Development and Validation of Comorbidity Severity Adjustment Methods in Mortality Models for Acute Cerebrovascular Disease Using Survival and Machine Learning Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Variables
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Recalibration of the Charlson Comorbidity Index (CCI) and the Development of the CCS-Based Comorbidity Index Using Survival Analysis
3.3. Distribution of CCI, m-CCI, and m-CCS Comorbidity Indices
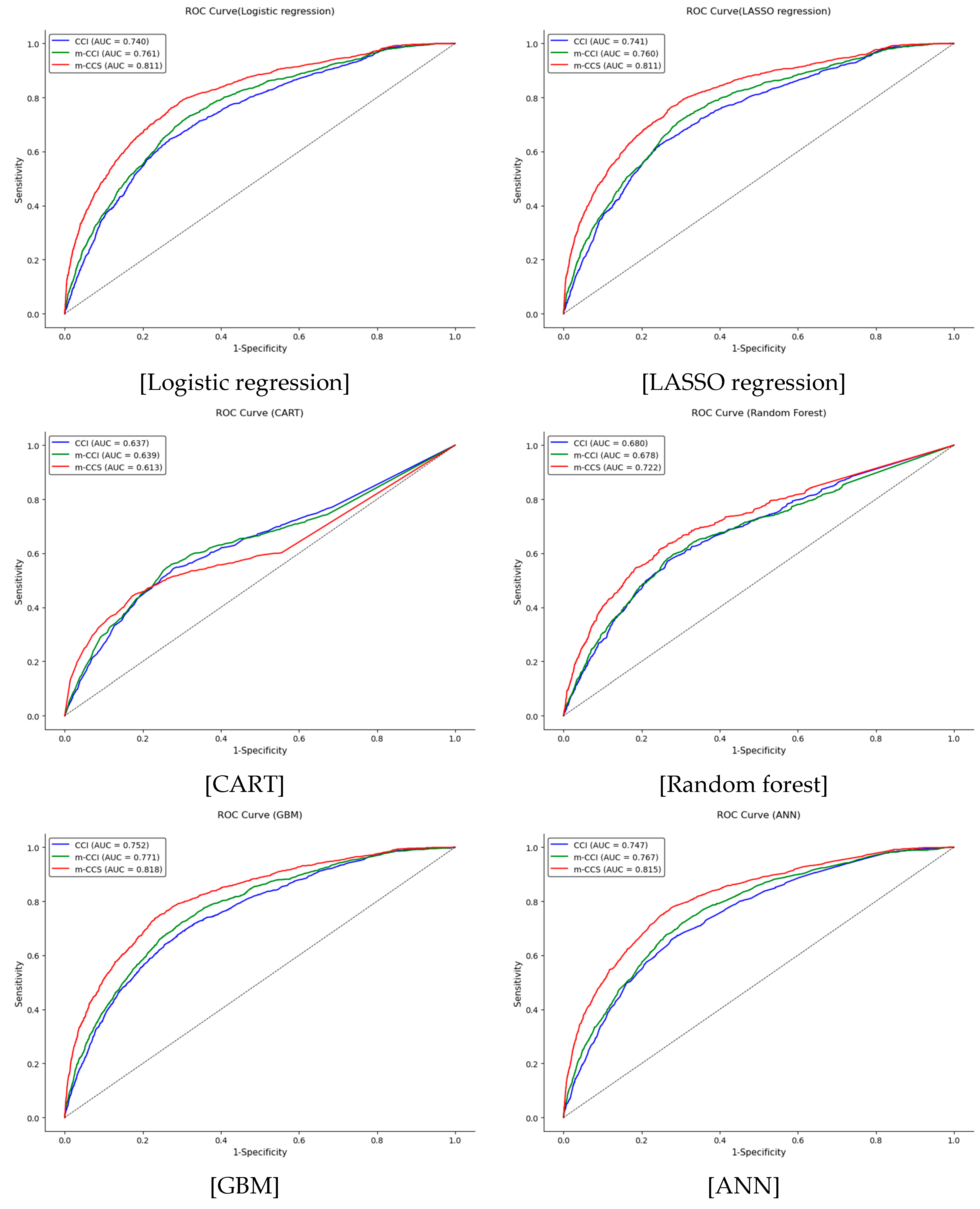
3.4. Validation of the Comorbidity Indices Using Machine Learning
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korean Statistical Information Service (KOSIS). KOSIS: Korean Statistical Information Service; KOSIS: Daejeon, Republic of Korea, 2023; Available online: https://kosis.kr/index/index.do (accessed on 28 February 2025).
- Donabedian, A. Evaluating the quality of medical care. Milbank Meml. Fund Q. 1966, 44, 166–203. [Google Scholar] [CrossRef]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Lin, Z.; Cohen, D.J. Achieving high-quality hospital care: The need for a more comprehensive approach to risk adjustment. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005371. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Clausen, A.; Möller, S.; Skjødt, M.K.; Bech, B.H.; Rubin, K.H. Evaluating the performance of the Charlson Comorbidity Index (CCI) in fracture risk prediction and developing a new Charlson Fracture Index (CFI): A register-based cohort study. Osteoporos. Int. 2022, 33, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, E.W.; Choi, Y.; Hong, S.Y.; Suh, S.; Hong, K.; Han, E.S.; Lee, J.M.; Hong, S.K.; Yi, N.J.; et al. Modified Charlson comorbidity index as a survival prediction tool for older patients after liver transplantation. Ann. Surg. Treat. Res. 2023, 104, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Alimenti, E.; Iavarone, M.; Canova, L.; Fracas, E.; Antonelli, B.; Ierardi, A.M.; Lampertico, P. A modified Charlson Comorbidity Index to improve management of patients with hepatocellular carcinoma: A step towards precision medicine. Dig. Liver Dis. 2024, 56, S81. [Google Scholar] [CrossRef]
- Agency for Healthcare Research and Quality (AHRQ). Clinical Classifications Software (CCS) for ICD-9-CM. In Healthcare Cost and Utilization Project (HCUP); 2024. Available online: https://hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp (accessed on 21 December 2024).
- Baek, S.K.; Park, H.J.; Kang, S.H.; Choi, J.Y.; Park, J.H. Convergence study in development of severity adjustment method for death with acute myocardial infarction patients using machine learning. J. Digit. Converg. 2019, 17, 217–230. [Google Scholar] [CrossRef]
- Campbell, M.J.; Jacques, R.M.; Fotheringham, J.; Maheswaran, R.; Nicholl, J. Developing a summary hospital mortality index: Retrospective analysis in English hospitals over five years. BMJ 2012, 344, e1001. [Google Scholar] [CrossRef] [PubMed]
- Health Insurance Review & Assessment Service (HIRA). 5th Hospital Standardized Mortality Ratio (HSMR) Appropriateness Evaluation Results; HIRA: Wonju, Republic of Korea, 2022; Available online: https://www.hira.or.kr/ra/eval/asmWrptPopup.do?evlCd=28&pgmid=HIRAA030004000000 (accessed on 24 March 2025).
- Desai, R.J.; Wang, S.V.; Vaduganathan, M.; Evers, T.; Schneeweiss, S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw. Open 2020, 3, e1918962. [Google Scholar] [CrossRef] [PubMed]
- Guo Ie, H.; Tang, C.H.; Sheu, M.L.; Liu, H.Y.; Lu, N.; Tsai, T.Y.; Huang, K.C. Evaluation of risk adjustment performance of diagnosis-based and medication-based comorbidity indices in patients with chronic obstructive pulmonary disease. PLoS ONE 2022, 17, e0270468. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Schwendimann, R.; Endrich, O.; Ausserhofer, D.; Simon, M. Comparing Charlson and Elixhauser comorbidity indices with different weightings to predict in-hospital mortality: An analysis of national inpatient data. BMC Health Serv. Res. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]

| CCS Category | Diagnosis | ICD-10 Code |
|---|---|---|
| 109 | Acute Cerebrovascular Disease | G46.0 *~G46.8 *, I60.0~I60.9, I61.0~I61.6, I61.8, I61.9, I62.0, I62.1, I62.9, I63.0~I63.6, I63.8, I63.9, I64, I66.0~I66.4, I66.8, I66.9 |
| Characteristics | Total (n = 30,084) | Training (n = 14,888) | Validation (n = 15,196) |
|---|---|---|---|
| Sex | |||
| Male | 17,159 (57.0) | 8390 (56.4) | 8769 (57.7) |
| Female | 12,925 (43.0) | 6498 (43.6) | 6427 (42.3) |
| Age, mean (SD), years | 65.9 (14.64) | 65.8 ± 14.65 | 65.9 ± 14.62 |
| Insurance type | |||
| National Health Insurance | 28,014 (93.1) | 13,854 (93.1) | 14,160 (93.2) |
| Medical Aid | 1899 (6.3) | 955 (6.4) | 944 (6.2) |
| Others | 171 (0.6) | 79 (0.5) | 92 (0.6) |
| Surgical Status | |||
| No | 25,886 (86.0) | 12,843 (86.3) | 13,043 (85.8) |
| Yes | 4198 (14.0) | 2045 (13.7) | 2153 (14.2) |
| Emergency admission | |||
| No | 5858 (19.5) | 2868 (19.3) | 2990 (19.7) |
| Yes | 24,226 (80.5) | 12,020 (80.7) | 12,206 (80.3) |
| Comorbidity | Total (n = 30,084) | |||||
|---|---|---|---|---|---|---|
| Parameter Estimate | Standard Error | Hazard Ratio | p Value | CCI | m-CCI * | |
| Myocardial infarct | 0.185 | 0.180 | 1.203 | 0.303 | - | - |
| Congestive heart failure | 0.547 | 0.110 | 1.729 | <0.0001 | 2 | 1 |
| Peripheral vascular disease | 0.061 | 0.207 | 1.063 | 0.767 | - | - |
| Cerebrovascular disease | 0.504 | 0.046 | 1.655 | <0.0001 | - | 1 |
| Dementia | −1.044 | 0.168 | 0.352 | <0.0001 | 2 | - |
| Chronic pulmonary disease | −0.064 | 0.156 | 0.938 | 0.682 | 1 | - |
| Connective tissue disease | 0.214 | 0.280 | 1.239 | 0.444 | 1 | - |
| Ulcer disease | −0.302 | 0.222 | 0.739 | 0.174 | - | - |
| Mild liver disease | 0.440 | 0.137 | 1.553 | 0.001 | 2 | 1 |
| Diabetes with end organ damage | −0.212 | 0.144 | 0.809 | 0.014 | 1 | - |
| Diabetes | −0.335 | 0.061 | 0.715 | <0.0001 | - | - |
| Hemiplegia | −1.753 | 0.103 | 0.173 | <0.0001 | 2 | - |
| Moderate or severe renal disease | 0.630 | 0.085 | 1.878 | <0.0001 | 1 | 1 |
| Any tumor, leukemia, lymphoma | 0.798 | 0.112 | 2.222 | <0.0001 | 2 | 1 |
| Moderate or severe liver disease | 1.058 | 0.295 | 2.880 | <0.0001 | 4 | 2 |
| Metastatic solid tumor | 0.090 | 0.205 | 1.094 | 0.663 | 6 | - |
| AIDS | 0.689 | 1.001 | 1.991 | 0.491 | 4 | - |
| Comorbidity | Total (n = 30,084) | ||||
|---|---|---|---|---|---|
| Parameter Estimate | Standard Error | Hazard Ratio | p Value | m-CCS * | |
| Other and ill-defined cerebrovascular disease | 0.211 | 0.080 | 1.235 | 0.009 | 1 |
| Pneumonia | 0.236 | 0.080 | 1.266 | 0.003 | 1 |
| Immunizations and screening for infectious disease | 0.285 | 0.117 | 1.330 | 0.015 | 1 |
| Residual codes; unclassified | 0.307 | 0.069 | 1.359 | <0.0001 | 1 |
| Respiratory failure; insufficiency; arrest | 0.430 | 0.173 | 1.537 | 0.013 | 1 |
| Other screening for suspected conditions | 0.445 | 0.132 | 1.561 | 0.001 | 1 |
| Coagulation and hemorrhagic disorders | 0.458 | 0.165 | 1.580 | 0.005 | 1 |
| Congestive heart failure; nonhypertensive | 0.464 | 0.125 | 1.590 | <0.0001 | 1 |
| septicemia | 0.531 | 0.106 | 1.701 | <0.0001 | 1 |
| Aspiration pneumonitis; food/vomitus | 0.565 | 0.090 | 1.759 | <0.0001 | 1 |
| Chronic renal failure | 0.572 | 0.089 | 1.772 | <0.0001 | 1 |
| Acute myocardial infarction | 0.657 | 0.208 | 1.930 | 0.002 | 2 |
| Acute and unspecified renal failure | 0.774 | 0.094 | 2.168 | <0.0001 | 2 |
| Acute cerebrovascular disease | 0.823 | 0.049 | 2.277 | <0.0001 | 2 |
| Cancer of liver and intrahepatic bile duct | 0.956 | 0.217 | 2.601 | <0.0001 | 2 |
| Cancer of bronchus; lung | 0.985 | 0.228 | 2.677 | <0.0001 | 2 |
| Shock | 1.016 | 0.385 | 2.761 | 0.008 | 2 |
| Non-Hodgkin’s lymphoma | 1.111 | 0.506 | 3.038 | 0.028 | 2 |
| Other mental conditions | 1.203 | 0.310 | 3.329 | <0.0001 | 3 |
| Cancer of cervix | 1.420 | 0.504 | 4.137 | 0.005 | 3 |
| Systemic lupus erythematosus and connective tissue disorders | 1.494 | 0.336 | 4.457 | <0.0001 | 4 |
| Coma; stupor; and brain damage | 1.519 | 0.193 | 4.569 | <0.0001 | 4 |
| Nervous system congenital anomalies | 1.536 | 0.580 | 4.648 | 0.008 | 4 |
| Cardiac arrest and ventricular fibrillation | 1.638 | 0.108 | 5.144 | <0.0001 | 4 |
| Pancreatic disorders | 1.671 | 0.357 | 5.316 | <0.0001 | 4 |
| Leukemias | 1.977 | 0.216 | 7.224 | <0.0001 | 6 |
| Training (n = 14,888) | Validation (n = 15,196) | |||||
|---|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | |||
| Lower | High | Lower | High | |||
| Logistic regression | ||||||
| CCI | 0.744 | 0.730 | 0.759 | 0.740 | 0.725 | 0.754 |
| m-CCI | 0.765 | 0.752 | 0.778 | 0.761 | 0.746 | 0.774 |
| m-CCS | 0.811 | 0.798 | 0.824 | 0.811 | 0.798 | 0.825 |
| LASSO regression | ||||||
| CCI | 0.743 | 0.729 | 0.758 | 0.741 | 0.725 | 0.755 |
| m-CCI | 0.764 | 0.752 | 0.778 | 0.760 | 0.746 | 0.774 |
| m-CCS | 0.811 | 0.798 | 0.824 | 0.811 | 0.798 | 0.825 |
| CART | ||||||
| CCI | 0.659 | 0.650 | 0.669 | 0.637 | 0.620 | 0.654 |
| m-CCI | 0.673 | 0.664 | 0.682 | 0.639 | 0.620 | 0.658 |
| m-CCS | 0.721 | 0.714 | 0.729 | 0.613 | 0.594 | 0.633 |
| Random forest | ||||||
| CCI | 0.652 | 0.642 | 0.663 | 0.680 | 0.664 | 0.696 |
| m-CCI | 0.667 | 0.658 | 0.677 | 0.678 | 0.661 | 0.695 |
| m-CCS | 0.713 | 0.706 | 0.721 | 0.722 | 0.705 | 0.739 |
| GBM | ||||||
| CCI | 0.773 | 0.760 | 0.788 | 0.752 | 0.737 | 0.766 |
| m-CCI | 0.792 | 0.780 | 0.805 | 0.771 | 0.757 | 0.785 |
| m-CCS | 0.835 | 0.822 | 0.847 | 0.818 | 0.806 | 0.831 |
| ANN | ||||||
| CCI | 0.773 | 0.760 | 0.787 | 0.747 | 0.733 | 0.761 |
| m-CCI | 0.789 | 0.776 | 0.801 | 0.767 | 0.752 | 0.780 |
| m-CCS | 0.830 | 0.818 | 0.843 | 0.815 | 0.802 | 0.828 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Park, J. Development and Validation of Comorbidity Severity Adjustment Methods in Mortality Models for Acute Cerebrovascular Disease Using Survival and Machine Learning Analyses. J. Clin. Med. 2025, 14, 3281. https://doi.org/10.3390/jcm14103281
Kim Y, Park J. Development and Validation of Comorbidity Severity Adjustment Methods in Mortality Models for Acute Cerebrovascular Disease Using Survival and Machine Learning Analyses. Journal of Clinical Medicine. 2025; 14(10):3281. https://doi.org/10.3390/jcm14103281
Chicago/Turabian StyleKim, Yeaeun, and Jongho Park. 2025. "Development and Validation of Comorbidity Severity Adjustment Methods in Mortality Models for Acute Cerebrovascular Disease Using Survival and Machine Learning Analyses" Journal of Clinical Medicine 14, no. 10: 3281. https://doi.org/10.3390/jcm14103281
APA StyleKim, Y., & Park, J. (2025). Development and Validation of Comorbidity Severity Adjustment Methods in Mortality Models for Acute Cerebrovascular Disease Using Survival and Machine Learning Analyses. Journal of Clinical Medicine, 14(10), 3281. https://doi.org/10.3390/jcm14103281






