al. The nasal microbiome in patients suffering from non-steroidal anti-inflammatory drugs-exacerbated respiratory disease in absence of corticosteroids. Front. Immunol. 2023, 14, 1112345. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, W.; Yu, C.; Li, J.; Yu, F.; Xia, M.; Liang, J.; Shi, J.; Lai, Y. Age-Associated Changes of Nasal Bacterial Microbiome in Patients with Chronic Rhinosinusitis. Front. Cell. Infect. Microbiol. 2022, 12, 786481. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, S.; Bassiouni, A.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.; et al. The international sinonasal microbiome study: A multicentre, multinational characterization of sinonasal bacterial ecology. Allergy 2020, 75, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Kim, D.Y.; Choi, S.; Won, S.; Kang, H.R.; Yi, H. Microbiome profiling of uncinate tissue and nasal polyps in patients with chronic rhinosinusitis using swab and tissue biopsy. PLoS ONE 2021, 16, e0249688. [Google Scholar] [CrossRef] [PubMed]
- Szaleniec, J.; Bezshapkin, V.; Krawczyk, A.; Kopera, K.; Zapała, B.; Gosiewski, T.; Kosciolek, T. Determinants of the microbiome spatial variability in chronic rhinosinusitis. Rhin. Rhinol. J. 2023, 62, 119–126. [Google Scholar] [CrossRef]
- Feazel, L.M.; Robertson, C.E.; Ramakrishnan, V.R.; Frank, D.N. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope 2012, 122, 467–472. [Google Scholar] [CrossRef]
- Liu, C.M.; Soldanova, K.; Nordstrom, L.; Dwan, M.G.; Moss, O.L.; Contente-Cuomo, T.L.; Keim, P.; Price, L.B.; Lane, A.P. Medical therapy reduces microbiota diversity and evenness in surgically recalcitrant chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2013, 3, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Michalik, M.; Podbielska-Kubera, A.; Basińska, A.M.; Szewc, M.; Gałęcka, M.; Schwiertz, A. Alteration of indicator gut microbiota in patients with chronic sinusitis. Immun. Inflam. Dis. 2023, 11, e996. [Google Scholar] [CrossRef]
- Brożek-Mądry, E.; Chmielik, L.P.; Gałązka, A.; Rogulska, J.; Frąckiewicz, M.; Biejat, A. Chronic rhinosinusitis in children—Bacteriological analysis in terms of cytological examination. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Tagg, J.R.; Harold, L.K.; Jain, R.; Hale, J.D.F. Beneficial modulation of human health in the oral cavity and beyond using bacteriocin-like inhibitory substance-producing streptococcal probiotics. Front. Microbiol. 2023, 14, 1161155. [Google Scholar] [CrossRef] [PubMed]
- Brożek-Mądry, E.; Burska, Z.; Życińska, K.; Sierdziński, J. Nasal Microbiome in Granulomatosis with Polyangiitis Compared to Chronic Rhinosinusitis. Diagnostics 2024, 14, 1673. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.; Stokken, J.; Sanford, T.; Aurora, R.; Sindwani, R. A Systematic Review of the Sinonasal Microbiome in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 161–166. [Google Scholar] [CrossRef]
- Mackenzie, B.W.; Chang, K.; Zoing, M.; Jain, R.; Hoggard, M.; Biswas, K.; Douglas, R.G.; Taylor, M.W. Longitudinal study of the bacterial and fungal microbiota in the human sinuses reveals seasonal and annual changes in diversity. Sci. Rep. 2019, 9, 17416. [Google Scholar] [CrossRef]
- Hoggard, M.; Biswas, K.; Zoing, M.; Wagner Mackenzie, B.; Taylor, M.W.; Douglas, R.G. Evidence of microbiota dysbiosis in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 7, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.S.; Peres, A.G.; Endam, L.M.; Cousineau, B.; Madrenas, J.; Desrosiers, M. Topical Probiotics as a Therapeutic Alternative for Chronic Rhinosinusitis: A Preclinical Proof of Concept. Am. J. Rhinol. Allergy 2016, 30, e202–e205. [Google Scholar] [CrossRef] [PubMed]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus Microbiome Diversity Depletion and Corynebacterium tuberculostearicum Enrichment Mediates Rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Pamp, S.J.; Fukuyama, J.; Hwang, P.H.; Cho, D.-Y.; Holmes, S.; Relman, D.A. Nasal Microenvironments and Interspecific Interactions Influence Nasal Microbiota Complexity and S. aureus Carriage. Cell Host Microbe 2013, 14, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Menberu, M.A.; Liu, S.; Cooksley, C.; Hayes, A.J.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Corynebacterium accolens Has Antimicrobial Activity against Staphylococcus aureus and Methicillin-Resistant S. aureus Pathogens Isolated from the Sinonasal Niche of Chronic Rhinosinusitis Patients. Pathogens 2021, 10, 207. [Google Scholar] [CrossRef]
- Uzunoğlu, E.; Kalkancı, A.; Kılıç, E.; Kızıl, Y.; Aydil, U.; Diker, K.S.; Uslu, S.S. Bacterial and fungal communities in chronic rhinosinusitis with nasal polyps. PLoS ONE 2024, 19, e0304634. [Google Scholar] [CrossRef]
- Koeller, K.; Herlemann, D.P.R.; Schuldt, T.; Ovari, A.; Guder, E.; Podbielski, A.; Kreikemeyer, B.; Olzowy, B. Microbiome and Culture Based Analysis of Chronic Rhinosinusitis Compared to Healthy Sinus Mucosa. Front. Microbiol. 2018, 9, 643. [Google Scholar] [CrossRef]
- Jain, R.; Hoggard, M.; Biswas, K.; Zoing, M.; Jiang, Y.; Douglas, R. Changes in the bacterial microbiome of patients with chronic rhinosinusitis after endoscopic sinus surgery. Int. Forum Allergy Rhinol. 2017, 7, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Hoggard, M.; Jain, R.; Taylor, M.W.; Douglas, R.G. The nasal microbiota in health and disease: Variation within and between subjects. Front. Microbiol. 2015, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.-J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Liver Physiol. 2010, 298, G851–G859. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Bang, S.-J.; Kim, J.-Y.; Choi, E.J.; Heo, K.; Shim, J.-J.; Lee, J.-L. The Probiotic Strain Bifidobacterium animalis ssp. lactis HY8002 Potentially Improves the Mucosal Integrity of an Altered Intestinal Microbial Environment. Front. Microbiol. 2022, 13, 817591. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.J.; Drilling, A.; Bassiouni, A.; James, C.; Vreugde, S.; Wormald, P. Probiotic manipulation of the chronic rhinosinusitis microbiome. Int. Forum Allergy Rhinol. 2014, 4, 309–314. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, S.S.; Pynnonen, M.A.; Kim, H.M.; Singer, A.; Tabor, M.; Terrell, J.E. Probiotics as adjunctive treatment for chronic rhinosinusitis: A randomized controlled trial. Otolaryngol. Head Neck Surg. 2009, 140, 202–208. [Google Scholar] [CrossRef]
- Habermann, W.; Zimmermann, K.; Skarabis, H.; Kunze, R.; Rusch, V. Verminderung der Rezidivhäufigkeit bei Patienten mit chronisch rezidivierender hypertrophischer Sinusitis unter Behandlung mit einem bakteriellen Immunstimulans (Enterococcus faecalis-Bakterien humaner Herkunft). Arzneimittelforschung 2011, 52, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Kitz, R.; Martens, U.; Zieseniß, E.; Enck, P.; Rose, M. Probiotic E.faecalis—adjuvant therapy in children with recurrent rhinosinusitis. Open Med. 2012, 7, 362–365. [Google Scholar] [CrossRef]
- Abolhalaj, M.; Lindstedt, M.; Mårtensson, A.; Olofsson, T.C.; Vásquez, A.; Greiff, L.; Cervin, A. Clinical efficacy of a topical lactic acid bacterial microbiome in chronic rhinosinusitis: A randomized controlled trial. Laryngoscope Investig. Otolaryngol. 2017, 2, 410–416. [Google Scholar] [CrossRef]
- Endam, L.M.; Alromaih, S.; Gonzalez, E.; Madrenas, J.; Cousineau, B.; Renteria, A.E.; Desrosiers, M. Intranasal Application of Lactococcus lactis W136 Is Safe in Chronic Rhinosinusitis Patients With Previous Sinus Surgery. Front. Cell. Infect. Microbiol. 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Brożek-Mądry, E.; Burska, Z.; Sosnowska, E. Nasal rinsing with probiotics in rhinosinusitis—Analysis of symptoms and safety assessment. Otolaryngol. Pol. 2025, 79, 1–5. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Martens, K.; Spacova, I.; Cauwenberghs, E.; Allonsius, C.N.; Jörissen, J.; Wuyts, S.; Van Beeck, W.; Dillen, J.; et al. The nasal mutualist Dolosigranulum pigrum AMBR11 supports homeostasis via multiple mechanisms. iScience 2021, 24, 102978. [Google Scholar] [CrossRef] [PubMed]
- Konovalovas, A.; Armalytė, J.; Klimkaitė, L.; Liveikis, T.; Jonaitytė, B.; Danila, E.; Bironaitė, D.; Mieliauskaitė, D.; Bagdonas, E.; Aldonytė, R. Human nasal microbiota shifts in healthy and chronic respiratory disease conditions. BMC Microbiol. 2024, 24, 150. [Google Scholar] [CrossRef] [PubMed]
- Passali, D.; Passali, G.C.; Vesperini, E.; Cocca, S.; Visconti, I.C.; Ralli, M.; Bellussi, L.M. The efficacy and tolerability of Streptococcus salivarius 24SMB and Streptococcus oralis 89a administered as nasal spray in the treatment of recurrent upper respiratory tract infections in children. Eur. Rev. Med. Pharmacol. Sci. 2019, 23 (Suppl. S1), 67–72. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torres-Maravilla, E.; Reyes-Pavón, D.; Benítez-Cabello, A.; González-Vázquez, R.; Ramírez-Chamorro, L.M.; Langella, P.; Bermúdez-Humarán, L.G. Strategies for the Identification and Assessment of Bacterial Strains with Specific Probiotic Traits. Microorganisms 2022, 10, 1389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
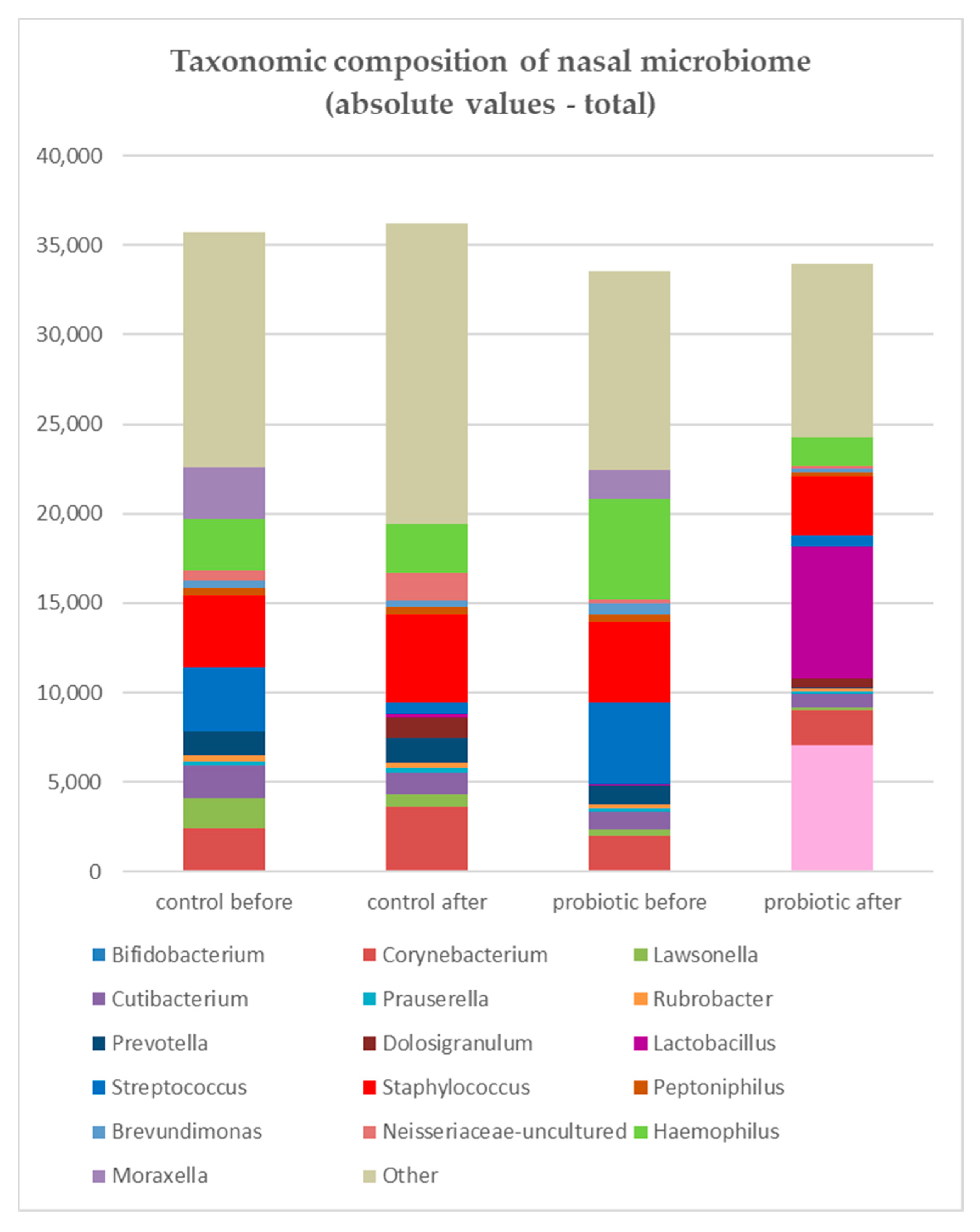
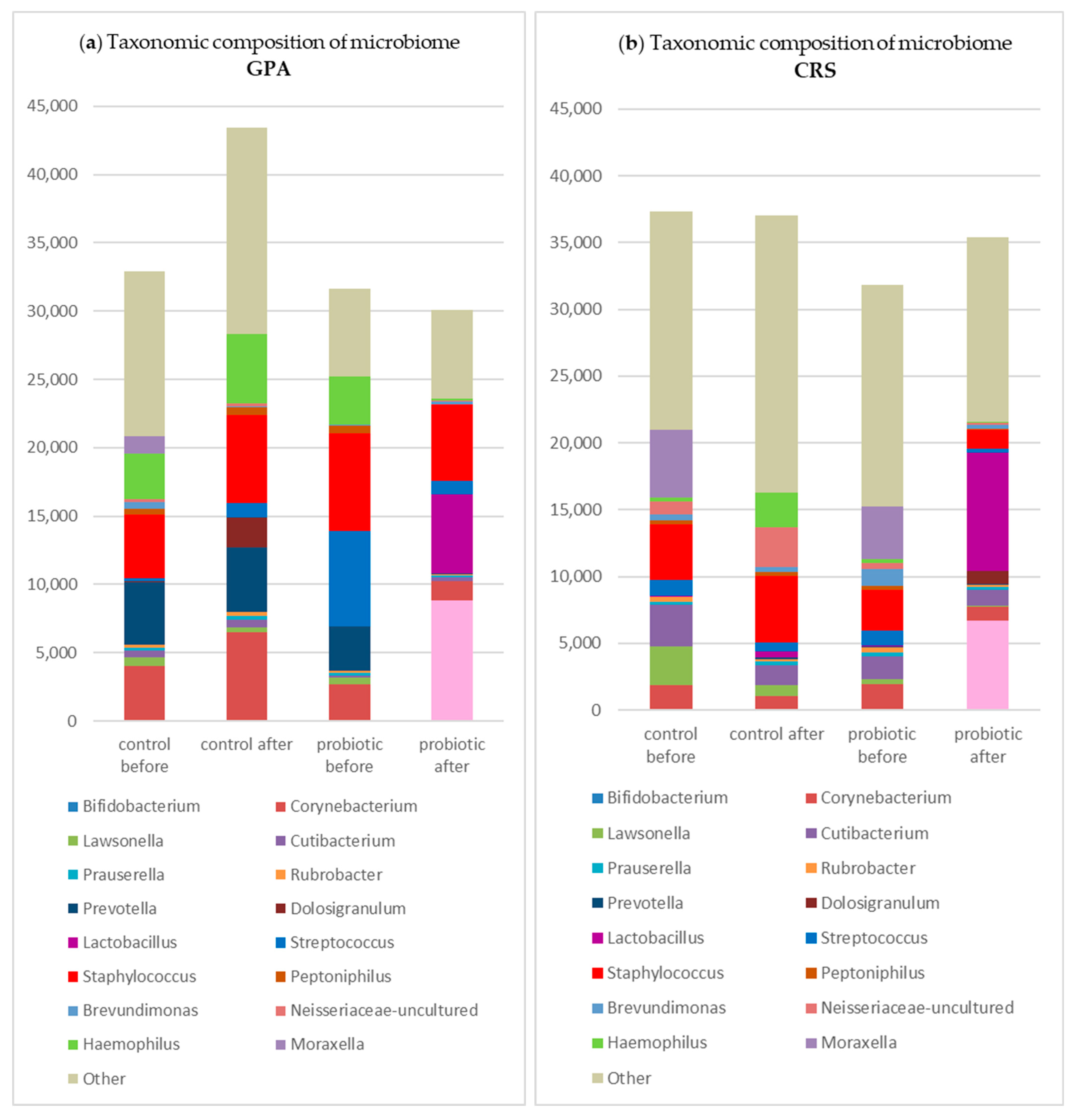

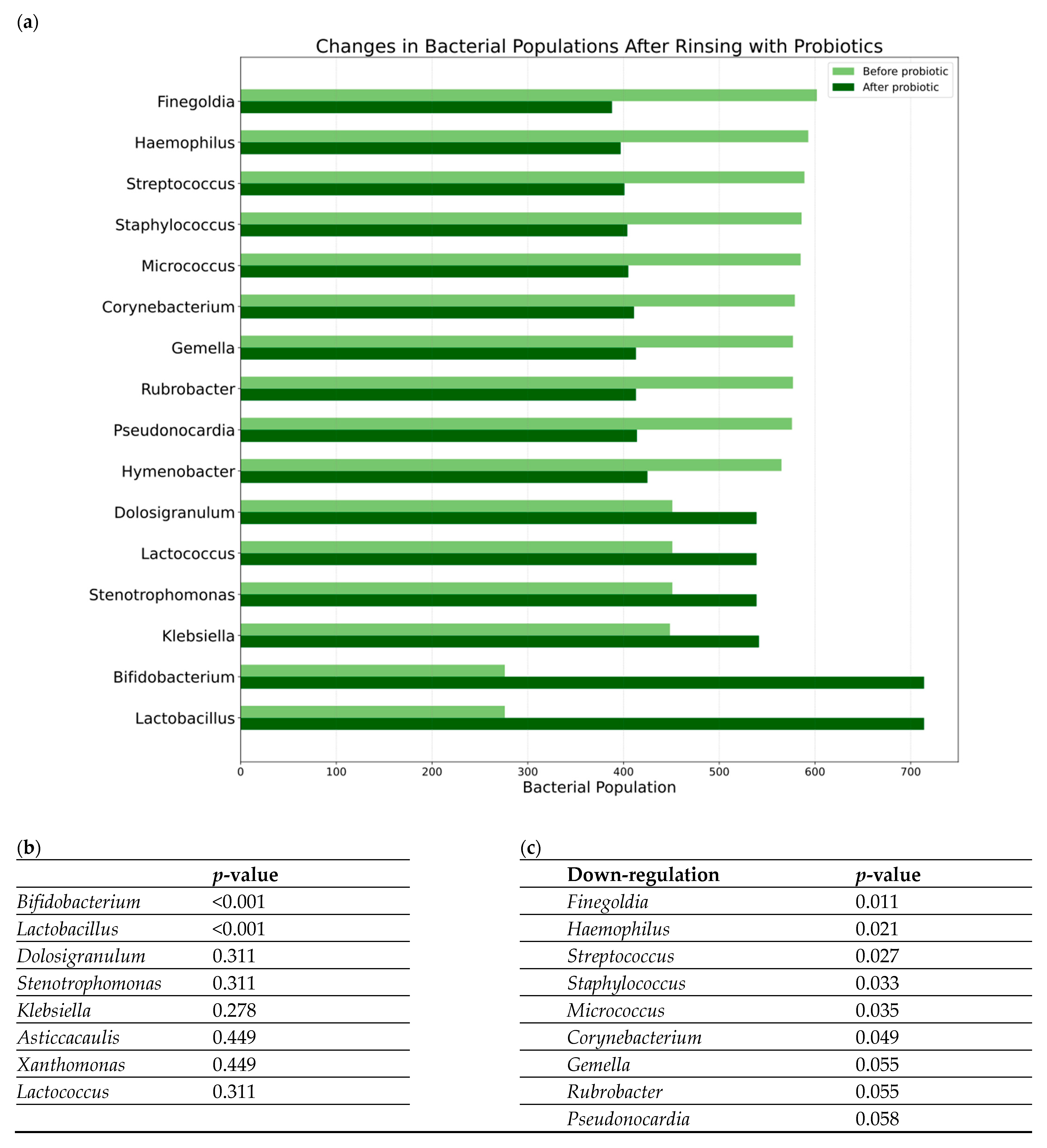
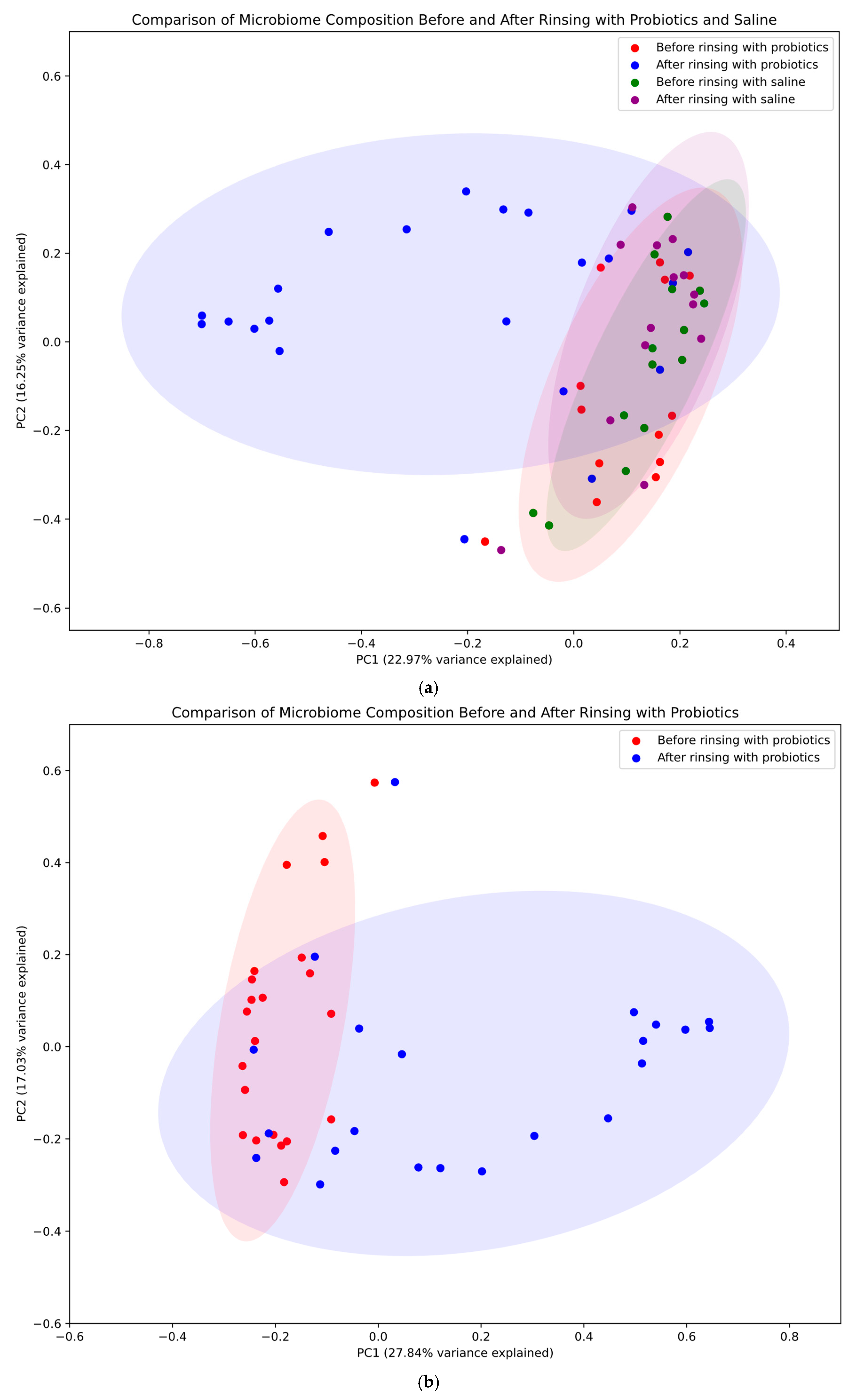
| Study Group n = 22 Mean (SD) | Control Group n = 14 Mean (SD) | p-Value | |
|---|---|---|---|
| Age | 44.0 (12.1) | 40.4 (12.6) | 0.393 |
| Symptoms and endoscopic scores before treatment | |||
| SNOT-22 total score | 34.3 (20.3) | 33.9 (20.4) | 0.955 |
| Lund–Kennedy score | 5.4(2.5) | 5.2 (2.5) | 0.800 |
| Symptoms and endoscopic scores after treatment | |||
| SNOT-22 total score | 27.2 (18.3) | 29.9 (21) | 0.681 |
| Lund–Kennedy score | 3.7 (1.8) | 5 (2.2) | 0.056 |
| GPA | NSP | CRS | ||||
|---|---|---|---|---|---|---|
| Study Group | Control Group | Study Group | Control Group | Study Group | Control Group | |
| n = 7 | n = 4 | n = 6 | n = 3 | n = 9 | n = 7 | |
| Age | 42.6 ± 11.7 | 40.5 ± 15.7 | 43.3 ± 13.5 | 50.3 ± 14.0 | 45.6 ± 12.9 | 35.4 ± 8.7 |
| SNOT-22 before treatment | 36.1 ± 22.0 | 31.5 ± 25.3 | 46.6 ± 21.3 | 53.0 ± 19.8 | 26.1 ± 16.3 | 25.7 ± 14.5 |
| SNOT-22 after treatment | 25.3 ± 20.1 | 23.0 ± 26.1 | 32.3 ± 19.5 | 44.7 ± 13.0 | 25.2 ± 17.7 | 27.6 ± 20.2 |
| Lund–Kennedy score before treatment | 7.1 ± 2.7 | 5.9 ± 4.2 | 4.8 ± 1.7 | 4.7 ± 2.1 | 4.5 ± 2.3 | 5.2 ± 1.7 |
| Lund–Kennedy score after treatment | 6.9 ± 5.4 | 5.1 ± 3.5 | 2.9 ± 1.6 | 5.0 ± 0.0 | 3.5 ± 1.1 | 5.0 ± 2.1 |
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GPA | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean | ||
| Staphylococcus | 65.0% | 9.4% | 7.4% | 58.6% | 12.1% | 26.5% | 8.9% | 26.8% | ||
| Haemophilus | 0.0% | 24.3% | 2.9% | 0.5% | 0.0% | 54.7% | 0.0% | 11.8% | ||
| Streptococcus | 0.2% | 0.7% | 0.2% | 3.9% | 74.9% | 2.1% | 0.0% | 11.7% | ||
| Corynebacterium | 16.4% | 1.0% | 37.4% | 4.6% | 0.3% | 0.4% | 1.6% | 8.8% | ||
| Unrecognised bacteria | 8.6% | 1.8% | 3.6% | 12.6% | 9.1% | 13.7% | 0.1% | 7.1% | ||
| Prevotella | 0.0% | 32.7% | 11.6% | 3.1% | 0.0% | 0.0% | 0.0% | 6.8% | ||
| Alteribacillus | 0.3% | 0.0% | 0.0% | 0.3% | 0.2% | 0.0% | 17.4% | 2.6% | ||
| Prauserella | 1.4% | 0.1% | 0.1% | 0.4% | 0.4% | 0.3% | 10.2% | 1.8% | ||
| Rubrobacter | 0.3% | 0.2% | 0.0% | 0.5% | 0.8% | 0.5% | 9.3% | 1.7% | ||
| Peptostreptococcus | 0.0% | 5.6% | 5.3% | 0.1% | 0.0% | 0.0% | 0.0% | 1.6% | ||
| Peptoniphilus | 0.8% | 2.7% | 7.0% | 0.0% | 0.0% | 0.0% | 0.0% | 1.5% | ||
| Cutibacterium | 0.6% | 0.2% | 0.3% | 1.6% | 0.2% | 0.1% | 7.2% | 1.5% | ||
| Campylobacter | 0.0% | 0.4% | 1.8% | 0.3% | 0.0% | 0.0% | 6.5% | 1.3% | ||
| Proteus | 0.0% | 2.1% | 6.4% | 0.0% | 0.0% | 0.0% | 0.0% | 1.2% | ||
| Micrococcus | 0.1% | 0.0% | 0.0% | 0.3% | 0.0% | 0.1% | 7.0% | 1.1% | ||
| Lawsonella | 1.5% | 4.7% | 0.7% | 0.0% | 0.0% | 0.0% | 0.0% | 1.0% | ||
| Fusobacterium | 0.0% | 6.8% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 1.0% | ||
| (b) | ||||||||||
| CRS | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Mean |
| Unrecognized bacteria | 48.58% | 31.03% | 0.05% | 40.82% | 9.77% | 34.32% | 27.74% | 22.23% | 26.57% | 26.79% |
| Staphylococcus | 2.24% | 7.17% | 16.21% | 12.69% | 26.54% | 1.79% | 5.62% | 1.76% | 41.70% | 12.86% |
| Corynebacterium | 1.08% | 18.94% | 7.03% | 15.90% | 9.02% | 4.18% | 3.17% | 0.30% | 2.63% | 6.92% |
| Brevundimonas | 1.20% | 1.68% | 3.78% | 3.22% | 0.74% | 0.41% | 1.14% | 46.27% | 0.34% | 6.53% |
| Cutibacterium | 7.09% | 7.15% | 9.53% | 2.35% | 20.21% | 1.77% | 1.56% | 0.50% | 0.41% | 5.62% |
| Moraxella | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 42.28% | 0.00% | 0.00% | 0.00% | 4.70% |
| Streptococcus | 1.80% | 0.66% | 7.70% | 0.39% | 6.11% | 4.38% | 0.88% | 0.00% | 5.43% | 3.04% |
| Rubrobacter | 4.63% | 1.58% | 0.37% | 0.44% | 0.34% | 0.45% | 1.36% | 4.65% | 1.08% | 1.65% |
| Lawsonella | 0.00% | 5.24% | 0.00% | 4.41% | 4.51% | 0.00% | 0.00% | 0.00% | 0.00% | 1.57% |
| Neisseriaceae; Genus uncultured | 0.00% | 0.00% | 0.48% | 1.06% | 9.95% | 0.00% | 0.96% | 0.00% | 0.00% | 1.38% |
| Pseudomonas | 0.00% | 0.00% | 0.15% | 0.00% | 0.00% | 0.00% | 1.59% | 10.46% | 0.00% | 1.36% |
| Prauserella | 0.58% | 1.44% | 0.58% | 0.46% | 0.11% | 0.30% | 0.64% | 6.43% | 1.23% | 1.31% |
| Peptoniphilus | 0.00% | 5.13% | 0.45% | 0.50% | 0.25% | 0.75% | 0.68% | 0.00% | 2.27% | 1.11% |
| Anaerococcus | 1.57% | 2.97% | 1.89% | 0.16% | 0.56% | 0.55% | 1.01% | 0.00% | 0.00% | 0.97% |
| Enhydrobacter | 0.37% | 0.18% | 0.11% | 0.00% | 2.05% | 0.07% | 5.67% | 0.00% | 0.23% | 0.96% |
| Chryseobacterium | 6.33% | 0.00% | 0.00% | 0.00% | 0.69% | 0.00% | 1.32% | 0.00% | 0.00% | 0.93% |
| Rothia | 0.30% | 0.34% | 3.68% | 0.17% | 0.41% | 2.68% | 0.37% | 0.00% | 0.10% | 0.89% |
| Paracoccus | 3.15% | 0.00% | 1.44% | 0.00% | 0.18% | 0.00% | 2.47% | 0.00% | 0.00% | 0.80% |
| Haemophilus | 0.00% | 0.29% | 2.78% | 0.03% | 1.72% | 0.88% | 0.05% | 0.00% | 1.43% | 0.80% |
| Phenylobacterium | 2.35% | 0.43% | 0.45% | 0.50% | 0.15% | 0.08% | 0.00% | 2.78% | 0.00% | 0.75% |
| Brochothrix | 0.00% | 0.00% | 0.00% | 4.20% | 0.00% | 0.00% | 2.03% | 0.00% | 0.42% | 0.74% |
| Alteribacillus | 1.14% | 0.38% | 0.52% | 0.76% | 0.12% | 0.23% | 0.31% | 2.34% | 0.60% | 0.71% |
| Micrococcus | 1.44% | 0.00% | 0.00% | 0.13% | 0.42% | 0.00% | 2.90% | 0.72% | 0.00% | 0.62% |
| Sphingomonas | 0.33% | 0.30% | 2.34% | 0.00% | 0.24% | 0.12% | 1.66% | 0.00% | 0.00% | 0.56% |
| Finegoldia | 0.00% | 2.24% | 0.59% | 0.99% | 0.24% | 0.16% | 0.45% | 0.00% | 0.31% | 0.55% |
| (c) | ||||||||||
| NSP | 17 | 18 | 19 | 20 | 21 | 22 | Mean | |||
| Haemophilus | 0.3% | 42.8% | 94.9% | 0.0% | 0.1% | 0.2% | 23.1% | |||
| Staphylococcus | 32.5% | 1.6% | 0.3% | 59.2% | 11.0% | 6.6% | 18.5% | |||
| Streptococcus | 11.2% | 41.9% | 1.2% | 0.5% | 38.1% | 0.9% | 15.7% | |||
| Unrecognised bacteria | 27.8% | 3.6% | 1.7% | 0.0% | 16.9% | 25.1% | 12.5% | |||
| Corynebacterium | 1.2% | 2.5% | 0.1% | 9.1% | 7.0% | 3.2% | 3.8% | |||
| Cutibacterium | 2.0% | 1.2% | 0.0% | 4.0% | 3.8% | 5.9% | 2.8% | |||
| Brevundimonas | 0.4% | 0.2% | 0.0% | 5.4% | 1.8% | 1.6% | 1.6% | |||
| Peptoniphilus | 0.6% | 0.4% | 0.0% | 0.6% | 4.7% | 0.7% | 1.2% | |||
| Rubrobacter | 3.8% | 0.1% | 0.1% | 0.3% | 0.3% | 0.5% | 0.8% | |||
| Sphingomonas | 0.4% | 0.0% | 0.0% | 0.8% | 0.4% | 3.1% | 0.8% | |||
| Anaerococcus | 0.0% | 0.4% | 0.0% | 0.2% | 3.5% | 0.5% | 0.8% | |||
| Prauserella | 2.2% | 0.3% | 0.1% | 0.7% | 0.4% | 0.8% | 0.7% | |||
| Micrococcus | 0.8% | 0.1% | 0.0% | 1.1% | 0.5% | 1.6% | 0.7% | |||
| Kocuria | 1.1% | 0.0% | 0.0% | 1.5% | 0.0% | 0.8% | 0.6% | |||
| Finegoldia | 0.0% | 0.5% | 0.0% | 0.0% | 1.6% | 1.0% | 0.5% | |||
| Rothia | 1.5% | 0.0% | 0.6% | 0.2% | 0.0% | 0.8% | 0.5% | |||
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GPA | a | b | c | d | e | f | g | Mean | ||
| Bifidobacterium | 42.6% | 31.3% | 4.8% | 24.0% | 27.2% | 11.5% | 51.5% | 27.5% | ||
| Lactobacillus | 14.0% | 49.1% | 2.1% | 47.8% | 10.5% | 9.4% | 32.0% | 23.6% | ||
| Staphylococcus | 6.7% | 15.2% | 68.4% | 1.5% | 1.1% | 10.9% | 3.9% | 15.4% | ||
| Unrecognizedbacteria | 24.0% | 1.4% | 3.9% | 20.5% | 43.6% | 16.3% | 0.0% | 15.7% | ||
| Streptococcus | 0.4% | 0.0% | 0.1% | 0.0% | 0.1% | 42.4% | 4.5% | 6.8% | ||
| Corynebacterium | 0.8% | 0.7% | 15.8% | 0.4% | 3.9% | 1.6% | 0.8% | 3.4% | ||
| Escherichia-Shigella | 6.3% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.9% | ||
| Cutibacterium | 0.8% | 0.9% | 0.0% | 0.8% | 0.5% | 0.3% | 1.5% | 0.7% | ||
| Brevundimonas | 0.1% | 0.0% | 0.1% | 0.2% | 1.4% | 2.2% | 0.1% | 0.6% | ||
| Neisseriaceae; Genus_uncultured | 0.0% | 0.0% | 0.0% | 3.8% | 0.2% | 0.0% | 0.0% | 0.6% | ||
| Prauserella | 0.7% | 0.0% | 0.1% | 0.2% | 0.3% | 1.5% | 0.9% | 0.5% | ||
| Lactococcus | 0.0% | 0.0% | 0.0% | 0.0% | 3.4% | 0.0% | 0.0% | 0.5% | ||
| Rubrobacter | 0.4% | 0.0% | 0.0% | 0.3% | 0.2% | 1.2% | 0.9% | 0.4% | ||
| Haemophilus | 0.0% | 0.0% | 0.0% | 0.0% | 2.3% | 0.0% | 0.0% | 0.3% | ||
| Klebsiella | 0.0% | 0.0% | 2.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.3% | ||
| (b) | ||||||||||
| CRS | h | i | j | k | l | m | n | o | p | mean |
| Unrecognized bacteria; | 42.36% | 1.74% | 41.98% | 1.54% | 48.66% | 0.68% | 30.82% | 29.33% | 25.10% | 24.69% |
| Lactobacillus | 0.00% | 33.83% | 15.10% | 53.29% | 1.84% | 50.03% | 0.18% | 7.99% | 6.33% | 18.73% |
| Bifidobacterium | 0.00% | 21.18% | 11.39% | 42.07% | 4.43% | 42.50% | 0.41% | 5.47% | 4.29% | 14.64% |
| Staphylococcus | 6.13% | 1.47% | 8.21% | 0.02% | 2.04% | 4.88% | 3.64% | 7.73% | 9.68% | 4.87% |
| Cutibacterium | 11.90% | 0.24% | 0.63% | 0.02% | 16.00% | 0.67% | 2.89% | 7.28% | 0.53% | 4.46% |
| Corynebacterium | 4.91% | 0.14% | 1.10% | 0.00% | 1.48% | 0.22% | 14.24% | 14.69% | 0.85% | 4.18% |
| Dolosigranulum | 0.00% | 0.00% | 0.00% | 0.00% | 4.15% | 0.00% | 28.93% | 0.00% | 0.00% | 3.68% |
| Pseudomonas | 0.00% | 31.41% | 0.33% | 0.02% | 0.00% | 0.00% | 0.00% | 0.00% | 0.77% | 3.61% |
| Marinomonas | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.11% | 20.01% | 2.24% |
| Brochothrix | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.05% | 9.66% | 7.09% | 1.87% |
| Anaerococcus | 1.00% | 0.02% | 0.19% | 0.00% | 9.83% | 0.00% | 0.38% | 0.00% | 0.50% | 1.32% |
| Brevundimonas | 2.96% | 0.16% | 0.84% | 0.18% | 1.50% | 0.04% | 1.90% | 1.15% | 0.58% | 1.03% |
| Streptococcus | 2.32% | 0.00% | 3.50% | 0.20% | 0.00% | 0.00% | 1.09% | 0.47% | 1.28% | 0.99% |
| Klebsiella | 0.00% | 8.40% | 0.00% | 0.00% | 0.09% | 0.00% | 0.00% | 0.00% | 0.00% | 0.94% |
| Campylobacter | 8.31% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.92% |
| Leuconostoc | 0.00% | 0.00% | 0.00% | 0.00% | 0.12% | 0.00% | 0.00% | 0.71% | 6.65% | 0.83% |
| Prauserella | 1.69% | 0.16% | 0.56% | 0.00% | 1.92% | 0.00% | 0.60% | 0.55% | 1.30% | 0.75% |
| Rubrobacter | 0.86% | 0.05% | 0.58% | 0.03% | 1.44% | 0.08% | 0.55% | 0.26% | 1.09% | 0.55% |
| (c) | ||||||||||
| NSP | q | r | s | t | u | w | mean | |||
| Lactobacillus | 2.62% | 4.88% | 1.36% | 46.59% | 61.34% | 0.50% | 19.55% | |||
| Bifidobacterium | 25.37% | 10.72% | 0.77% | 35.88% | 30.86% | 0.00% | 17.27% | |||
| Haemophilus | 0.00% | 0.04% | 95.93% | 0.00% | 0.00% | 1.15% | 16.19% | |||
| Unrecognized bacteria | 29.94% | 8.68% | 1.24% | 8.65% | 0.00% | 14.85% | 10.56% | |||
| Staphylococcus | 3.88% | 22.71% | 0.18% | 3.55% | 0.48% | 13.04% | 7.31% | |||
| Corynebacterium | 0.00% | 35.72% | 0.12% | 0.21% | 0.05% | 4.69% | 6.80% | |||
| Cutibacterium | 2.49% | 1.68% | 0.16% | 0.63% | 0.19% | 9.09% | 2.37% | |||
| Streptococcus | 3.61% | 0.65% | 0.02% | 0.14% | 0.00% | 7.91% | 2.05% | |||
| Anaerococcus | 0.00% | 1.01% | 0.00% | 0.07% | 0.01% | 8.28% | 1.56% | |||
| Peptoniphilus | 0.00% | 0.61% | 0.00% | 0.03% | 0.00% | 8.44% | 1.51% | |||
| Finegoldia | 0.00% | 0.08% | 0.00% | 0.00% | 0.00% | 6.66% | 1.12% | |||
| Neisseria | 0.00% | 0.00% | 0.00% | 0.00% | 5.34% | 0.89% | 1.04% | |||
| Prauserella | 4.68% | 0.18% | 0.07% | 0.12% | 0.08% | 0.41% | 0.92% | |||
| Family Bacillaceae | 4.37% | 0.13% | 0.00% | 0.00% | 0.00% | 0.04% | 0.76% | |||
| Rubrobacter | 3.55% | 0.31% | 0.05% | 0.03% | 0.05% | 0.25% | 0.71% | |||
| Rothia | 0.77% | 0.34% | 0.00% | 0.32% | 0.00% | 2.73% | 0.69% | |||
| Lawsonella | 0.00% | 0.00% | 0.03% | 0.07% | 0.00% | 3.52% | 0.60% | |||
| Phenylobacterium | 2.81% | 0.00% | 0.00% | 0.18% | 0.00% | 0.26% | 0.54% | |||
| Genus | p-Value * |
|---|---|
| Bifidobacterium | <0.001 |
| Lactobacillus | <0.001 |
| Gemella | 0.003 |
| Nocardioides | 0.027 |
| Finegoldia | 0.027 |
| Tepidimonas | 0.027 |
| Methylobacterium-Methylorubrum | 0.035 |
| Haemophilus | 0.045 |
| Deinococcus | 0.053 |
| Cutibacterium | 0.057 |
| Neisseria | 0.057 |
| (a) | ||||||
|---|---|---|---|---|---|---|
| Genus | SNOT22 Change Correlation | SNOT22 Change p Value | LK Change Correlation | LK Change p Value | SNOT22 Significant | LK Significant |
| Coprococcus | 0.584 | 0.005 | 0.145 | 0.519 | True | False |
| Ruminococcus | 0.496 | 0.022 | 0.367 | 0.093 | True | False |
| Agathobacter | 0.461 | 0.035 | 0.072 | 0.751 | True | False |
| Roseburia | 0.435 | 0.049 | 0.072 | 0.751 | True | False |
| Dietzia | −0.435 | 0.049 | −0.304 | 0.169 | True | False |
| Rathayibacter | −0.435 | 0.049 | −0.191 | 0.394 | True | False |
| Haliangium | −0.435 | 0.049 | −0.191 | 0.394 | True | False |
| Prosthecobacter | −0.435 | 0.049 | −0.191 | 0.394 | True | False |
| Janibacter | −0.506 | 0.019 | −0.109 | 0.629 | True | False |
| Cloacibacterium | −0.512 | 0.018 | 0.275 | 0.216 | True | False |
| Faecalibacterium | 0.366 | 0.103 | 0.424 | 0.049 | False | True |
| Finegoldia | 0.206 | 0.371 | 0.489 | 0.021 | False | True |
| Peptoniphilus | 0.110 | 0.635 | 0.513 | 0.015 | False | True |
| Xanthomonas | 0.033 | 0.886 | 0.436 | 0.042 | False | True |
| Lawsonella | −0.025 | 0.914 | 0.466 | 0.029 | False | True |
| Luteimonas | −0.203 | 0.378 | −0.433 | 0.044 | False | True |
| Terrimonas | −0.343 | 0.128 | −0.479 | 0.024 | False | True |
| (b) | ||||||
| Genus | SNOT22 Change Correlation | SNOT22 Change p Value | LK Change Correlation | LK Change p Value | SNOT22 Significant | LK Significant |
| Pseudoxanthomonas | 0.662 | 0.014 | 0.397 | 0.160 | True | False |
| Veillonella | 0.610 | 0.027 | −0.265 | 0.360 | True | False |
| Moraxella | 0.566 | 0.044 | 0.425 | 0.130 | True | False |
| Pseudopropio-nibacterium | 0.561 | 0.047 | 0.194 | 0.506 | True | False |
| Tannerella | −0.568 | 0.043 | −0.372 | 0.191 | True | False |
| Rubrobacter | −0.576 | 0.040 | −0.474 | 0.087 | True | False |
| Alteribacillus | −0.781 | 0.002 | −0.394 | 0.163 | True | False |
| Negativicoccus | 0.372 | 0.211 | 0.543 | 0.045 | False | True |
| Exiguobacterium | 0.345 | 0.248 | 0.543 | 0.045 | False | True |
| Altererythrobacter | 0.345 | 0.248 | 0.543 | 0.045 | False | True |
| Vibrionimonas | 0.133 | 0.666 | 0.543 | 0.045 | False | True |
| Abiotrophia | 0.018 | 0.953 | 0.662 | 0.010 | False | True |
| Phenylobacterium | −0.139 | 0.650 | −0.571 | 0.033 | False | True |
| Lautropia | −0.163 | 0.594 | −0.574 | 0.032 | False | True |
| Micrococcus | −0.363 | 0.223 | −0.632 | 0.015 | False | True |
| Pontibacter | −0.372 | 0.211 | −0.614 | 0.019 | False | True |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brożek-Mądry, E.; Ziuzia-Januszewska, L.; Misztal, O.; Burska, Z.; Sosnowska-Turek, E.; Sierdziński, J. Nasal Rinsing with Probiotics—Microbiome Evaluation in Patients with Inflammatory Diseases of the Nasal Mucosa. J. Clin. Med. 2025, 14, 3341. https://doi.org/10.3390/jcm14103341
Brożek-Mądry E, Ziuzia-Januszewska L, Misztal O, Burska Z, Sosnowska-Turek E, Sierdziński J. Nasal Rinsing with Probiotics—Microbiome Evaluation in Patients with Inflammatory Diseases of the Nasal Mucosa. Journal of Clinical Medicine. 2025; 14(10):3341. https://doi.org/10.3390/jcm14103341
Chicago/Turabian StyleBrożek-Mądry, Eliza, Laura Ziuzia-Januszewska, Oliwier Misztal, Zofia Burska, Ewelina Sosnowska-Turek, and Janusz Sierdziński. 2025. "Nasal Rinsing with Probiotics—Microbiome Evaluation in Patients with Inflammatory Diseases of the Nasal Mucosa" Journal of Clinical Medicine 14, no. 10: 3341. https://doi.org/10.3390/jcm14103341
APA StyleBrożek-Mądry, E., Ziuzia-Januszewska, L., Misztal, O., Burska, Z., Sosnowska-Turek, E., & Sierdziński, J. (2025). Nasal Rinsing with Probiotics—Microbiome Evaluation in Patients with Inflammatory Diseases of the Nasal Mucosa. Journal of Clinical Medicine, 14(10), 3341. https://doi.org/10.3390/jcm14103341





