Efficacy of Physical Therapy Rehabilitation in the Cardiovascular Deconditioning of Post-Stroke Survivors: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approval—Registrations
2.2. Data Sources, Search, and Study Selection
2.3. Quality Control, Bias Assessment, and Data Extraction
2.4. Outcomes
2.5. Statistical Analysis
2.6. Data Availability Statement
3. Results
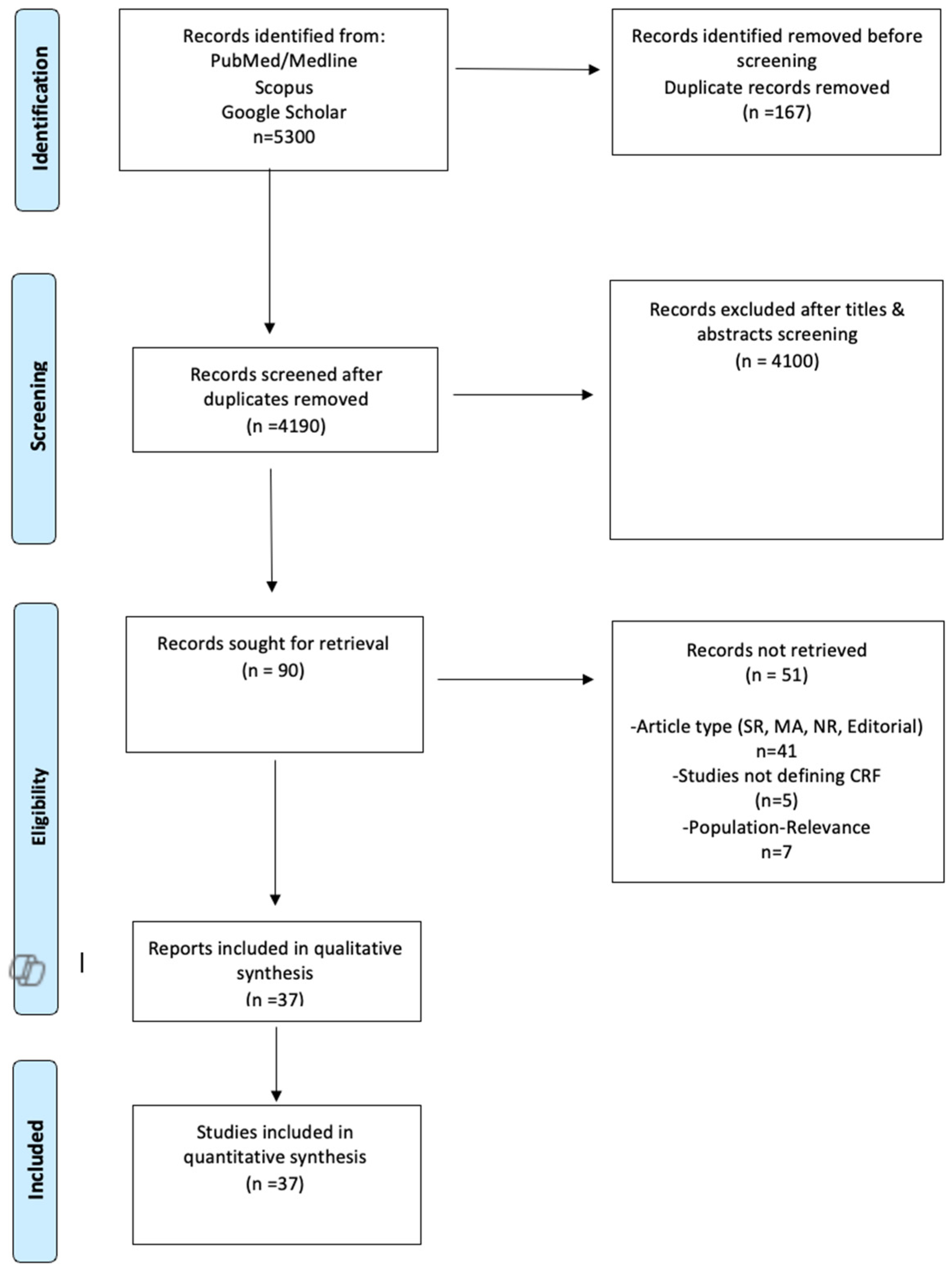
3.1. Literature Search
3.2. Quality Control of Included Studies
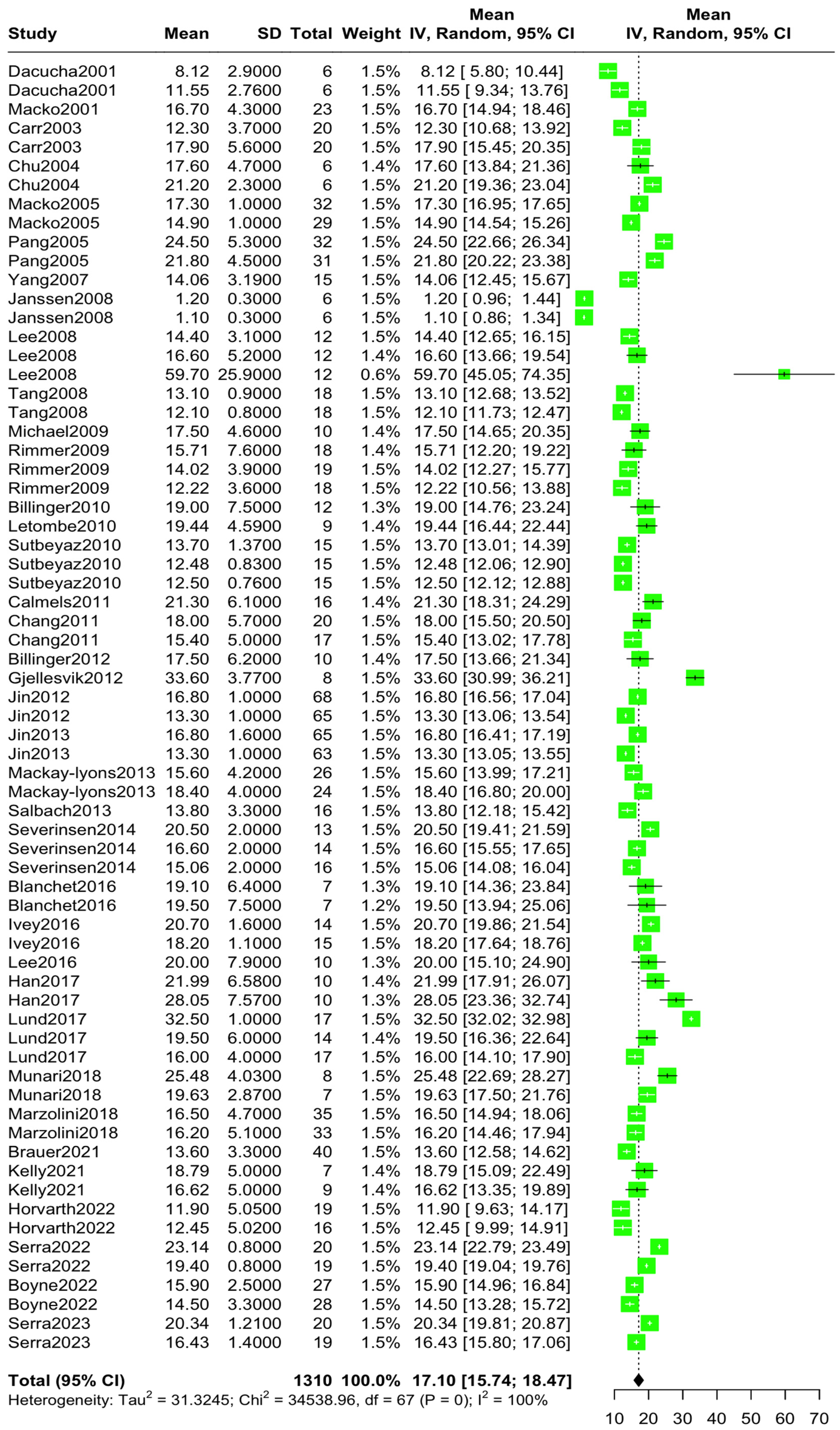
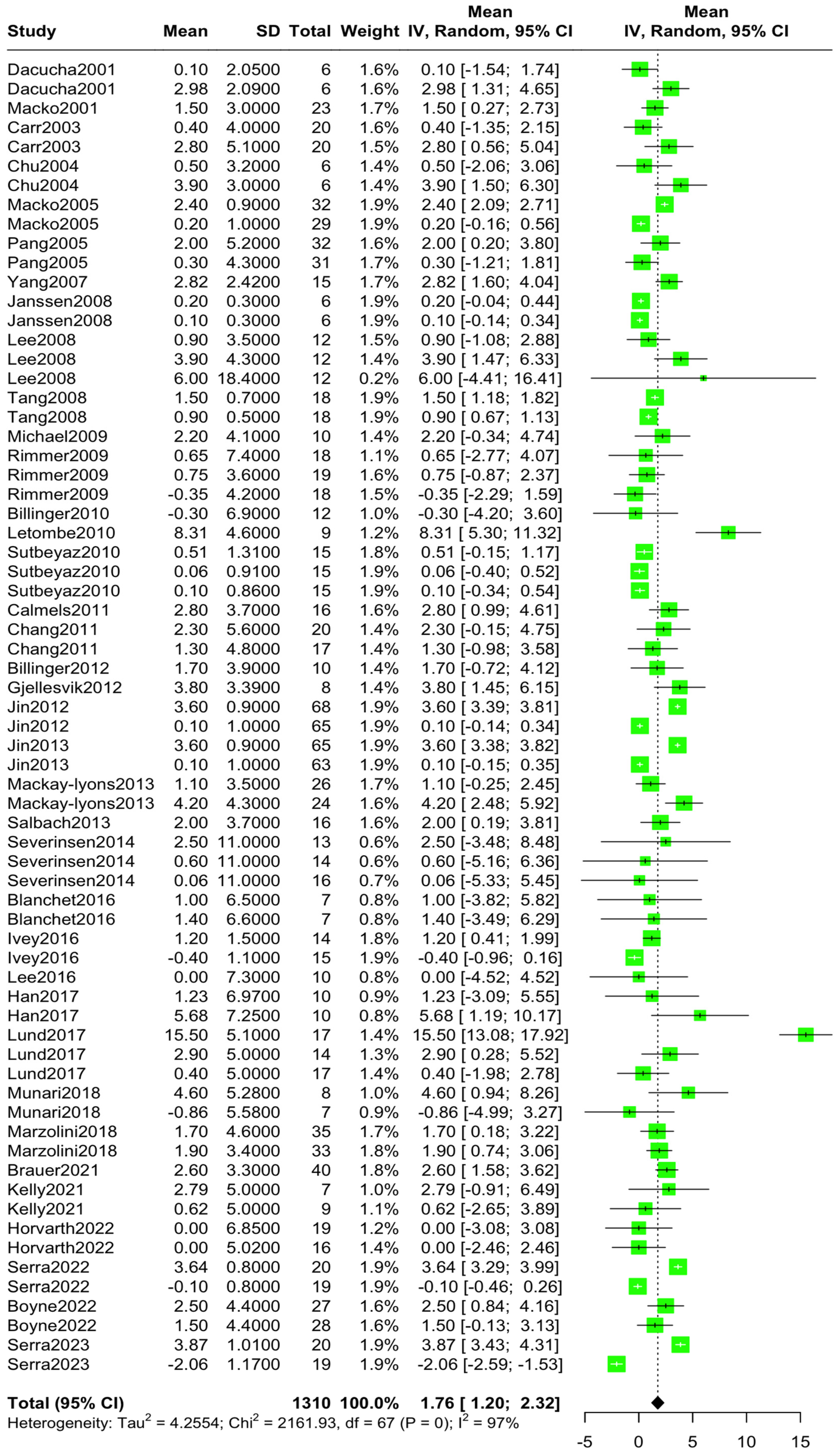
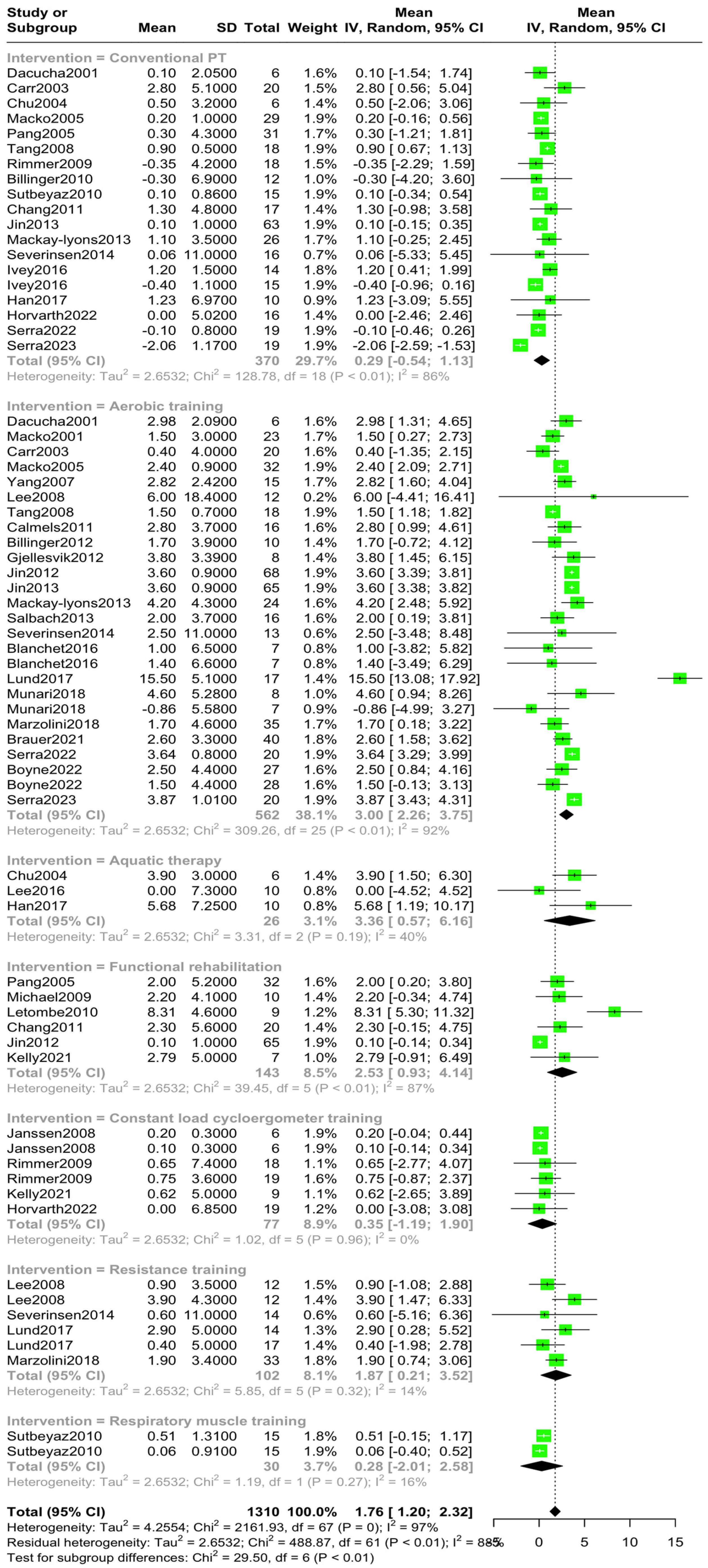
3.3. Overall and Subgroup Analysis
3.4. Sensitivity Analyses
3.5. Publication Bias
4. Discussion
4.1. Clinical Significance of PeakVO2 in Post-Stroke Recovery
4.2. Limitations—Confounding Factors
4.3. Future Research Direction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Capirossi, C.; Laiso, A.; Renieri, L.; Capasso, F.; Limbucci, N. Epidemiology, organization, diagnosis and treatment of acute ischemic stroke. Eur. J. Radiol. Open. 2023, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, T.; Piechowski-Jóźwiak, B.; Bonita, R.; Mathers, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe: A review of available data. Eur. J. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef] [PubMed]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated with Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef]
- Rahman, F.B.A.; Jones, A.Y.M.; Pang, M.Y.C. Oxygen consumption and peak heart rate in stroke patients during the completion of the Modified Rivermead Mobility Index (MRMI). Hong Kong Physiother. J. 2012, 30, 76–82. [Google Scholar] [CrossRef]
- Blokland, I.J.; Groot, F.P.; Logt, N.H.G.; Van Bennekom, C.A.M.; De Koning, J.J.; Van Dieen, J.H.; Houdijk, H. Cardiorespiratory Fitness in Individuals Post-stroke: Reference Values and Determinants. Arch. Phys. Med. Rehabil. 2023, 104, 1612–1619. [Google Scholar] [CrossRef]
- Sushmitha, S.; Kothari, R.; Mittal, G.; Gopani, M.; Prashanth, A.; Bokariya, P.; Vemparala, S.S.; Tamrakar, S.; Abishek, S.; Bennita, A. Exploring the Relationship Between the Indices of Body Composition with Grip Strength Performance and Peak VO2. Cureus 2023, 15, e40874. [Google Scholar] [CrossRef]
- Billinger, S.A.; Coughenour, E.; MacKay-Lyons, M.J.; Ivey, F.M. Reduced cardiorespiratory fitness after stroke: Biological consequences and exercise-induced adaptations. Stroke Res. Treat. 2011, 2012, 959120. [Google Scholar] [CrossRef]
- Mendoza, M.F.; Suan, N.M.; Lavie, C.J. Exploring the Molecular Adaptations, Benefits, and Future Direction of Exercise Training: Updated Insights into Cardiovascular Health. J. Funct. Morphol. Kinesiol. 2024, 9, 131. [Google Scholar] [CrossRef]
- MacKay-Lyons, M.; McDonald, A.; Matheson, J.; Eskes, G.; Klus, M.A. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: A randomized controlled trial. Neurorehabilit. Neural Repair 2013, 27, 644–653. [Google Scholar] [CrossRef]
- Calmels, P.; Degache, F.; Courbon, A.; Roche, F.; Ramas, J.; Fayolle-Minon, I.; Devillard, X. The feasibility and the effects of cycloergometer interval-training on aerobic capacity and walking performance after stroke. Ann. Phys. Rehabil. Med. 2011, 54, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.C.; Hafer-Macko, C.E.; Robbins, R.; Jason, C.; Connor, O.; Ryan, A.S. Stress in Stroke. J. Am. Geriatr. Soc. 2023, 681, S1–S385. [Google Scholar] [CrossRef]
- Michael, K.; Goldberg, A.P.; Treuth, M.S.; Beans, J.; Normandt, P.; Macko, R.F. Progessive Adaptive Physical Activity in Stroke Improves Balance, Gait and Fitness: Preliminary Results. Top. Stroke Rehabil. 2009, 16, 133–139. [Google Scholar] [CrossRef]
- Letombe, A.; Cornille, C.; Delahaye, H.; Khaled, A.; Morice, O.; Tomaszewski, A.; Olivier, N. Early post-stroke physical conditioning in hemiplegic patients: A preliminary study. Ann. Phys. Rehabil. Med. 2010, 53, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, S.; Brooks, D.; Oh, P.; Jagroop, D.; MacIntosh, B.J.; Anderson, N.D.; Alter, D.; Corbett, D. Aerobic with Resistance Training or Aerobic Training Alone Poststroke: A Secondary Analysis from a Randomized Clinical Trial. Neurorehabilit. Neural Repair 2018, 32, 209–222. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Research Methods And Reporting PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting—Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 366, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Giannopapas, V.; Stefanou, M.-I.; Smyrni, V.; Kitsos, D.K.; Kosmidou, M.; Stasi, S.; Chasiotis, A.K.; Stavrogianni, K.; Papagiannopoulou, G.; Tzartos, J.S.; et al. Waist Circumference and Body Mass Index as Predictors of Disability Progression in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 1739. [Google Scholar] [CrossRef]
- Teixeira da Cunha Filho, I.; Lim, P.A.; Qureshy, H.; Henson, H.; Monga, T.; Protas, E.J. A comparison of regular rehabilitation and regular rehabilitation with supported treadmill ambulation training for acute stroke patients. J. Rehabil. Res. Dev. 2001, 38, 245–255. [Google Scholar] [PubMed]
- Macko, R.F.; Smith, G.V.; Dobrovolny, C.L.; Sorkin, J.D.; Goldberg, A.P.; Silver, K.H. Treadmill training improves fitness reserve in chronic stroke patients. Arch. Phys. Med. Rehabil. 2001, 82, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.; Jones, J. Physiological Effects of Exercise on Stroke Survivors. Top. Stroke Rehabil. 2003, 9, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.S.; Eng, J.J.; Dawson, A.S.; Harris, J.E.; Ozkaplan, A.; Gylfadóttir, S. Water-based exercise for cardiovascular fitness in people with chronic stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2004, 85, 870–874. [Google Scholar] [CrossRef]
- Macko, R.F.; Ivey, F.M.; Forrester, L.W.; Hanley, D.; Sorkin, J.D.; Katzel, L.I.; Silver, K.H.; Goldberg, A.P. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: A randomized, controlled trial. Stroke 2005, 36, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.C.; Eng, J.J.; Dawson, A.S.; McKay, H.A.; Harris, J.E. A community-based fitness and mobility exercise program for older adults with chronic stroke: A randomized, controlled trial. J. Am. Geriatr. Soc. 2005, 53, 1667–1674. [Google Scholar] [CrossRef]
- Yang, A.L.; Lee, S.D.; Su, C.T.; Wang, J.L.; Lin, K.L. Effects of exercise intervention on patients with stroke with prior coronary artery disease: Aerobic capacity, functional ability, and lipid profile: A pilot study. J. Rehabil. Med. 2007, 39, 88–90. [Google Scholar] [CrossRef][Green Version]
- Janssen, T.W.; Beltman, J.M.; Elich, P.; Koppe, P.A.; Konijnenbelt, H.; de Haan, A.; Gerrits, K.H. Effects of Electric Stimulation-Assisted Cycling Training in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 463–469. [Google Scholar] [CrossRef]
- Lee, M.J.; Kilbreath, S.L.; Singh, M.F.; Zeman, B.; Lord, S.R.; Raymond, J.; Davis, G.M. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: A randomized sham exercise-controlled study. J. Am. Geriatr. Soc. 2008, 56, 976–985. [Google Scholar] [CrossRef]
- Tang, A.; Sibley, K.M.; Thomas, S.G.; Bayley, M.T.; Richardson, D.; McIlroy, W.E.; Brooks, D. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabilit. Neural Repair 2009, 23, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, J.H.; Rauworth, A.E.; Wang, E.C.; Nicola, T.L.; Hill, B. A Preliminary Study to Examine the Effects of Aerobic and Therapeutic (Nonaerobic) Exercise on Cardiorespiratory Fitness and Coronary Risk Reduction in Stroke Survivors. Arch. Phys. Med. Rehabil. 2009, 90, 407–412. [Google Scholar] [CrossRef]
- Billinger, S.; Guo, L.X.; Pohl, P.S.; Kluding, P.M. Single limb exercise: Pilot study of physiological and functional responses to forced use of the hemiparetic lower extremity. Top. Stroke Rehabil. 2010, 17, 128–139. [Google Scholar] [CrossRef]
- Sutbeyaz, S.T.; Koseoglu, F.; Inan, L.; Coskun, O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: A randomized controlled trial. Clin. Rehabil. 2010, 24, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.H.; Kim, M.S.; Huh, J.P.; Lee, P.K.W.; Kim, Y.H. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients: A randomized controlled study. Neurorehabilit. Neural Repair 2012, 26, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Mattlage, A.E.; Ashenden, A.L.; Lentz, A.A.; Harter, G.; Rippee, M.A. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J. Neurol. Phys. Ther. 2012, 36, 159–165. [Google Scholar] [CrossRef]
- Gjellesvik, T.I.; Brurok, B.; Hoff, J.; Tørhaug, T.; Helgerud, J. Effect of high aerobic intensity interval treadmill walking in people with chronic stroke: A pilot study with one year follow-up. Top. Stroke Rehabil. 2012, 19, 353–360. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, Y.; Wei, Q.; Wang, B.; Ma, G. Intensive aerobic cycling training with lower limb weights in Chinese patients with chronic stroke: Discordance between improved cardiovascular fitness and walking ability. Disabil. Rehabil. 2012, 34, 1665–1671. [Google Scholar] [CrossRef]
- Jin, H.; Jiang, Y.; Wei, Q.; Chen, L.; Ma, G. Effects of aerobic cycling training on cardiovascular fitness and heart rate recovery in patients with chronic stroke. NeuroRehabilitation 2013, 32, 327–335. [Google Scholar] [CrossRef]
- Salbach, N.M.; Brooks, D.; Romano, J.; Woon, L.; Dolmage, T.E. Cardiorespiratory responses during the 6-minute walk and ramp cycle ergometer tests and their relationship to physical activity in stroke. Neurorehabilit. Neural Repair 2014, 28, 111–119. [Google Scholar] [CrossRef]
- Severinsen, K.; Jakobsen, J.K.; Pedersen, A.R.; Overgaard, K.; Andersen, H. Effects of resistance training and aerobic training on ambulation in chronic stroke. Am. J. Phys. Med. Rehabil. 2014, 93, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, S.; Richards, C.L.; Leblond, J.; Olivier, C.; Maltais, D.B. Cardiorespiratory fitness and cognitive functioning following short-term interventions in chronic stroke survivors with cognitive impairment: A pilot study. Int. J. Rehabil. Res. 2016, 39, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ivey, F.M.; Prior, S.J.; Hafer-Macko, C.E.; Katzel, L.I.; Macko, R.F.; Ryan, A.S. Strength Training for Skeletal Muscle Endurance after Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 787–794. [Google Scholar] [CrossRef]
- Lee, Y.K.; Kim, B.R.; Han, E.Y. Peak Cardiorespiratory Responses of Patients with Subacute Stroke during Land and Aquatic Treadmill Exercise. Am. J. Phys. Med. Rehabil. 2017, 96, 289–293. [Google Scholar] [CrossRef]
- Han, E.Y.; Im, S.H. Effects of a 6-Week Aquatic Treadmill Exercise Program on Cardiorespiratory Fitness and Walking Endurance in Subacute Stroke Patients A PILOT TRIAL. J. Cardiopulm. Rehabil. Prev. 2018, 38, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Lund, C.; Dalgas, U.; Grønborg, T.K.; Andersen, H.; Severinsen, K.; Riemenschneider, M.; Overgaard, K. Balance and walking performance are improved after resistance and aerobic training in persons with chronic stroke. Disabil. Rehabil. 2018, 40, 2408–2415. [Google Scholar] [CrossRef]
- Munari, D.; Pedrinolla, A.; Smania, N.; Picelli, A.; Gandolfi, M.; Saltuari, L.; Schena, F. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: Preliminary results of a pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 408–418. [Google Scholar] [CrossRef]
- Brauer, S.G.; Kuys, S.S.; Paratz, J.D.; Ada, L. High-intensity treadmill training and self-management for stroke patients undergoing rehabilitation: A feasibility study. Pilot Feasibility Stud. 2021, 7, 215. [Google Scholar] [CrossRef]
- Kelly, L.P.; Devasahayam, A.J.; Chaves, A.R.; Curtis, M.E.; Randell, E.W.; McCarthy, J.; Basset, F.A.; Ploughman, M. Task-oriented circuit training as an alternative to ergometer-type aerobic exercise training after stroke. J. Clin. Med. 2021, 10, 2423. [Google Scholar] [CrossRef]
- Horváth, J.; Debreceni Nagy, A.; Fülöp, P.; Jenei, Z. Effectiveness of hospital-based low intensity and inspected aerobic training on functionality and cardiorespiratory fitness in unconditioned stroke patients: Importance of submaximal aerobic fitness markers. Medicine 2022, 101, E31035. [Google Scholar] [CrossRef]
- Boyne, P.; Billinger, S.A.; Reisman, D.S.; Awosika, O.O.; Buckley, S.; Burson, J.B.; Carl, D.; DeLange, M.; Doren, S.; Earnest, M.; et al. A Multicenter Randomized Comparison of High-Intensity Interval Training and Moderate-Intensity Exercise to Recover. Walking Post-Stroke: Results of the HIT-Stroke Trial. MedRxiv 2022. [Google Scholar] [CrossRef]
- Serra, M.C.; Hafer-Macko, C.E.; Robbins, R.; O’Connor, J.C.; Ryan, A.S. Randomization to Treadmill Training Improves Physical and Metabolic Health in Association with Declines in Oxidative Stress in Stroke. Arch. Phys. Med. Rehabil. 2022, 103, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Balady, G.J.; Amsterdam, E.A.; Chaitman, B.; Eckel, R.; Fleg, J.; Froelicher, V.F.; Leon, A.S.; Piña, I.L.; Rodney, R.; et al. Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 104, 1694–1740. [Google Scholar] [CrossRef]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Wang, J.; Zhao, W.; Zeng, Z.; Hao, L.; Yuan, Y.; Lin, Y.; Wu, Y.; Wang, Z. Normal References of Peak Oxygen Uptake for Cardiorespiratory Fitness Measured with Cardiopulmonary Exercise Testing in Chinese Adults. J. Clin. Med. 2022, 21, 4904. [Google Scholar] [CrossRef]
- Rivas, E.; Huynh, H.; Galassetti, P.R. Obesity Affects Submaximal Oxygen Uptake-Heart Rate Relationship and Exercise Economy Differently in Pre- and Post-pubescent Boys and Girls. Int. J. Exerc. Sci. 2019, 12, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Mishra, S.P. Effect of BMI, body fat percentage and fat free mass on maximal oxygen consumption in healthy young adults. J. Clin. Diagn. Res. 2017, 11, CC17–CC20. [Google Scholar] [CrossRef]
- He, Z.H.; Ma, L.H. The aerobic fitness (VO2 peak) and α-fibrinogen genetic polymorphism in obese and non-obese chinese boys. Int. J. Sports Med. 2005, 26, 253–257. [Google Scholar] [CrossRef]
- Seiler, S. A Brief History of Endurance Testing in Athletes. Sportscience 2011, 15, 40–86. [Google Scholar]
- Shehjar, F.; Maktabi, B.; Rahman, Z.A.; Bahader, G.A.; James, A.W.; Naqvi, A.; Mahajan, R.; Shah, Z.A. Stroke: Molecular mechanisms and therapies: Update on recent developments. Neurochem. Int. 2023, 162, 105458. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Megarbane, B.; Rezaee, R. Molecular Mecahnisms Mediating Adaptation to Exercise. Adv. Exp. Med. Biol. 2020, 1228, 45–61. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.W.; Steimle, A.E.; Fonarow, G.; Kermani, M.; Kermani, D.; Hamilton, M.A.; Moriguchi, J.D.; Walden, J.; Tillisch, J.H.; Drinkwater, D.C.; et al. Improvement in exercise capacity of candidates awaiting heart transplantation. J. Am. Coll. Cardiol. 1995, 25, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.C.; et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]


| Author | Region | Study Type | Sample | Post-Stroke Rehabilitation Latency | Rehabilitation Protocol |
|---|---|---|---|---|---|
| Dacucha et al., 2001 [22] | Texas | RCT* | 12 | <1 | Group 1: conventional PT* Group 2: aerobic training |
| Macko et al., 2001 [23] | Baltimore | CT* | 23 | 2.2 | Aerobic training |
| Carr et al., 2003 [24] | Chicago | RCT* | 40 | <1 | Group 1: conventional PT* Group 2: aerobic training |
| Chu et al., 2004 [25] | Columbia | RCT* | 12 | <1 | Group 1: conventional PT* Group 2: aquatic therapy |
| Macho et al., 2005 [26] | Baltimore | RCT* | 61 | 3.2 | Group 1: conventional PT* Group 2: aerobic training |
| Pang et al., 2005 [27] | Canada | RCT* | 32 | 5.2 | Group 1: conventional PT* Group 2: functional rehabilitation training |
| Yang et al., 2007 [28] | Taiwan | CT* | 15 | 1.0 | Aerobic training |
| Janssen et al., 2008 [29] | Netherlands | RCT* | 12 | 1.8 | Group 1: constant-load cycloergometer training Group 2: electrical constant-load cycloergometer training |
| Lee et al., 2008 [30] | Sydney | RCT* | 36 | 3.6 | Group 1: sham resistance training Group 2: resistance training Group C (n = 12): Aerobic training |
| Tang et al., 2008 [31] | Canada | RCT* | 36 | <1 | Group 1 conventional PT* Group 1: aerobic training |
| Michael et al., 2009 [13] | Baltimore | CT* | 10 | 7.5 | Functional rehabilitation training |
| Rimmer et al., 2009 [32] | Chicago | RCT* | 18 | <1 | Group 1: conventional PT* Group 2: constant-load cycloergometer training Group 3: constant-load cycloergometer training |
| Billinger et al., 2010 [33] | Kansas | CT* | 12 | 5.7 | Conventional PT* training |
| Letombe et al., 2010 [14] | France | RCT* | 9 | <1 | Functional rehabilitation training |
| Sutbeyaz et al., 2010 [34] | Turkey | RCT* | 45 | <1 | Group 1: conventional PT* training Group 2: respiratory muscle training Group 3: respiratory muscle training |
| Calmels et al., 2011 [11] | France | CT* | 16 | 1.0 | Aerobic cycloergometer interval training |
| Chang et al., 2011 [35] | Korea | RCT* | 37 | <1 | Group 1: conventional PT* training Group 2: functional rehabilitation training |
| Billinger et al., 2012 [36] | Kansas | CT* | 10 | <1 | Aerobic training |
| Gjellevsnik et al., 2012 [37] | Norway | CT* | 8 | 7.2 | Aerobic training |
| Jin et al., 2012 [38] | China | RCT* | 133 | 1.5 | Group 1: aerobic training Group 2: functional rehabilitation training |
| Jin et al., 2013 [39] | China | RCT* | 65 | <1 | Group 1: aerobic training Group 2: conventional PT* |
| Mackay-Lyons et al., 2013 [10] | Canada | RCT* | 26 | 6 | Group 1: conventional PT* Group 2: aerobic training |
| Salbach et al., 2013 [40] | Toronto | RCT* | 16 | 2.0 | Aerobic training |
| Severinsen et al., 2014 [41] | Denmark | RCT* | 43 | 1.5 | Group 1: aerobic training Group 2: resistance training Group 3: conventional PT* |
| Blanchet et al., 2016 [42] | Canada | RCT* | 14 | 4.2 | Group 1: aerobic training Group 2: aerobic and cognitive training |
| Ivey et al., 2016 [43] | Baltimore | RCT* | 29 | <1 | Group 1: conventional PT* Group 2: conventional PT* |
| Lee et al., 2016 [44] | Korea | RCT* | 10 | <1 | Aquatic therapy |
| Han et al., 2017 [45] | Korea | RCT* | 20 | <1 | Group 1: conventional PT* Group 2: aquatic therapy |
| Lund et al., 2017 [46] | Denmark | RCT* | 48 | 1.8 | Group 1: aerobic training Group 2: high-intensity resistance training Group 3: low-intensity resistance training |
| Munari et al., 2018 [47] | Italy | RCT* | 15 | 6.4 | Group 1: aerobic HITT* training Group 2: aerobic LITT* training |
| Marzolini et al., 2018 [15] | Canada | RCT* | 68 | 1.2 | Group 1: aerobic training Group 2: resistance training |
| Brauer et al., 2021 [48] | Australia | CT* | 40 | <1 | Aerobic training |
| Kelly et al., 2021 [49] | Canada | RCT* | 7 | <1 | Group 1: constant-load cycloergometer training Group 2: functional rehabilitation training |
| Horvarth et al., 2022 [50] | Hungary | RCT* | 35 | 8 | Group 1: conventional PT* Group 2: constant-load cycloergometer training |
| Serra et al., 2023 [12] | San Antonio | RCT* | 39 | 2.7 | Group 1: conventional PT* Group 2: constant-load cycloergometer training |
| Boyne et al., 2022 [51] | Kansas | RCT* | 27 | 2.2 | Group 1: aerobic HIT* training Group 2: aerobic MAT* training |
| Serra et al., 2022 [52] | San Antonio | RCT* | 20 | 7.0 | Group 1: conventional PT* Group 2: aerobic HIT* training |
| Intervention | Sample | Mean δVO2peak Pre–Post-Intervention | 95%CI | I2 | sig. |
|---|---|---|---|---|---|
| Conventional PT [10,12,22,23,24,25,26,27,31,32,33,34,35,39,41,43,45,51] | 370 | 0.29 | −0.54, 1.13 | 86% | p < 0.01 |
| Aerobic training [10,11,12,15,22,23,24,26,28,30,31,36,37,38,39,40,41,42,46,47,48,51,52] | 562 | 3.00 | 2.26, 3.75 | 91.9% | p < 0.01 |
| Aquatic therapy [25,44,45] | 26 | 3.36 | 0.57, 6.16 | 39.6% | p = 0.19 |
| Functional Rehabilitation [13,14,27,35,38,49] | 143 | 2.53 | 0.93, 4.14 | 87.3% | p < 0.01 |
| Constant-load cycloergometer training [29,32,49,50] | 77 | 0.35 | −1.19, 1.90 | 0% | p = 0.96 |
| Resistance training [15,30,41,46] | 102 | 1.87 | 0.21, 3.52 | 14.5% | p = 0.32 |
| Respiratory muscle training [34] | 30 | 0.28 | −2.01, 2.58 | 16.2% | p = 0.27 |
| Total | 1310 | 1.76 | 1.20, 2.32 | 88% | b: p < 0.001 w: p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chasiotis, A.K.; Papadopoulou, M.; Giannopapas, V.; Smyrni, V.; Theodorou, A.; Bakola, E.; Kitsos, D.K.; Stavrogianni, K.; Stasinopoulos, D.; Bakalidou, D.; et al. Efficacy of Physical Therapy Rehabilitation in the Cardiovascular Deconditioning of Post-Stroke Survivors: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 3327. https://doi.org/10.3390/jcm14103327
Chasiotis AK, Papadopoulou M, Giannopapas V, Smyrni V, Theodorou A, Bakola E, Kitsos DK, Stavrogianni K, Stasinopoulos D, Bakalidou D, et al. Efficacy of Physical Therapy Rehabilitation in the Cardiovascular Deconditioning of Post-Stroke Survivors: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(10):3327. https://doi.org/10.3390/jcm14103327
Chicago/Turabian StyleChasiotis, Athanasios K., Marianna Papadopoulou, Vasileios Giannopapas, Vassiliki Smyrni, Aikaterini Theodorou, Eleni Bakola, Dimitrios K. Kitsos, Konstantina Stavrogianni, Dimitrios Stasinopoulos, Daphne Bakalidou, and et al. 2025. "Efficacy of Physical Therapy Rehabilitation in the Cardiovascular Deconditioning of Post-Stroke Survivors: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 10: 3327. https://doi.org/10.3390/jcm14103327
APA StyleChasiotis, A. K., Papadopoulou, M., Giannopapas, V., Smyrni, V., Theodorou, A., Bakola, E., Kitsos, D. K., Stavrogianni, K., Stasinopoulos, D., Bakalidou, D., Tsivgoulis, G., & Giannopoulos, S. (2025). Efficacy of Physical Therapy Rehabilitation in the Cardiovascular Deconditioning of Post-Stroke Survivors: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(10), 3327. https://doi.org/10.3390/jcm14103327












