Patient-Centred Management of Well-Controlled Haemophilia: Obtaining Opinions and Definitions Through a Delphi Consensus
Abstract
1. Introduction
2. Materials and Methods
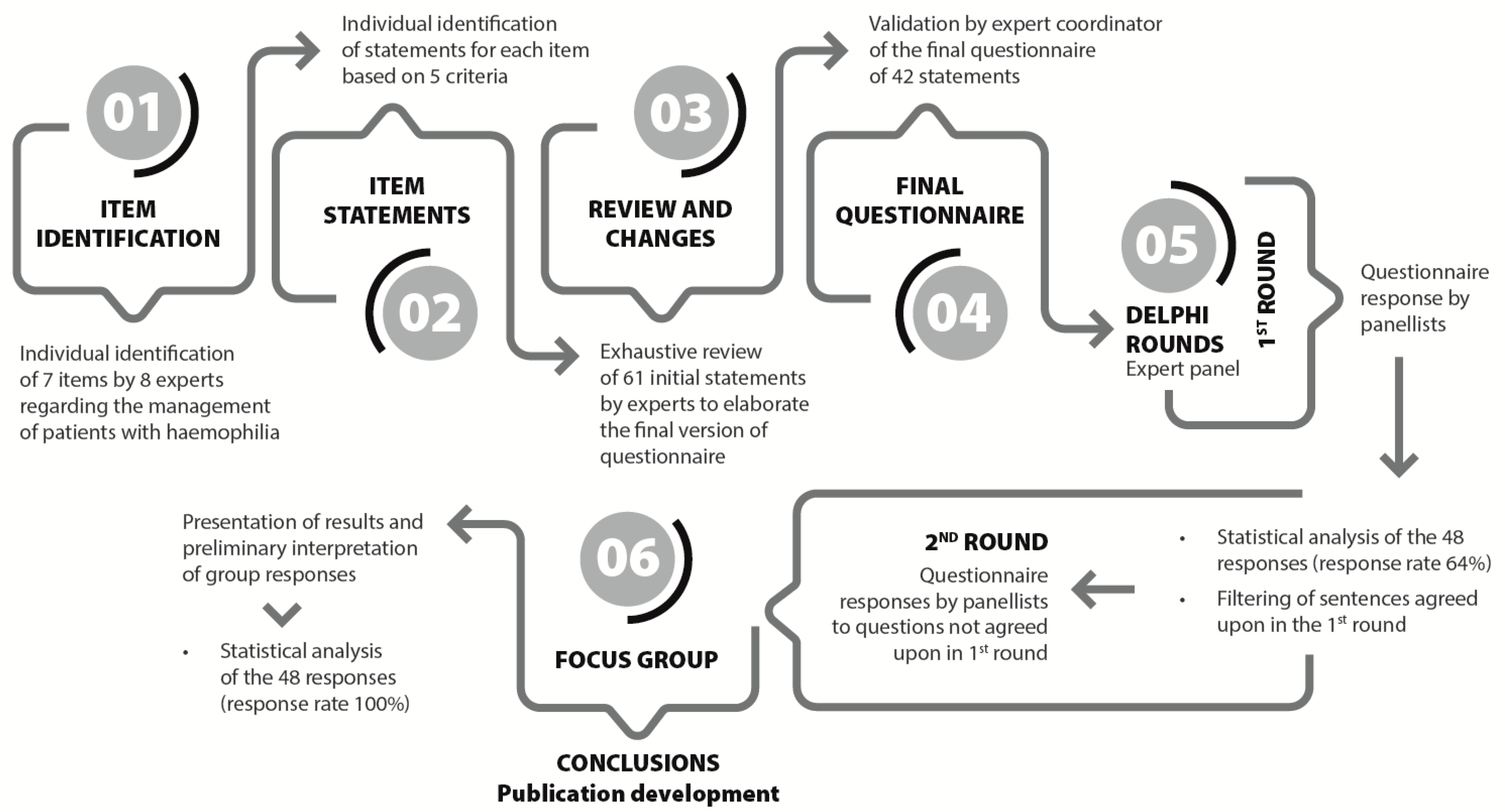
2.1. Project Flowchart
2.2. Expert Panel
2.3. Data Management
2.4. Statistical Analysis
3. Results
3.1. Internal Consistency of Questionnaire
3.2. Consensus by Terciles
3.3. Inter-Round Correlation Analysis
4. Discussion
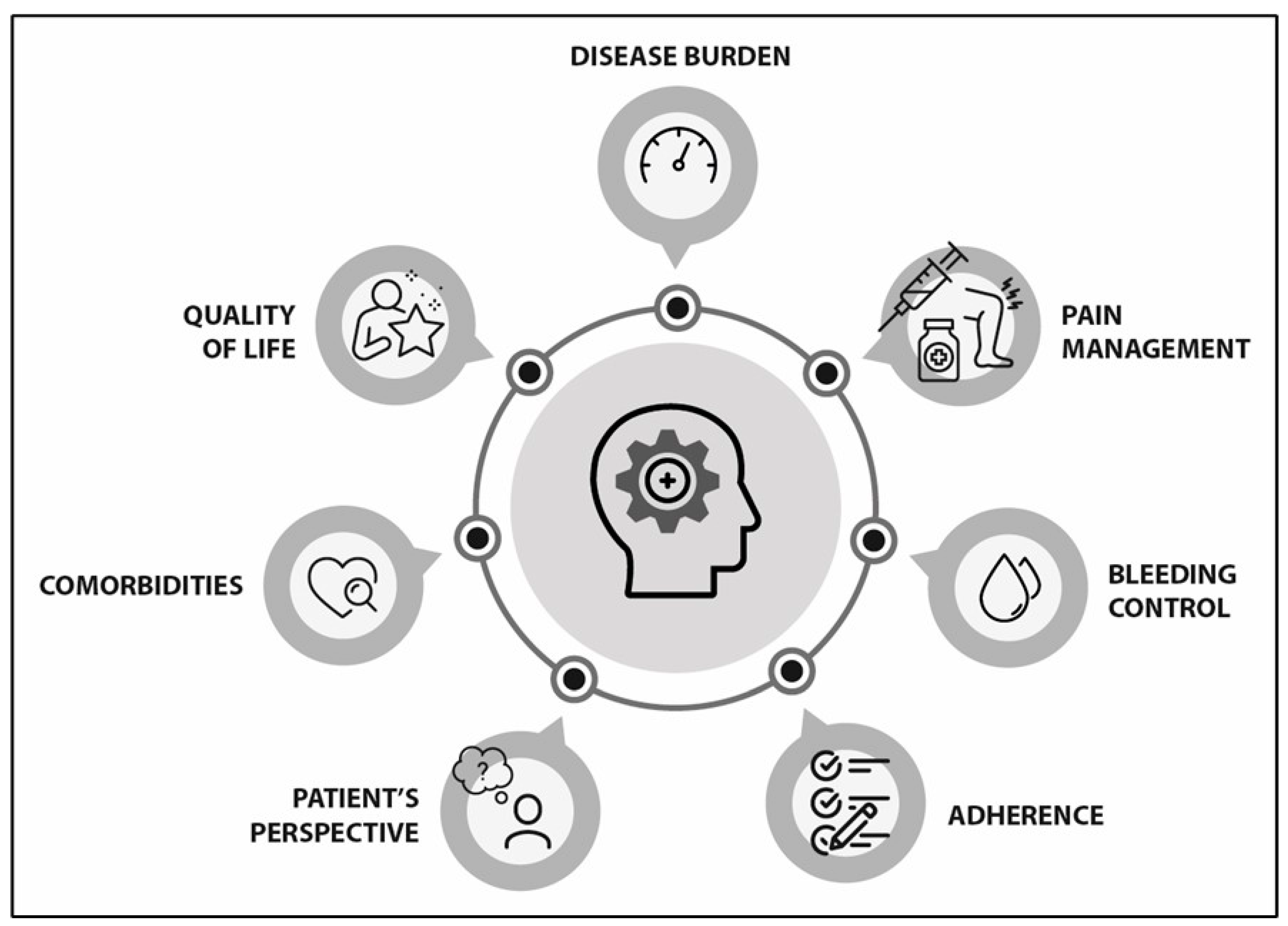
4.1. Disease Burden (See Supplementary Table S1, S1–S4)
4.2. Pain Management (See Supplementary Table S1, S5–S13)
4.3. Bleeding Control (See Supplementary Table S1, S14–S26)
4.4. Adherence (See Supplementary Table S1, S27–S31)
4.5. Patient Perspective (See Supplementary Table S1, S32–S34)
4.6. Comorbidities (See Supplementary Table S1, S35–S38)
4.7. Quality of Life (See Supplementary Table S1, S38–S42)
4.8. Strengths and Limitations
4.9. Implications and Clinical Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Annual bleeding rate |
| AJBR | Annual joint bleeding rate |
| CV | Coefficient of variation |
| EHL | Extended half-life |
| FVIII | Factor VIII |
| FIX | Factor IX |
| HCV | Hepatitis C virus |
| HIV | Human immunodeficiency virus |
| HRQL | Health-related QoL |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PwH | Patients with haemophilia |
| PwHA | Patients with haemophilia A |
| QoL | Quality of life |
| SD | Standard deviation |
| SHL | Standard half-life |
| WFH | World Federation of Haemophilia |
| WHO | World Health Organization |
References
- Peyvandi, F.; Garagiola, I.; Young, G. The past and future of haemophilia: Diagnosis, treatments, and its complications. Lancet 2016, 388, 187–197. [Google Scholar] [CrossRef]
- Blanchette, V.S.; Key, N.S.; Ljung, L.R.; Manco-Johnson, M.J.; Berg, H.M.v.D.; Srivastava, A. Definitions in hemophilia: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2014, 12, 1935–1939. [Google Scholar] [CrossRef]
- Klamroth, R.; Pollmann, H.; Hermans, C.; Faradji, A.; Yarlas, A.S.; Epstein, J.D.; Ewenstein, B.M. The relative burden of haemophilia A and the impact of target joint development on health-related quality of life: Results from the ADVATE Post-Authorization Safety Surveillance (PASS) study. Haemophilia 2011, 17, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Santagostino, E.; Dougall, A.; Kitchen, S.; Sutherland, M.; Pipe, S.W.; Carcao, M.; Mahlangu, J.; Ragni, M.V.; Windyga, J.; et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020, 26, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Manco-Johnson, M.J.; Kempton, C.L.; Reding, M.T.; Lissitchkov, T.; Goranov, S.; Gercheva, L.; Rusen, L.; Ghinea, M.; Uscatescu, V.; Rescia, V.; et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J. Thromb. Haemost. 2013, 11, 1119–1127. [Google Scholar] [CrossRef]
- Oldenburg, J. Optimal treatment strategies for hemophilia: Achievements and limitations of current prophylactic regimens. Blood 2015, 125, 2038–2044. [Google Scholar] [CrossRef] [PubMed]
- Thornburg, C.D.; Duncan, N.A. Treatment adherence in hemophilia. Patient Prefer. Adherence 2017, 11, 1677–1686. [Google Scholar] [CrossRef]
- Berntorp, E.; Hermans, C.; Solms, A.; Poulsen, L.; Mancuso, M.E. Optimising prophylaxis in haemophilia A: The ups and downs of treatment. Blood Rev. 2021, 50, 100852. [Google Scholar] [CrossRef]
- Coffin, D.; Gouider, E.; Konkle, B.; Hermans, C.; Lambert, C.; Diop, S.; Ayoub, E.; Tootoonchian, E.; Youttananukorn, T.; Dakik, P.; et al. The World Federation of Hemophilia World Bleeding Disorders Registry: Insights from the first 10,000 patients. Res. Pract. Thromb. Haemost. 2023, 7, 102264. [Google Scholar] [CrossRef]
- Mancuso, M.E.; Santagostino, E. Outcome of Clinical Trials with New Extended Half-Life FVIII/IX Concentrates. J. Clin. Med. 2017, 6, 39. [Google Scholar] [CrossRef]
- Pipe, S.W.; Shima, M.; Lehle, M.; Shapiro, A.; Chebon, S.; Fukutake, K.; Key, N.S.; Portron, A.; Schmitt, C.; Podolak-Dawidziak, M.; et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): A multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019, 6, e295–e305. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Mahlangu, J.N.; Bujan, W.; Trask, P.; Callaghan, M.U.; Young, G.; Asikanius, E.; Peyvandi, F.; Santagostino, E.; Kruse-Jarres, R.; et al. The effect of emicizumab prophylaxis on health-related outcomes in persons with haemophilia A with inhibitors: HAVEN 1 Study. Haemophilia 2019, 25, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Shima, M.; Nogami, K.; Nagami, S.; Yoshida, S.; Yoneyama, K.; Ishiguro, A.; Suzuki, T.; Taki, M. A multicentre, open-label study of emicizumab given every 2 or 4 weeks in children with severe haemophilia A without inhibitors. Haemophilia 2019, 25, 979–987. [Google Scholar] [CrossRef]
- Mahlangu, J.; Oldenburg, J.; Paz-Priel, I.; Negrier, C.; Niggli, M.; Mancuso, M.E.; Schmitt, C.; Jiménez-Yuste, V.; Kempton, C.; Dhalluin, C.; et al. Emicizumab Prophylaxis in Patients Who Have Hemophilia A without Inhibitors. N. Engl. J. Med. 2018, 379, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Malec, L.; Matino, D. Targeting higher factor VIII levels for prophylaxis in haemophilia A: A narrative review. Haemophilia 2023, 29, 1419–1429. [Google Scholar] [CrossRef]
- Dargaud, Y.; Leuci, A.; Ruiz, A.R.; Lacroix-Desmazes, S. Efanesoctocog alfa: The renaissance of Factor VIII replacement therapy. Haematologica 2024, 109, 2436–2444. [Google Scholar] [CrossRef]
- Von Drygalski, A.; Chowdary, P.; Kulkarni, R.; Susen, S.; Konkle, B.A.; Oldenburg, J.; Matino, D.; Klamroth, R.; Weyand, A.C.; Jimenez-Yuste, V.; et al. Efanesoctocog Alfa Prophylaxis for Patients with Severe Hemophilia A. N. Engl. J. Med. 2023, 388, 310–318. [Google Scholar] [CrossRef]
- Croteau, S.E.; Wang, M.; Wheeler, A.P. 2021 clinical trials update: Innovations in hemophilia therapy. Am. J. Hematol. 2021, 96, 128–144. [Google Scholar] [CrossRef]
- Marchesini, E.; Morfini, M.; Valentino, L. Recent Advances in the Treatment of Hemophilia: A Review. Biologics 2021, 15, 221–235. [Google Scholar] [CrossRef]
- Manco-Johnson, M.J.; Warren, B.B.; Buckner, T.W.; Funk, S.M.; Wang, M. Outcome measures in Haemophilia: Beyond ABR (Annualized Bleeding Rate). Haemophilia 2021, 27 (Suppl. 3), 87–95. [Google Scholar] [CrossRef]
- Van Balen, E.C.; O’Mahony, B.; Cnossen, M.H.; Dolan, G.; Blanchette, V.S.; Fischer, K.; Gue, D.; O’Hara, J.; Iorio, A.; Jackson, S.; et al. Patient-relevant health outcomes for hemophilia care: Development of an international standard outcomes set. Res. Pract. Thromb. Haemost. 2021, 5, e12488. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.; Chai-Adisaksopha, C.; Curtis, R.; Frick, N.; Nichol, M.; Noone, D.; O’mahony, B.; Page, D.; Stonebraker, J.S.; Iorio, A. The Patient Reported Outcomes, Burdens and Experiences (PROBE) Project: Development and evaluation of a questionnaire assessing patient reported outcomes in people with haemophilia. Pilot Feasibility Stud. 2018, 4, 58. [Google Scholar] [CrossRef]
- Linstone, H.; Turoff, M. The Delphi Method: Techniques and Applications; Addison-Wesley Publishing Company, University of Southern California: Los Angeles, CA, USA, 1975. [Google Scholar]
- Schmitt, N. Uses and abuses of coefficient alpha. Psychol. Assess 1996, 8, 350–353. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research. Practical Statistics for Medical Research; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Coppola, A.; Franchini, M.; Pappagallo, G.; Borchiellini, A.; De Cristofaro, R.; Molinari, A.C.; Santoro, R.C.; Santoro, C.; Tagliaferri, A. Current Choices and Management of Treatment in Persons with Severe Hemophilia A without Inhibitors: A Mini-Delphi Consensus. J. Clin. Med. 2022, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, L.H.; Schuurmans, M.J.; Fischer, K. Promoting self-management and adherence during prophylaxis: Evidence-based recommendations for haemophilia professionals. Haemophilia 2016, 22, 499–506. [Google Scholar] [CrossRef]
- Isidro de Pedro, A.I. Afrontamiento y mejora de la calidad de vida en afectados de hemofilia. Psychosoc. Interv. 2002, 11, 333–347. [Google Scholar]
- Stromer, W.; Pabinger, I.; Ay, C.; Crevenna, R.; Donnerer, J.; Feistritzer, C.; Hemberger, S.; Likar, R.; Sevelda, F.; Thom, K.; et al. Pain management in hemophilia: Expert recommendations. Wien Klin. Wochenschr. 2021, 133, 1042–1056. [Google Scholar] [CrossRef]
- SSantoro, C.; Di Minno, M.N.D.; Corcione, A.; Di Minno, G.; Martinelli, M.; Mancuso, M.E.; Acone, B.; Molinari, A.C.; Passeri, E.V.; Rocino, A.; et al. Improving assessment and management of pain in hemophilia: An Italian Delphi consensus statement. Blood Rev. 2022, 51, 100885. [Google Scholar] [CrossRef]
- Paredes, A.C.; Costa, P.; Almeida, A.; Pinto, P.R. A new measure to assess pain in people with haemophilia: The Multidimensional Haemophilia Pain Questionnaire (MHPQ). PLoS ONE 2018, 13, e0207939. [Google Scholar] [CrossRef]
- Humphries, T.J.; Kessler, C.M. Pain in haemophilia: Are we listening? Haemophilia 2016, 22, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Holstein, K.; Klamroth, R.; Richards, M.; Carvalho, M.; Pérez-Garrido, R.; Gringeri, A. Pain management in patients with haemophilia: A European survey. Haemophilia 2012, 18, 743–752. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Tafuri, F.; Arcudi, S.; Solimeno, P.L.; Acquati, J.; Landi, L.; Peyvandi, F. Current and Emerging Approaches for Pain Management in Hemophilic Arthropathy. Pain Ther. 2022, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.R.J.; Makris, M. Choosing and using non-steroidal anti-inflammatory drugs in haemophilia. Haemophilia 2016, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; van der Bom, J.G.; Mauser-Bunschoten, E.P.; Roosendaal, G.; Prejs, R.; De Kleijn, P.; Grobbee, D.E.; van den Berg, M. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood 2002, 99, 2337–2341. [Google Scholar] [CrossRef]
- Fischer, K.; Van der Bom, J.G.; Molho, P.; Negrier, C.; Mauser-Bunschoten, E.P.; Roosendaal, G.; De Kleijn, P.; Grobbee, D.E.; Berg, H.M.V.D. Prophylactic versus on-demand treatment strategies for severe haemophilia: A comparison of costs and long-term outcome. Haemophilia 2002, 8, 745–752. [Google Scholar] [CrossRef]
- Abshire, T.C.; Shapiro, A.D.; Riske, B.; Hacker, M.R.; Kilcoyne, R.; Ingram, J.D.; Manco-Johnson, M.L.; Funk, S.; Jacobson, L.; Valentino, L.A.; et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007, 357, 535–544. [Google Scholar] [CrossRef]
- McEneny-King, A.; Chelle, P.; Goggans, M.H.; Barker, P.J.; Jacobs, T.W.; Neufeld, E.J.; Reiss, U.M.; Panetta, J.C. Limited sampling strategies for accurate determination of extended half-life factor VIII pharmacokinetics in severe haemophilia A patients. Haemophilia 2021, 27, 408–416. [Google Scholar] [CrossRef]
- Iorio, A.; Carcao, M.D. Individualizing Factor Replacement Therapy in Severe Hemophilia. Semin. Thromb. Hemost. 2015, 41, 864–871. [Google Scholar] [CrossRef]
- Young, G.; Mahlangu, J.; Kulkarni, R.; Nolan, B.; Liesner, R.; Pasi, J.; Barnes, C.; Neelakantan, S.; Gambino, G.; Cristiano, L.M.; et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J. Thromb. Haemost. 2015, 13, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Krishnan, S.; Myrén, K.; Lethagen, S.; McCormick, N.; Yermakov, S.; Karner, P. Indirect comparisons of efficacy and weekly factor consumption during continuous prophylaxis with recombinant factor VIII Fc fusion protein and conventional recombinant factor VIII products. Haemophilia 2017, 23, 408–416. [Google Scholar] [CrossRef]
- Powell, J.S.; Pasi, K.J.; Ragni, M.V.; Ozelo, M.C.; Valentino, L.A.; Mahlangu, J.N.; Josephson, N.C.; Perry, D.; Manco-Johnson, M.J.; Apte, S.; et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N. Engl. J. Med. 2013, 369, 2313–2323. [Google Scholar] [CrossRef]
- Nolan, B.; Klukowska, A.; Shapiro, A.; Rauch, A.; Recht, M.; Ragni, M.; Curtin, J.; Gunawardena, S.; Mukhopadhyay, S.; Jayawardene, D.; et al. Final results of the PUPs B-LONG study: Evaluating safety and efficacy of rFIXFc in previously untreated patients with hemophilia B. Blood Adv. 2021, 5, 2732–2739. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Adherence to Long-Term Therapies: Evidence for Action; WHO: Geneva, Switzerland, 2003; 196p. [Google Scholar]
- Krishnan, S.; Vietri, J.; Furlan, R.; Duncan, N. Adherence to prophylaxis is associated with better outcomes in moderate and severe haemophilia: Results of a patient survey. Haemophilia 2015, 21, 64–70. [Google Scholar] [CrossRef]
- Collins, P.W.; Blanchette, V.S.; Fischer, K.; Björkman, S.; Oh, M.; Fritsch, S.; Schroth, P.; Spotts, G.; Astermark, J.; Ewenstein, B. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J. Thromb. Haemost. 2009, 7, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Schrijvers, L.H.; Uitslager, N.; Schuurmans, M.J.; Fischer, K. Barriers and motivators of adherence to prophylactic treatment in haemophilia: A systematic review. Haemophilia 2013, 19, 355–361. [Google Scholar] [CrossRef]
- Powell, J.; Miguelino, M. Clinical utility and patient perspectives on the use of extended half-life rFIXFc in the management of hemophilia B. Patient Prefer. Adherence 2014, 8, 1073–1083. [Google Scholar] [CrossRef]
- Duncan, N.; Kronenberger, W.; Roberson, C.; Shapiro, A. VERITAS-Pro: A new measure of adherence to prophylactic regimens in haemophilia. Haemophilia 2010, 16, 247–255. [Google Scholar] [CrossRef]
- Qian, W.; Lam, T.T.-N.; Lam, H.H.W.; Li, C.-K.; Cheung, Y.T. Telehealth Interventions for Improving Self-Management in Patients With Hemophilia: Scoping Review of Clinical Studies. J. Med. Internet Res. 2019, 21, e12340. [Google Scholar] [CrossRef]
- Konkle, B.A.; Skinner, M.; Iorio, A. Hemophilia trials in the twenty-first century: Defining patient important outcomes. Res. Pract. Thromb. Haemost. 2019, 3, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Plug, I.; van der Bom, J.G.; Peters, M.; Mauser-Bunschoten, E.P.; DE Goede-Bolder, A.; Heijnen, L.; Smit, C.; Willemse, J.; Rosendaal, F.R. Mortality and causes of death in patients with hemophilia, 1992-2001: A prospective cohort study. J. Thromb. Haemost. 2006, 4, 510–516. [Google Scholar] [CrossRef]
- Shapiro, S.; Benson, G.; Evans, G.; Harrison, C.; Mangles, S.; Makris, M. Cardiovascular disease in hereditary haemophilia: The challenges of longevity. Br. J. Haematol. 2022, 197, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Van de Putte, D.E.F.; Fischer, K.; Makris, M.; Tait, C.R.; Chowdary, P.; Collins, P.W.; Meijer, K.; Roosendaal, G.; Schutgens, R.E.G.; Mauser-Bunschoten, E.P. Unfavourable cardiovascular disease risk profiles in a cohort of Dutch and British haemophilia patients. Thromb. Haemost. 2013, 109, 16–23. [Google Scholar] [CrossRef]
- Staritz, P.; de Moerloose, P.; Schutgens, R.; Dolan, G. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia—An assessment by the ADVANCE Working Group. Haemophilia 2013, 19, 833–840. [Google Scholar] [CrossRef]
- Schutgens, R.E.; Jimenez-Yuste, V.; Escobar, M.; Falanga, A.; Gigante, B.; Klamroth, R.; Lassila, R.; Leebeek, F.W.; Makris, M.; Owaidah, T.; et al. Antithrombotic Treatment in Patients With Hemophilia: An EHA-ISTH-EAHAD-ESO Clinical Practice Guidance. Hemasphere 2023, 7, E900. [Google Scholar] [CrossRef]
- Skinner, M.W.; Nugent, D.; Wilton, P.; O’mahony, B.; Dolan, G.; O’Hara, J.; Berntorp, E. Achieving the unimaginable: Health equity in haemophilia. Haemophilia 2020, 26, 17–24. [Google Scholar] [CrossRef]
- Hermans, C.; Pierce, G.F. Towards achieving a haemophilia-free mind. Haemophilia 2023, 29, 951–953. [Google Scholar] [CrossRef]
- Limperg, P.; Terwee, C.; Young, N.; Price, V.; Gouw, S.; Peters, M.; Grootenhuis, M.; Blanchette, V.; Haverman, L. Health-related quality of life questionnaires in individuals with haemophilia: A systematic review of their measurement properties. Haemophilia 2017, 23, 497–510. [Google Scholar] [CrossRef]
- Hasson, F.; Keeney, S.; McKenna, H. Research guidelines for the Delphi survey technique. J. Adv. Nurs. 2000, 32, 1008–1015. [Google Scholar] [CrossRef]
- Powell, C. The Delphi technique: Myths and realities. J. Adv. Nurs. 2003, 41, 376–382. [Google Scholar] [CrossRef] [PubMed]
| Rounds 1 and 2 | |
|---|---|
| Age (years) (mean ± SD) | 48.9 ± 11.8 |
| Years of experience (median (25th–75th percentiles)) | 12 (8–21) |
| Percentage of clinical practice time dedicated to haemophilia (median (25th–75th percentiles)) | 30 (20–50) |
| Percentage of patients receiving prophylaxis in moderate haemophilia (median (25th–75th percentiles)) | 37.5 (10–75) |
| Percentage of patients receiving prophylaxis in severe haemophilia (median (25th–75th percentiles)) | 100 (95–100) |
| Percentage of patients on SHL treatment (median (25th–75th percentiles)) | 15 (5–20) |
| Percentage of patients on EHL treatment (median (25th–75th percentiles)) | 79.5 (51–84) |
| Round 1 | Round 2 | |||
|---|---|---|---|---|
| α of C (p) | ri (p) | α of C (p) | ri (p) | |
| Disease burden (S1–S4) | 0.593 (<0.001) | 0.567 (<0.001) | 0.593 (<0.001) | 0.567 (<0.001) |
| Pain management (S5–S13) | 0.582 (<0.001) | 0.534 (<0.001) | 0.617 (<0.001) | 0.543 (<0.001) |
| Bleeding control (S14–S26) | 0.708 (<0.001) | 0.657 (<0.001) | 0.573 (<0.001) | 0.582 (<0.001) |
| Adherence (S27–S31) | 0.665 (<0.001) | 0.684 (<0.001) | 0.669 (<0.001) | 0.688 (<0.001) |
| Patient’s perspective (S32–S34) | 0.728 (<0.001) | 0.671 (<0.001) | 0.743 (<0.001) | 0.691 (<0.001) |
| Comorbidities (S35–S38) | 0.492 (<0.001) | 0.495 (<0.001) | 0.492 (<0.001) | 0.495 (<0.001) |
| Quality of life (S39–S42) | 0.654 (<0.001) | 0.621 (<0.001) | 0.654 (<0.001) | 0.621 (<0.001) |
| Total (42S and 44S in rounds 1 and 2, respectively) | 0.882 (<0.001) | 0.852 (<0.001) | 0.868 (<0.001) | 0.830 (<0.001) |
| Spearman’s Rank Correlation | p | Kappa Index | p | |
|---|---|---|---|---|
| Burden Disease (S1–S4) | 1 | - | 1 | - |
| Pain management (S5–S13) | 0.771 | <0.001 | 0.345 | 0.001 |
| S9: The first-line pharmacological treatment of chronic arthropathic pain in patients with haemophilia is paracetamol. | 0.514 | <0.001 | 0.248 | 0.011 |
| S10: In the second-line pharmacological treatment of chronic arthropathic pain in adult patients, the combination of paracetamol and a weak opioid is used. | 0.587 | <0.001 | 0.227 | 0.059 |
| S12: Treatment of exacerbation of chronic arthropathic pain in patients with haemophilia may include oral non-steroidal anti-inflammatory drugs. | 0.478 | 0.001 | 0.216 | 0.041 |
| Bleeding (S14–S26) | 0.771 | <0.001 | 0.374 | <0.001 |
| S14: The controlled haemophilia patient should have balanced haemostasis. | 0.156 | 0.288 | 0.030 | 0.279 |
| S19: The most appropriate prophylaxis for optimal haemostatic protection is replacement therapy with deficient clotting factor concentrates for patients with haemophilia. | 0.471 | <0.001 | 0.205 | 0.049 |
| S20a: One of the options for improving hemostatic protection with factor replacement therapy prophylaxis is to increase the administration frequency. | 0.195 | 0.183 | 0.086 | 0.132 |
| S20b: One of the options for improving hemostatic protection with prophylaxis by factor replacement therapy is to increase the dose and maintain the frequency of administrations. | −0.028 | 0.848 | −0.059 | 0.514 |
| S21a: One of the options to improve haemostatic protection with prophylaxis by factor replacement therapy is to use products with a longer half-life. | 0.238 | 0.103 | 0.001 | 0.999 |
| S21b: To improve haemostatic protection with factor replacement therapy prophylaxis, the frequency of administrations should be increased or products with a longer half-life should be used. | 0.006 | 0.970 | −0.072 | 0.438 |
| Adherence (S27–S31) | 0.930 | <0.001 | 0.843 | <0.001 |
| S31: Home delivery of medication may improve adherence in patients with haemophilia | 0.571 | <0.001 | 0.603 | <0.001 |
| Patient Perspective (S32–S34) | 0.891 | <0.001 | 0.475 | <0.001 |
| S32: The haemophilia patient under control is one who is aware of the burden of the disease and the potential consequences | 0.383 | 0.007 | 0.110 | 0.084 |
| Comorbidities (S35–S38) | 1 | - | 1 | - |
| Quality of life (S39–S42) | 1 | - | 1 | - |
| Total (42S and 44S in rounds 1 and 2, respectively) | 0.922 | <0.001 | 0.781 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berrueco, R.; Soto, I.; Bastida, J.M.; Calvo Villas, J.M.; de Cos, C.; Haya, S.; García, F.S.; Mateo Arranz, J. Patient-Centred Management of Well-Controlled Haemophilia: Obtaining Opinions and Definitions Through a Delphi Consensus. J. Clin. Med. 2025, 14, 3300. https://doi.org/10.3390/jcm14103300
Berrueco R, Soto I, Bastida JM, Calvo Villas JM, de Cos C, Haya S, García FS, Mateo Arranz J. Patient-Centred Management of Well-Controlled Haemophilia: Obtaining Opinions and Definitions Through a Delphi Consensus. Journal of Clinical Medicine. 2025; 14(10):3300. https://doi.org/10.3390/jcm14103300
Chicago/Turabian StyleBerrueco, Rubén, Inmaculada Soto, José María Bastida, José Manuel Calvo Villas, Carmen de Cos, Saturnino Haya, Francisco Sierra García, and José Mateo Arranz. 2025. "Patient-Centred Management of Well-Controlled Haemophilia: Obtaining Opinions and Definitions Through a Delphi Consensus" Journal of Clinical Medicine 14, no. 10: 3300. https://doi.org/10.3390/jcm14103300
APA StyleBerrueco, R., Soto, I., Bastida, J. M., Calvo Villas, J. M., de Cos, C., Haya, S., García, F. S., & Mateo Arranz, J. (2025). Patient-Centred Management of Well-Controlled Haemophilia: Obtaining Opinions and Definitions Through a Delphi Consensus. Journal of Clinical Medicine, 14(10), 3300. https://doi.org/10.3390/jcm14103300







