The Effect of a Kasai Procedure on Liver Transplantation in Children with Biliary Atresia: A Cohort Study
Abstract
1. Introduction
2. Materials and Methods
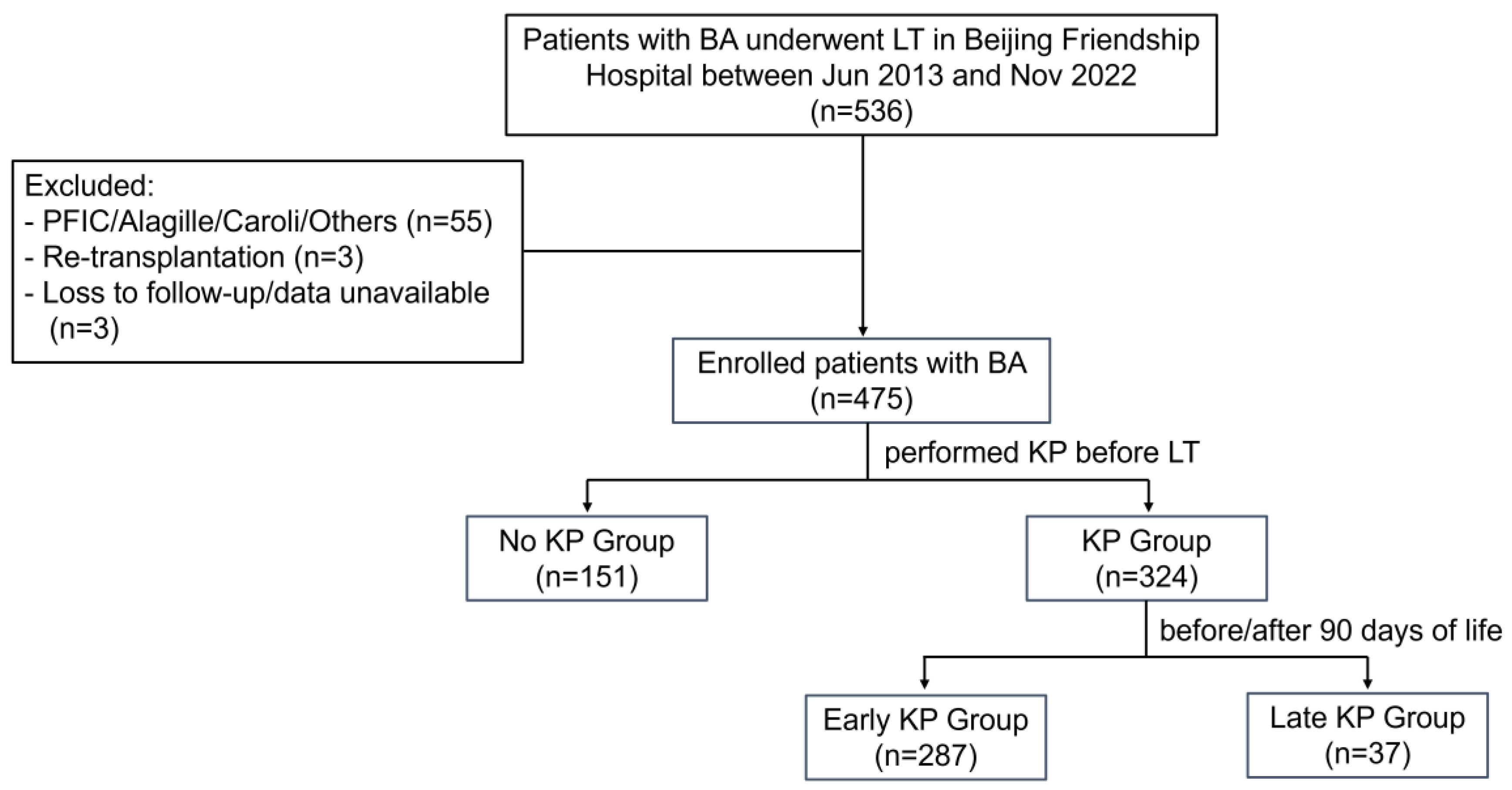
2.1. Study Population
2.2. Clinical Data and Follow-Up
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Identification of Predictive Risk Factors Using the Cox Model
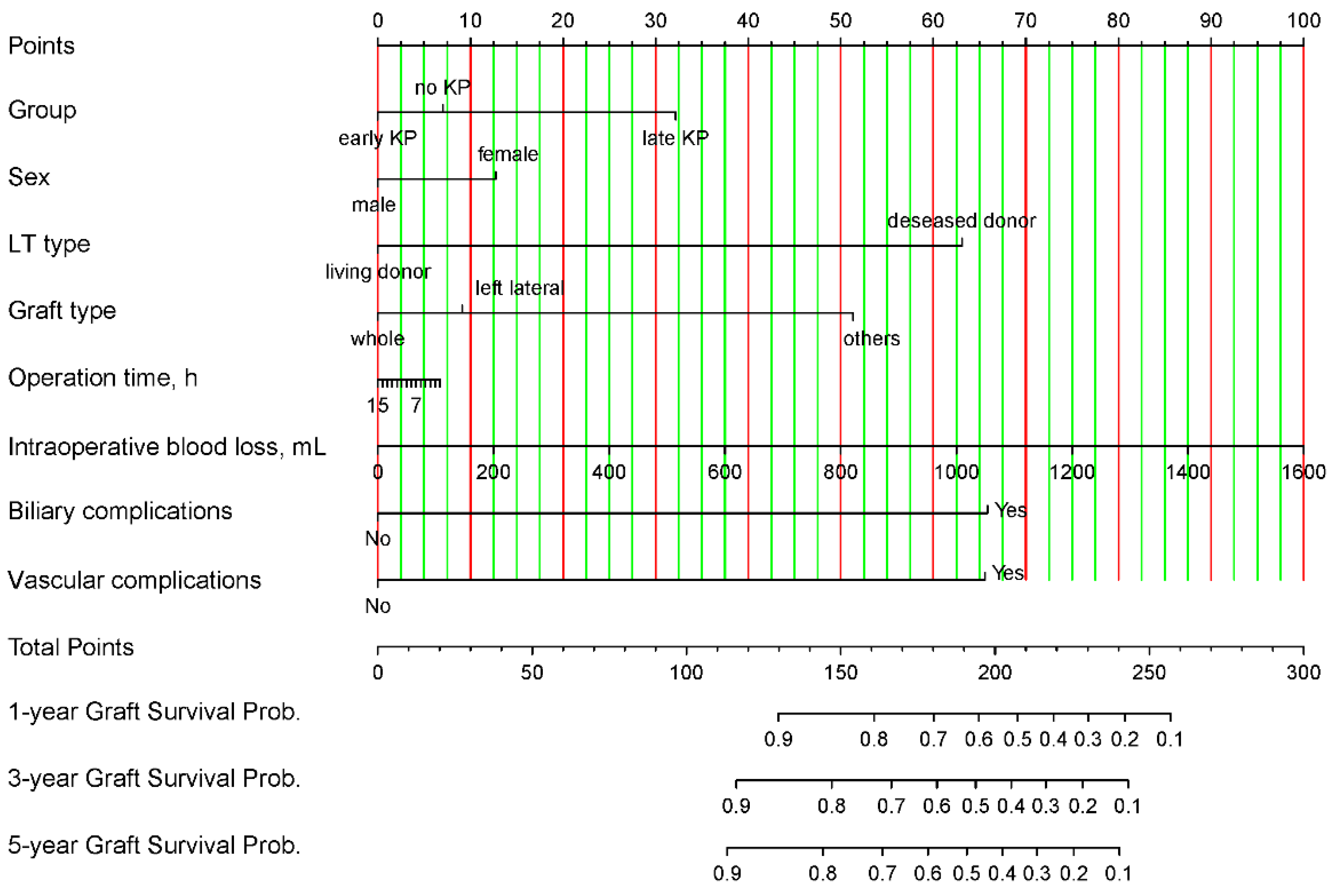
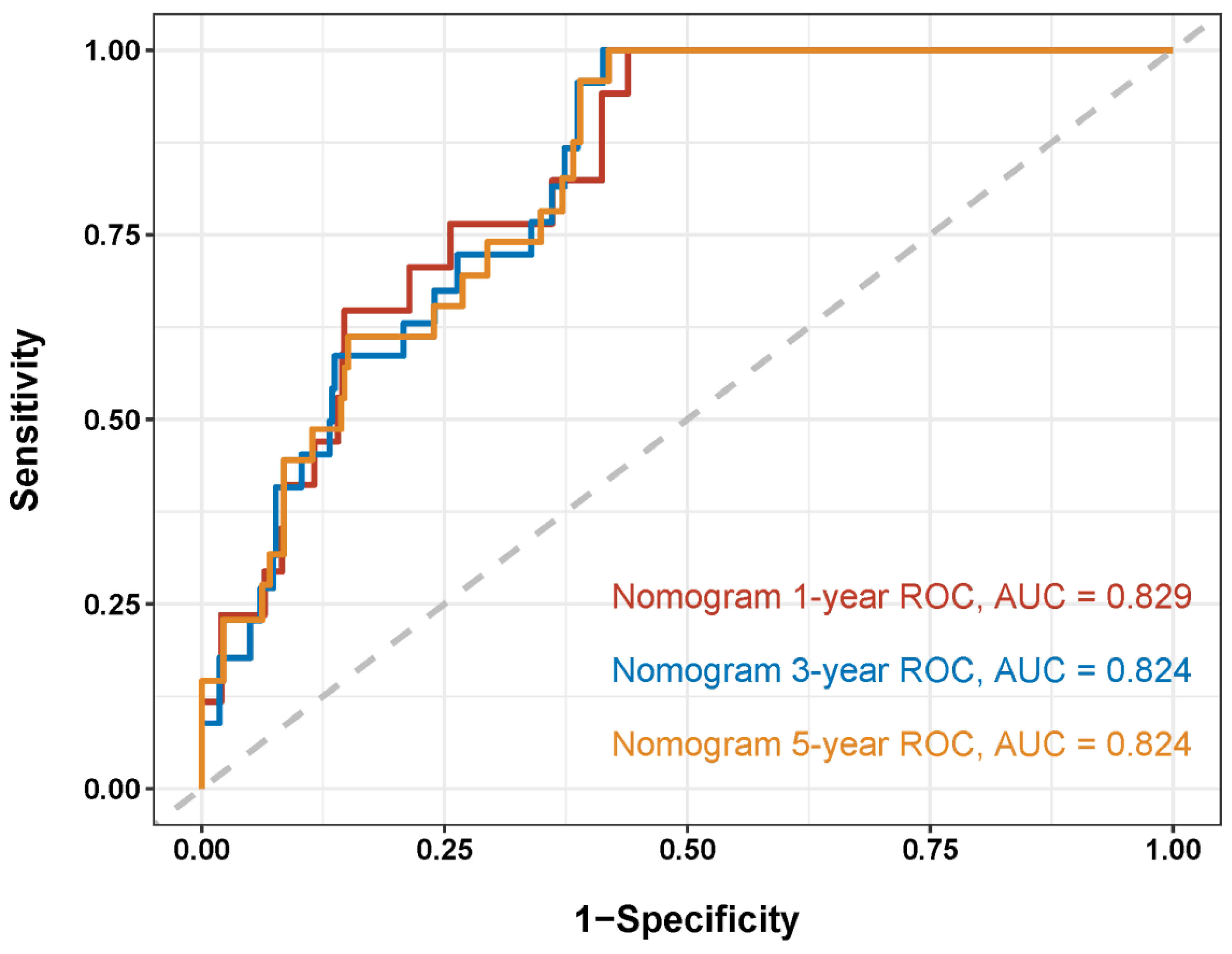
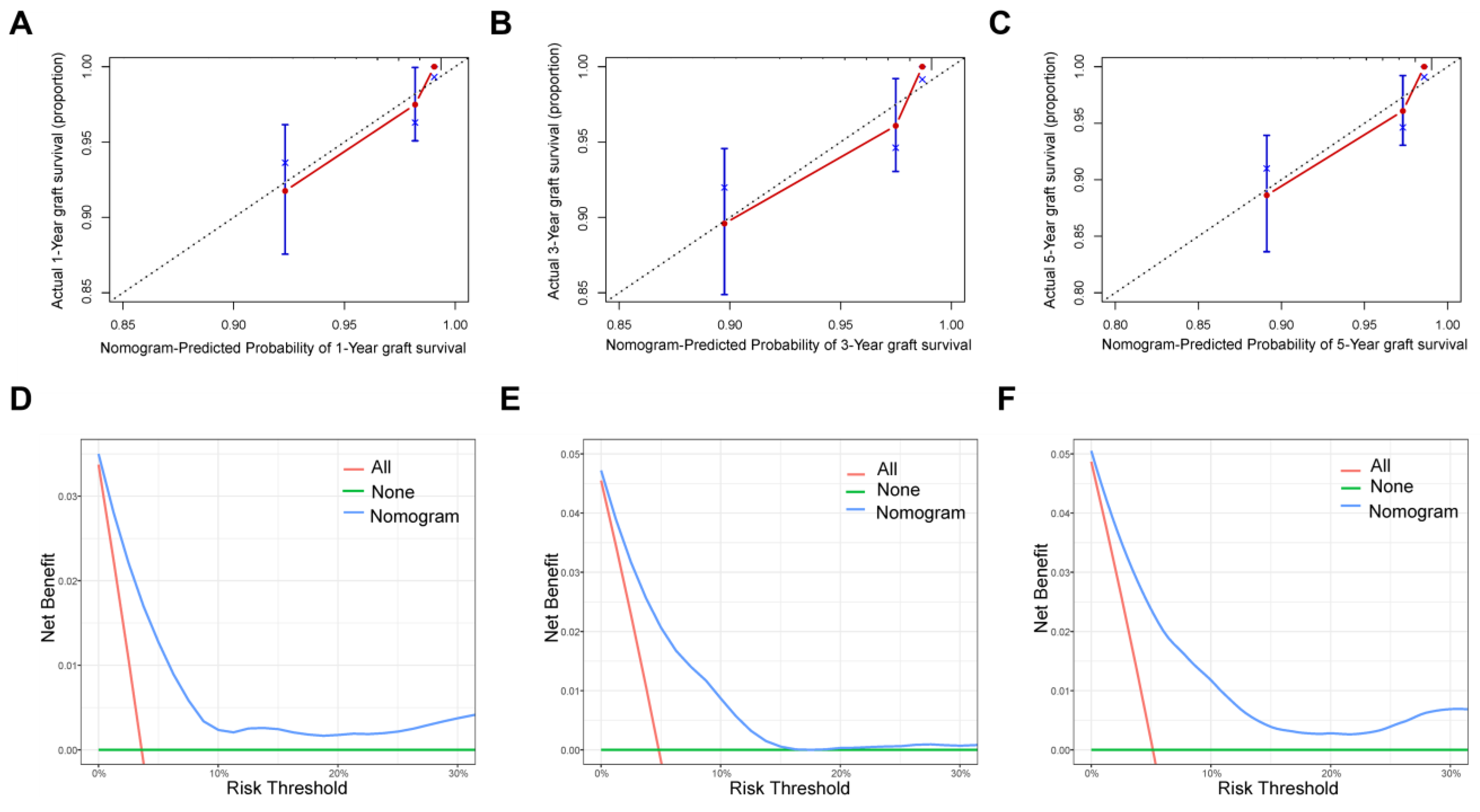
3.3. Nomogram Development and Validation
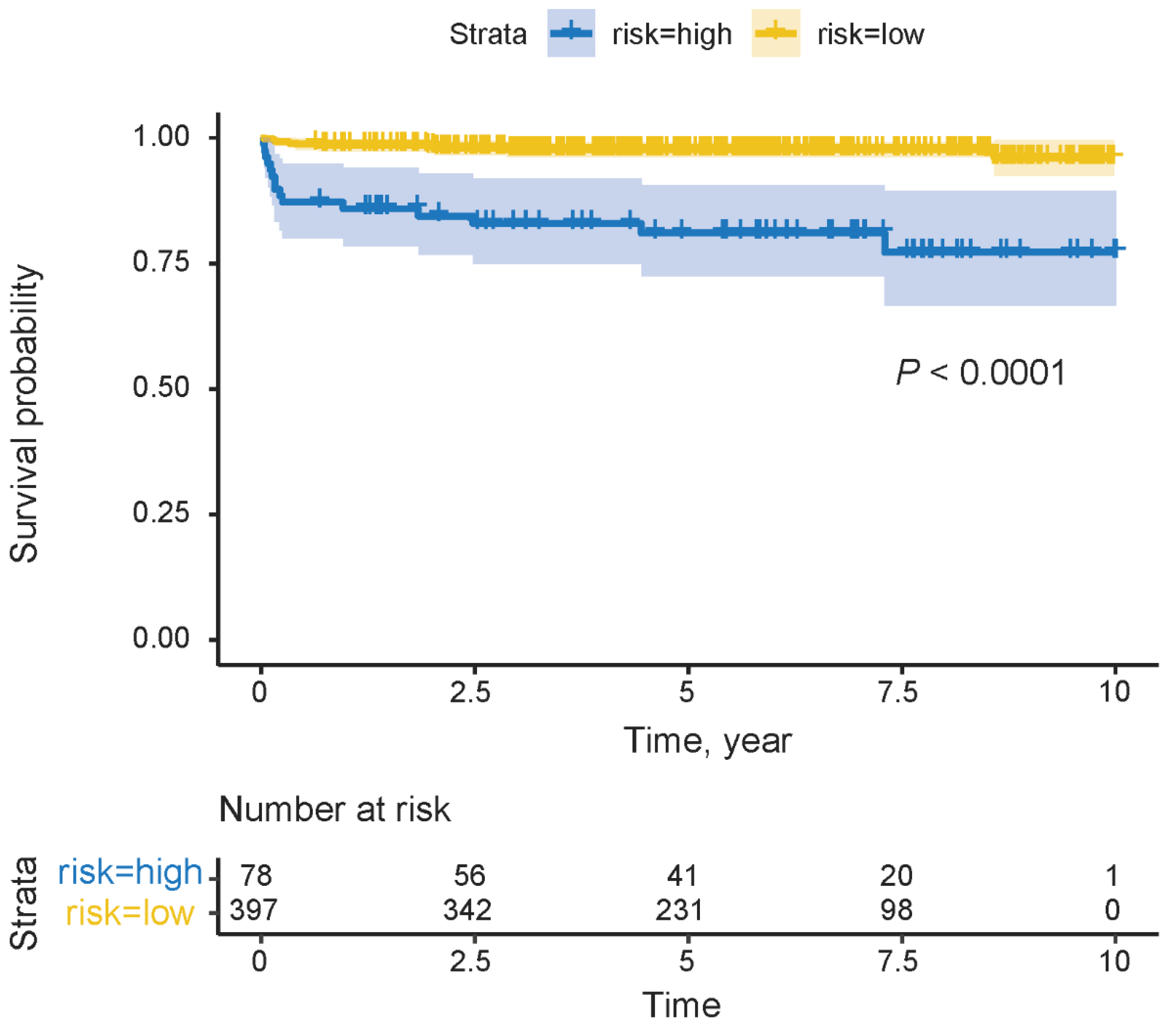
3.4. Risk Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | Biliary atresia |
| LT | Liver transplantation |
| KP | Kasai hepatoportoenterostomy |
| DCA | Decision curve analysis |
| GRWR | Graft-to-recipient weight ratio |
| CIT | Cold ischemic time |
| WIT | Warm ischemic time |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| GGT | Gamma-glutamyl transpeptidase |
| TB | Total bilirubin |
| SD | Standard deviation |
| IQR | Interquartile range |
| ROC | Receiver operating characteristic |
| WBC | White blood cell |
| HB | Hemoglobin |
| LDLT | Living donor liver transplantation |
| DDLT | Deceased donor liver transplantation |
| HAT | Hepatic artery thrombosis |
| PVT | Portal vein thrombosis |
References
- Shi, Y.; Jiang, Y.Z.; Zhou, G.P.; Shi, Y.; Gan, L.X.; Kong, Y.Y.; Wang, H.B.; Zhu, Z.J.; Sun, L.Y. Prognostic Factors Related to In-hospital Death in Children with Biliary Atresia: Analysis of a Nationwide Inpatient Database. J. Clin. Transl. Hepatol. 2023, 11, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Watayo, H.; Tsukui, T.; Suda, K.; Abe, E.; Fujimoto, T.; Ochi, T.; Lane, G.J.; Koga, H.; Yamataka, A. Native liver survivors of portoenterostomy for biliary atresia with excellent outcome: Redefining “successful” portoenterostomy. Pediatr. Surg. Int. 2022, 39, 24. [Google Scholar] [CrossRef]
- Matcovici, M.; Stoica, I.; Smith, K.; Davenport, M. What Makes A “Successful” Kasai Portoenterostomy “Unsuccessful”? J. Pediatr. Gastroenterol. Nutr. 2023, 76, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Umeshita, K.; Sakamoto, S.; Fukuda, A.; Furukawa, H.; Uemoto, S. Liver transplantation for biliary atresia: A systematic review. Pediatr. Surg. Int. 2017, 33, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Umeshita, K.; Sakamoto, S.; Fukuda, A.; Furukawa, H.; Sakisaka, S.; Kobayashi, E.; Tanaka, E.; Inomata, Y.; Kawasaki, S.; et al. Living donor liver transplantation for biliary atresia: An analysis of 2085 cases in the registry of the Japanese Liver Transplantation Society. Am. J. Transpl. 2018, 18, 659–668. [Google Scholar] [CrossRef]
- Utz Melere, M.; Sanha, V.; Farina, M.; da Silva, C.S.; Nader, L.; Trein, C.; Lucchese, A.M.; Ferreira, C.; Kalil, A.N.; Feier, F.H. Primary liver transplantation vs transplant after Kasai portoenterostomy in children with biliary atresia: A retrospective Brazilian single-center cohort. World J. Transpl. 2024, 14, 88734. [Google Scholar] [CrossRef]
- Davenport, M.; Superina, R. Primary Liver Transplant in Biliary Atresia: The Case for and Against. J. Pediatr. Surg. 2024, 59, 1418–1426. [Google Scholar] [CrossRef]
- Fuchs, J.; Mrad, C.; Gonzales, E.; Ndiaye, D.; Fouquet, V.; Héry, G.; Baujard, C.; Guérin, F.; Branchereau, S. Biliary drainage surgery before or after 3 months of life versus primary liver transplantation in children with biliary atresia: Comparative cohort study. BJS Open 2023, 7, zrac175. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Q.; Ji, Q.; Wang, Z.; Sun, R.; Zhan, J. Effect of Kasai procedure on liver transplantation in children with biliary atresia: A systematic review and updated meta-analysis. Transl. Pediatr. 2024, 13, 10–25. [Google Scholar] [CrossRef]
- Takeda, M.; Sakamoto, S.; Uchida, H.; Shimizu, S.; Yanagi, Y.; Fukuda, A.; Uchida, H.; Yamataka, A.; Kasahara, M. Comparative study of open and laparoscopic Kasai portoenterostomy in children undergoing living donor liver transplantation for biliary atresia. Pediatr. Surg. Int. 2021, 37, 1683–1691. [Google Scholar] [CrossRef]
- Jiang, Y.-Z.; Zhao, X.-Y.; Zhou, G.-P.; Wei, L.; Qu, W.; Zeng, Z.-G.; Wu, S.-S.; Zhang, H.-M.; Liu, Y.; Tan, Y.-L.; et al. Impact of immunosuppression level on liver allograft fibrosis after pediatric liver transplantation: A retrospective cohort study. Int. J. Surg. 2023, 109, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.G.; Zhou, G.P.; Wei, L.; Qu, W.; Liu, Y.; Tan, Y.L.; Wang, J.; Sun, L.Y.; Zhu, Z.J. Therapeutic potential of living donor liver transplantation from heterozygous carrier donors in children with propionic acidemia. Orphanet J. Rare Dis. 2022, 17, 62. [Google Scholar] [CrossRef]
- Oh, S.H.; Jeong, I.S.; Kim, D.Y.; Namgoong, J.M.; Jhang, W.K.; Park, S.J.; Jung, D.H.; Moon, D.B.; Song, G.W.; Park, G.C.; et al. Recent Improvement in Survival Outcomes and Reappraisal of Prognostic Factors in Pediatric Living Donor Liver Transplantation. Liver Transpl. 2022, 28, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.E.; Malamon, J.S.; Kaplan, B.; Saben, J.L.; Schold, J.D.; Pomposelli, J.J.; Pomfret, E.A. Survival Benefit of Living-Donor Liver Transplant. JAMA Surg. 2022, 157, 926–932. [Google Scholar] [CrossRef]
- Barbetta, A.; Butler, C.; Barhouma, S.; Hogen, R.; Rocque, B.; Goldbeck, C.; Schilperoort, H.; Meeberg, G.; Shapiro, J.; Kwon, Y.K.; et al. Living Donor Versus Deceased Donor Pediatric Liver Transplantation: A Systematic Review and Meta-analysis. Transpl. Direct 2021, 7, e767. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Schnellinger, E.M.; Handarova, D.; Weiss, S.; Cafarella, M.; et al. OPTN/SRTR 2021 Annual Data Report: Liver. Am. J. Transplant. 2023, 23 (Suppl. S1), S178–S263. [Google Scholar] [CrossRef]
- Shingina, A.; Vutien, P.; Uleryk, E.; Shah, P.S.; Renner, E.; Bhat, M.; Tinmouth, J.; Kim, J. Long-term Outcomes of Pediatric Living Versus Deceased Donor Liver Transplantation Recipients: A Systematic Review and Meta-analysis. Liver Transpl. 2022, 28, 437–453. [Google Scholar] [CrossRef]
- Anouti, A.; Patel, M.S.; VanWagner, L.B.; Lee, W.M.; Fung, J.J.; Cholankeril, G.; Hwang, C.S.; Mufti, A.R.; Tujios, S.; Kerr, T.; et al. Biliary atresia and liver transplantation in the United States: A contemporary analysis. Liver Int. 2023, 43, 2198–2209. [Google Scholar] [CrossRef]
- Taylor, S.A.; Venkat, V.; Arnon, R.; Gopalareddy, V.V.; Rosenthal, P.; Erinjeri, J.; Anand, R.; Daniel, J.F. Improved Outcomes for Liver Transplantation in Patients with Biliary Atresia Since Pediatric End-Stage Liver Disease Implementation: Analysis of the Society of Pediatric Liver Transplantation Registry. J. Pediatr. 2020, 219, 89–97. [Google Scholar] [CrossRef]
- Tang, W.; Qiu, J.G.; Cai, Y.; Cheng, L.; Du, C.Y. Increased Surgical Complications but Improved Overall Survival with Adult Living Donor Compared to Deceased Donor Liver Transplantation: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 2020, 1320830. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q.; Wang, B.; Zhou, A.; Zhou, T.; Liu, Y.; Luo, Y.; Xia, Q. Development of a Survival Predictive Model After Pediatric Liver Transplantation: A Single-center Retrospective Cohort Study. Transplantation 2025, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, S.; Mogawer, S.; Hosny, A.; El-Shazli, M.; Al-Jarhi, U.M.; Abdel-Hamed, S.; Salah, A.; El-Garem, N.; Sholkamy, A.; El-Amir, M.; et al. Predictors of Mortality in Living Donor Liver Transplantation. Transplant. Proc. 2017, 49, 1376–1382. [Google Scholar] [CrossRef]
- Avanaz, A.; Ünal, D.S.; Kisaoglu, A.; Yilmaz, V.T.; Moharer, P.S.; Demiryilmaz, I.; Aydinli, B. The Factors Affecting Mortality After Pediatric Liver Transplantation and Long-Term Survival Outcomes: A Single Center Experience. Transpl. Proc. 2023, 55, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Astarcıoglu, I.; Egeli, T.; Gulcu, A.; Ozbilgin, M.; Agalar, C.; Cesmeli, E.B.; Kaya, E.; Karademir, S.; Unek, T. Vascular Complications After Liver Transplantation. Exp. Clin. Transpl. 2023, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Nayeem, M.A.; Agarwal, S.; Goyal, N.; Gupta, S. Vascular complications in living donor liver transplantation at a high-volume center: Evolving protocols and trends observed over 10 years. Liver Transpl. 2017, 23, 457–464. [Google Scholar] [CrossRef]
- Calinescu, A.M.; Monluc, S.; Franchi-Abella, S.; Habes, D.; Weber, G.; Almes, M.F.; Waguet, J.; Jacquemin, E.; Fouquet, V.; Miatello, J.; et al. Long-term outcome of combined radiologic and surgical strategy for the management of biliary complications after pediatric liver transplantation. BMC Res. Notes 2024, 17, 86. [Google Scholar] [CrossRef]
- Boeva, I.; Karagyozov, P.I.; Tishkov, I. Post-liver transplant biliary complications: Current knowledge and therapeutic advances. World J. Hepatol. 2021, 13, 66–79. [Google Scholar] [CrossRef]
- Li, Z.; Rammohan, A.; Gunasekaran, V.; Hong, S.; Chih-Yi Chen, I.; Kim, J.; Hervera Marquez, K.A.; Hsu, S.C.; Kirimker, E.O.; Akamatsu, N.; et al. Biliary complications after adult-to-adult living-donor liver transplantation: An international multicenter study of 3633 cases. Am. J. Transpl. 2024, 24, 1233–1246. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Kelly, Y.; Braun, H.; Maluf, D.; Freise, C.; Ascher, N.; Roberts, J.; Roll, G. Comparison of Biliary Complications Rates After Brain Death, Donation After Circulatory Death, and Living-Donor Liver Transplantation: A Single-Center Cohort Study. Transpl. Int. 2022, 35, 10855. [Google Scholar] [CrossRef]
- Manay, P.; Seth, A.; Jackson, K.; Lentine, K.L.; Schnitzler, M.A.; Xiao, H.; Segev, D.L.; Axelrod, D.A. Biliary Complications After Liver Transplantation in the United States: Changing Trends and Economic Implications. Transplantation 2023, 107, e127–e138. [Google Scholar] [CrossRef]
- Axelrod, D.A.; Dzebisashvili, N.; Lentine, K.L.; Xiao, H.; Schnitzler, M.; Tuttle-Newhall, J.E.; Segev, D.L. Variation in biliary complication rates following liver transplantation: Implications for cost and outcome. Am. J. Transpl. 2015, 15, 170–179. [Google Scholar] [CrossRef] [PubMed]
| Parameter | No Kasai, n = 151 | Early Kasai, n = 287 | Late Kasai, n = 37 | p-Value | |
|---|---|---|---|---|---|
| Male, n (%) | 71 (47.0) | 145 (50.5) | 23 (62.2) | 0.25 | |
| Age at Kasai, days | - | 61 (51–72) | 107 (95–118) | <0.001 | |
| Weight, kg | 7.3 (6.5–8.2) | 9.3 (7.0–15.5) | 8.5 (6.7–16.0) | <0.001 | No vs. early KP: <0.001 No vs. late KP: 0.02 |
| Age at LT, months | 7.7 (6.2–9.5) | 15.0 (8.5–46.9) | 9.9 (7.8–56.4) | <0.001 | No vs. early KP: <0.001 No vs. late KP: <0.001 |
| ALT, U/L | 340 (171–581) | 317 (117–616) | 321 (164–692) | 0.43 | |
| AST, U/L | 530.4 (310.5–978.9) | 491.7 (186.1–909.1) | 398.5 (220.0–965.6) | 0.18 | |
| GGT, U/L | 106 (29–237) | 102 (46–201) | 128 (48–349) | 0.34 | |
| TBIL, µmol/L | 167.0 (96.2–261.3) | 72.7 (39.1–139.2) | 118.6 (46.9–204.4) | <0.001 | No vs. early KP: <0.001 No vs. late KP: 0.04 |
| WBC, ×109/L | 9.1 (6.4–13.3) | 9.5 (6.1–13.4) | 9.1 (6.4–12.8) | 0.97 | |
| Neutrophils % | 58.3 (48.0–68.9) | 63.8 (49.5–75.7) | 68.8 (53.8–77.4) | 0.003 | No vs. early KP: 0.01 No vs. late KP: 0.02 |
| RBC, ×1012/L | 3.4 (3.0–3.9) | 3.8 (3.2–4.2) | 3.8 (3.0–4.4) | <0.001 | No vs. early KP: <0.001 |
| HB, g/L | 100 (86–113) | 103 (89–116) | 101 (84–121) | 0.58 | |
| Platelet count, ×109/L | 132 (93–201) | 129 (85–190) | 125 (95–222) | 0.49 |
| Parameter | No Kasai, n = 151 | Early Kasai, n = 287 | Late Kasai, n = 37 | p-Value | |
|---|---|---|---|---|---|
| LT type | 0.001 | ||||
| LDLT | 126 (83.4) | 197 (68.6) | 23 (62.2) | ||
| DDLT | 25 (16.6) | 90 (31.4) | 14 (37.8) | ||
| GRWR, % | 3.4 (3.0–4.0) | 2.8 (2.1–3.6) | 3.1 (2.1–4.0) | <0.001 | No vs. early KP: <0.001 No vs. late KP: 0.03 |
| Graft type | 0.002 | ||||
| Whole liver | 23 (15.2) | 81 (28.2) | 12 (32.4) | ||
| Left lateral segment | 117 (77.5) | 170 (59.2) | 22 (59.5) | ||
| Others | 11 (7.3) | 36 (12.5) | 3 (8.1) | ||
| Warm ischemia time, min | 3.0 (2.0–5.0) | 3.0 (2.0–5.0) | 3.0 (3.0–5.0) | 0.92 | |
| Cold ischemia time, h | 1.4 (1.1–2.1) | 2.0 (1.4–6.0) | 2.2 (1.5–6.3) | <0.001 | No vs. early KP: <0.001 No vs. late KP: 0.002 |
| Operation time, h | 6.8 (5.8–7.5) | 6.7 (5.8–7.6) | 6.3 (5.4–7.5) | 0.31 | |
| Intraoperative blood loss, mL | 240 (150–300) | 200 (120–300) | 160 (120–300) | 0.23 | |
| Duration of ventilation, h | 3.0 (2.0–6.0) | 3.0 (2.0–5.0) | 3.0 (1.5–5.0) | 0.03 | No vs. early KP: 0.03 |
| ICU stay, hours | 92.0 (69.5–120.0) | 78.3 (60.0–107.8) | 87.0 (59.0–100.0) | <0.001 | No vs. early KP: <0.001 |
| Hospitalization, days | 24 (19–35) | 22 (17–32) | 23 (18–30) | 0.06 | |
| Follow-up period, years | 6.7 (4.0–8.5) | 5.2 (3.2–6.6) | 5.2 (3.0–6.6) | <0.001 | No vs. early KP: <0.001 No vs. late KP: 0.004 |
| Postoperative complications | |||||
| Vascular complications | 8 (5.3) | 5 (1.7) | 1 (2.7) | 0.11 | |
| Biliary complications | 3 (2.0) | 19 (6.6) | 4 (10.8) | 0.03 | |
| Infection | 28 (18.5) | 92 (32.1) | 11 (29.7) | 0.01 | |
| Re-transplantation | 1 (0.7) | 4 (1.4) | 1 (2.7) | 0.40 | |
| Bleeding | 3 (2.0) | 17 (5.9) | 1 (2.7) | 0.15 | |
| Patient mortality | 6 (4.0) | 9 (3.1) | 4 (10.8) | 0.06 | |
| Graft loss | 7 (4.6) | 13 (4.5) | 5 (13.5) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, J.-Y.; Jiang, Y.-Z.; Sun, L.-Y.; Wang, Y.-X. The Effect of a Kasai Procedure on Liver Transplantation in Children with Biliary Atresia: A Cohort Study. J. Clin. Med. 2025, 14, 3328. https://doi.org/10.3390/jcm14103328
Dong H, Liu J-Y, Jiang Y-Z, Sun L-Y, Wang Y-X. The Effect of a Kasai Procedure on Liver Transplantation in Children with Biliary Atresia: A Cohort Study. Journal of Clinical Medicine. 2025; 14(10):3328. https://doi.org/10.3390/jcm14103328
Chicago/Turabian StyleDong, Hao, Jing-Yi Liu, Yi-Zhou Jiang, Li-Ying Sun, and You-Xin Wang. 2025. "The Effect of a Kasai Procedure on Liver Transplantation in Children with Biliary Atresia: A Cohort Study" Journal of Clinical Medicine 14, no. 10: 3328. https://doi.org/10.3390/jcm14103328
APA StyleDong, H., Liu, J.-Y., Jiang, Y.-Z., Sun, L.-Y., & Wang, Y.-X. (2025). The Effect of a Kasai Procedure on Liver Transplantation in Children with Biliary Atresia: A Cohort Study. Journal of Clinical Medicine, 14(10), 3328. https://doi.org/10.3390/jcm14103328








