Efficacy of Repeated Low-Level Red Light (RLRL) Therapy in Managing Childhood Myopia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
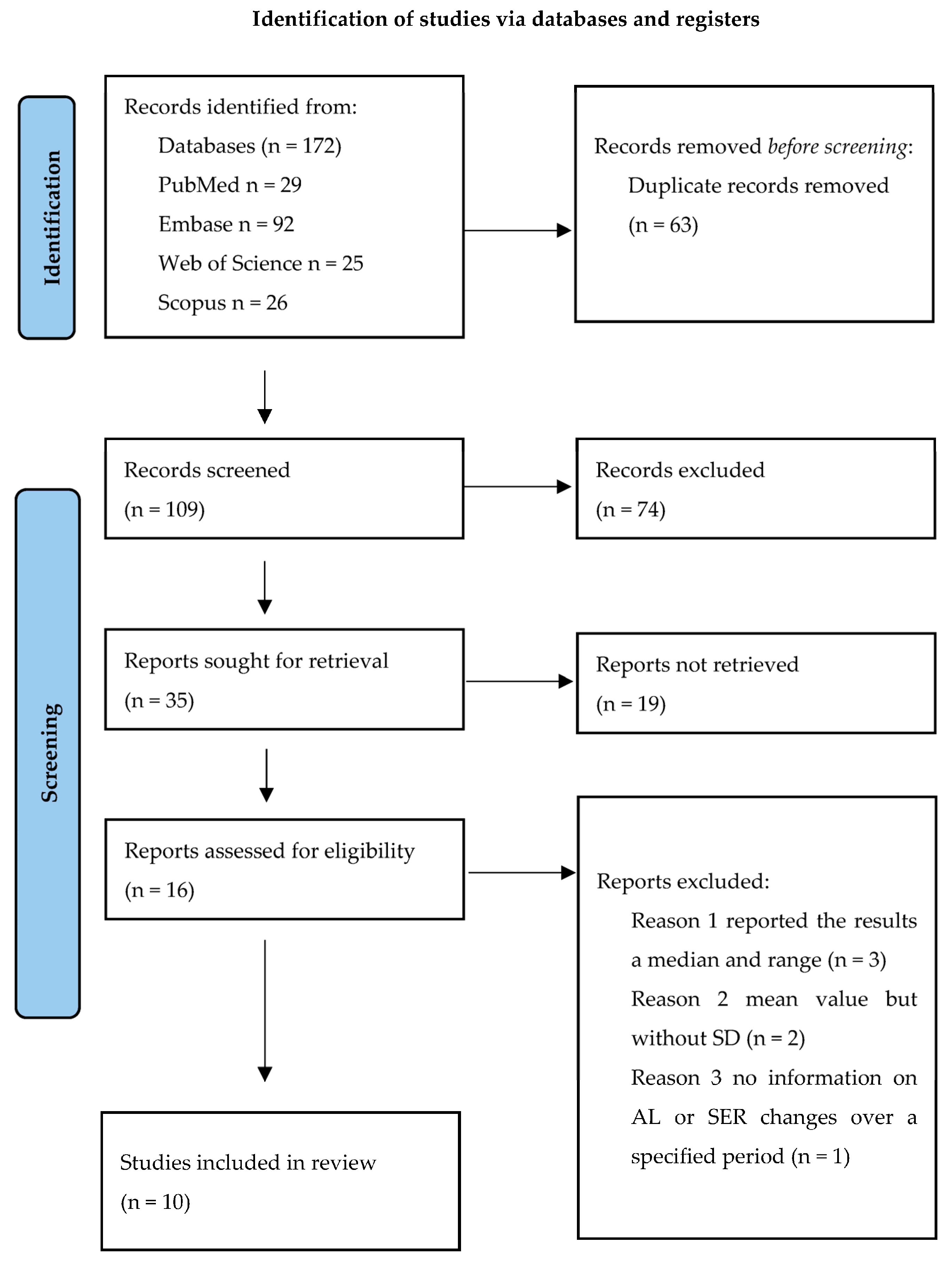
2. Materials and Methods
2.1. Study Selection
2.2. Statistical Analysis
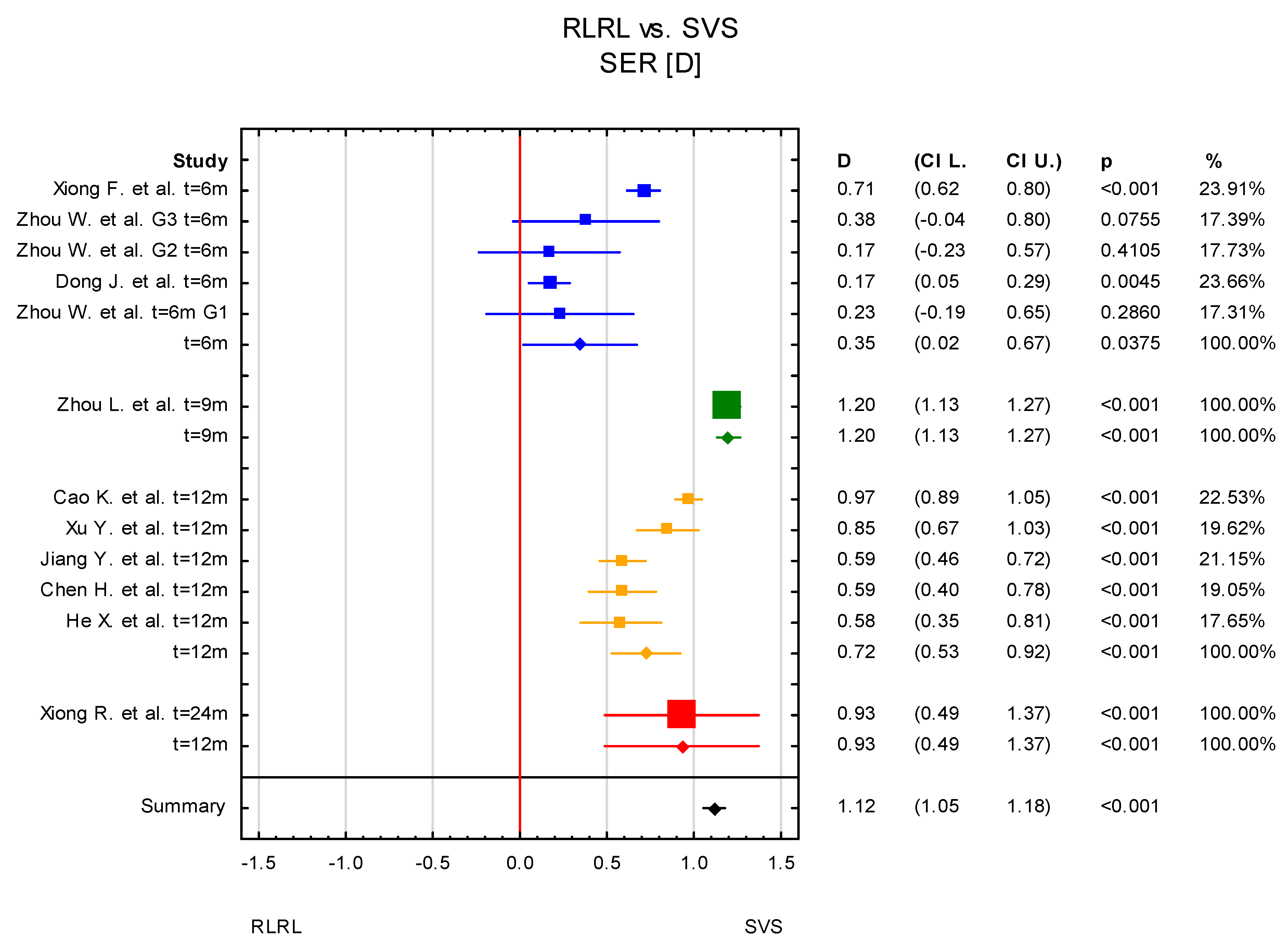
3. Results
3.1. Risk-of-Bias Assessment
3.2. Subgroup Analysis
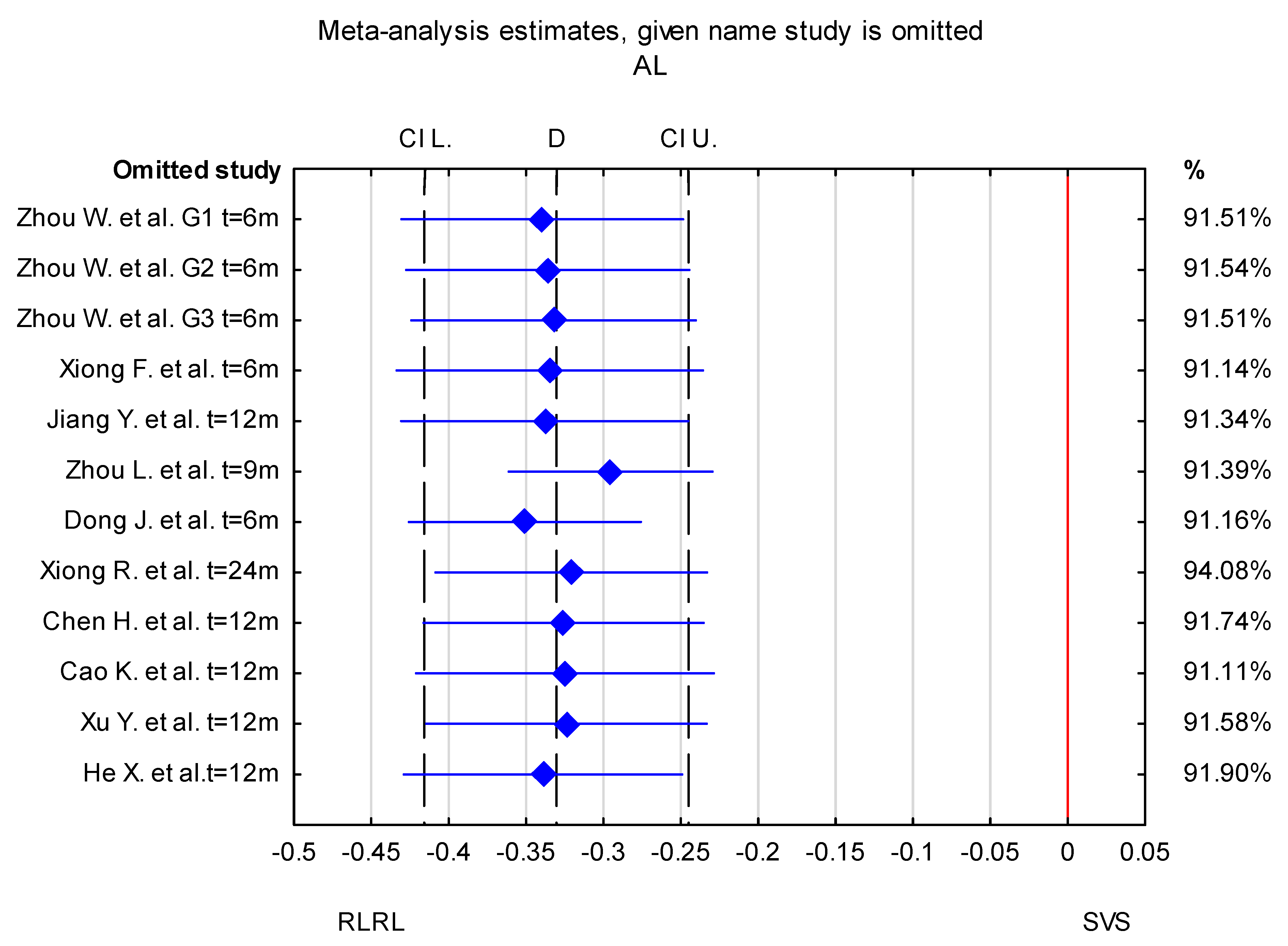
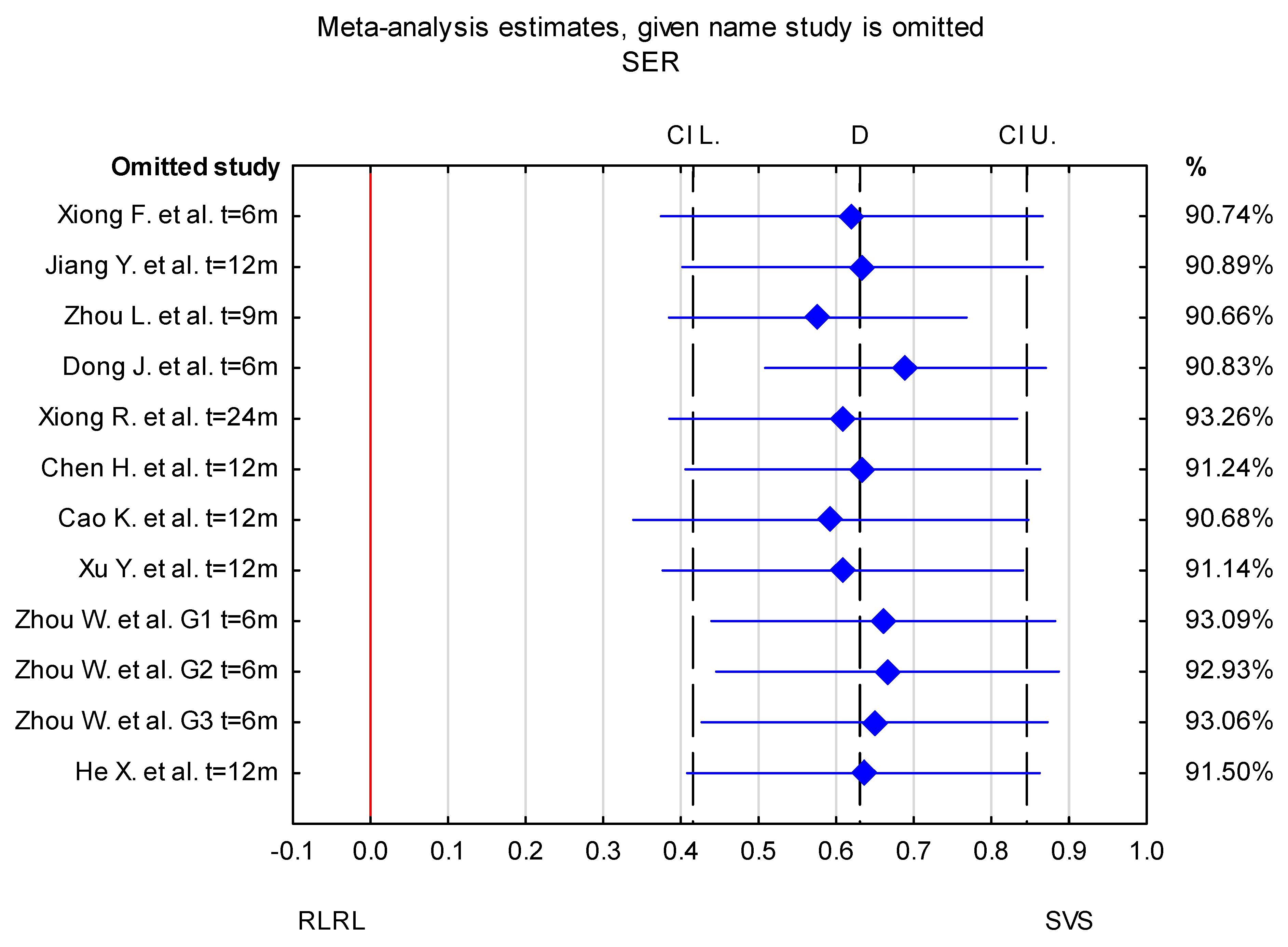
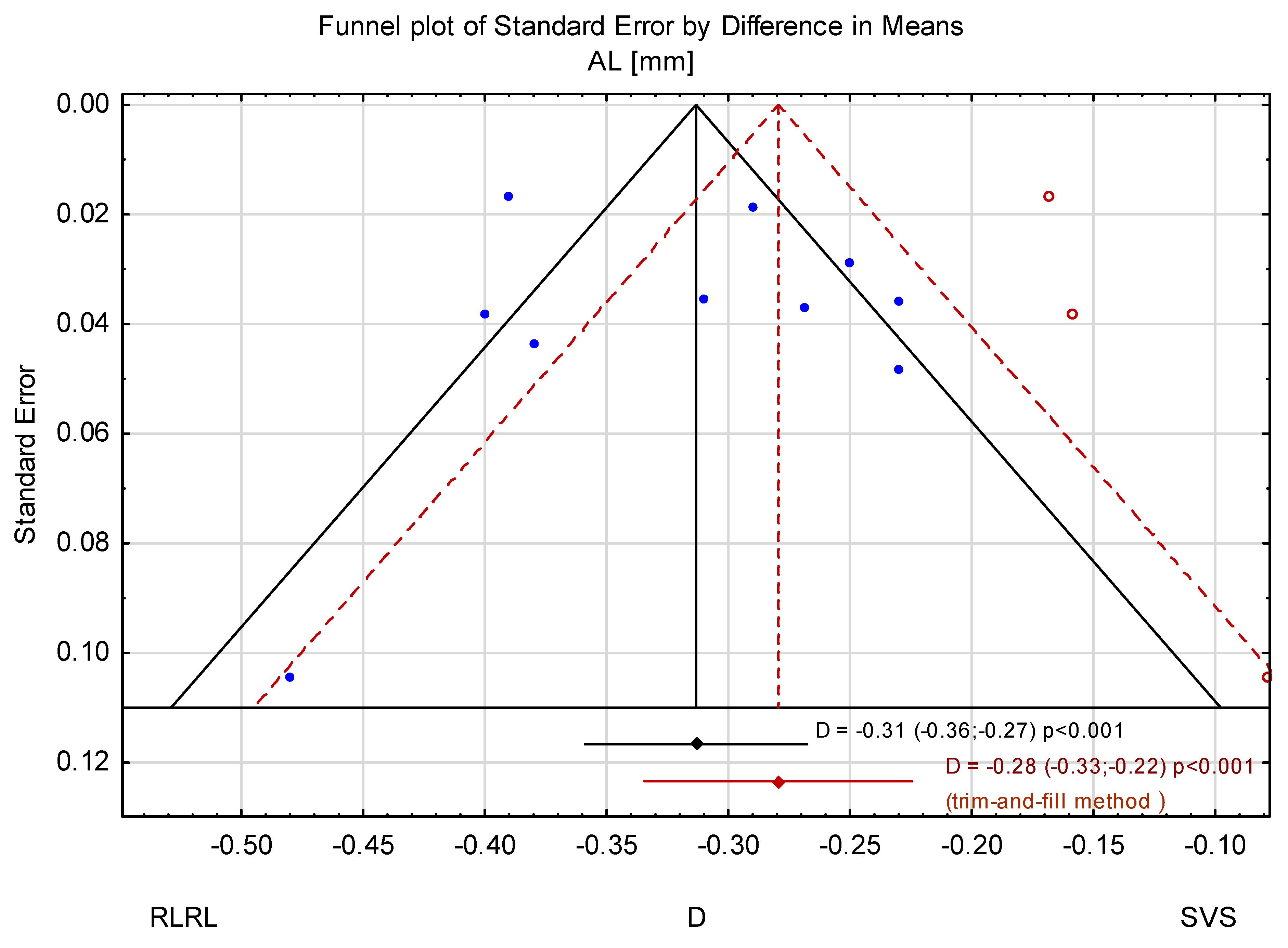
3.3. Trim-and-Fill Method
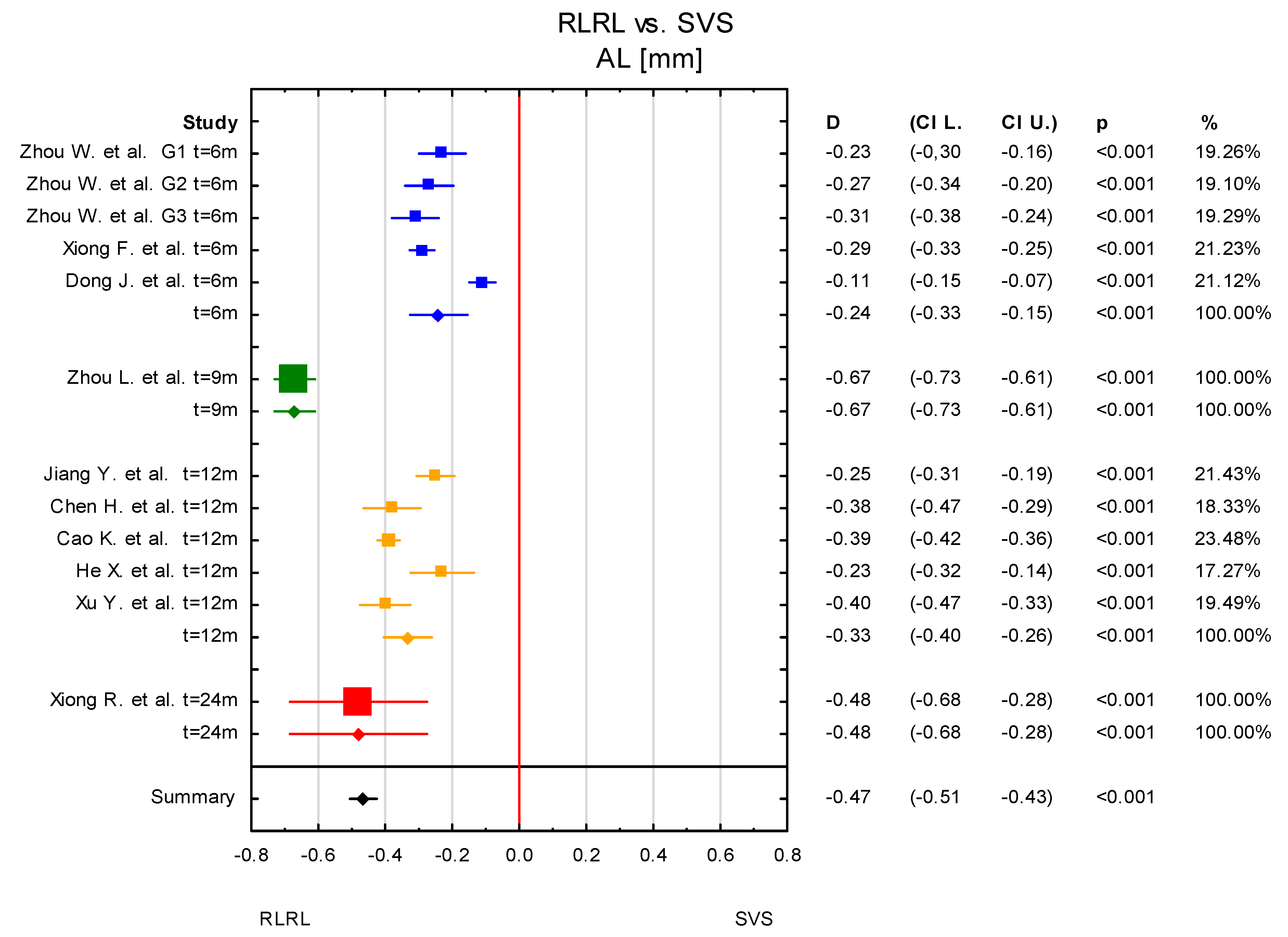
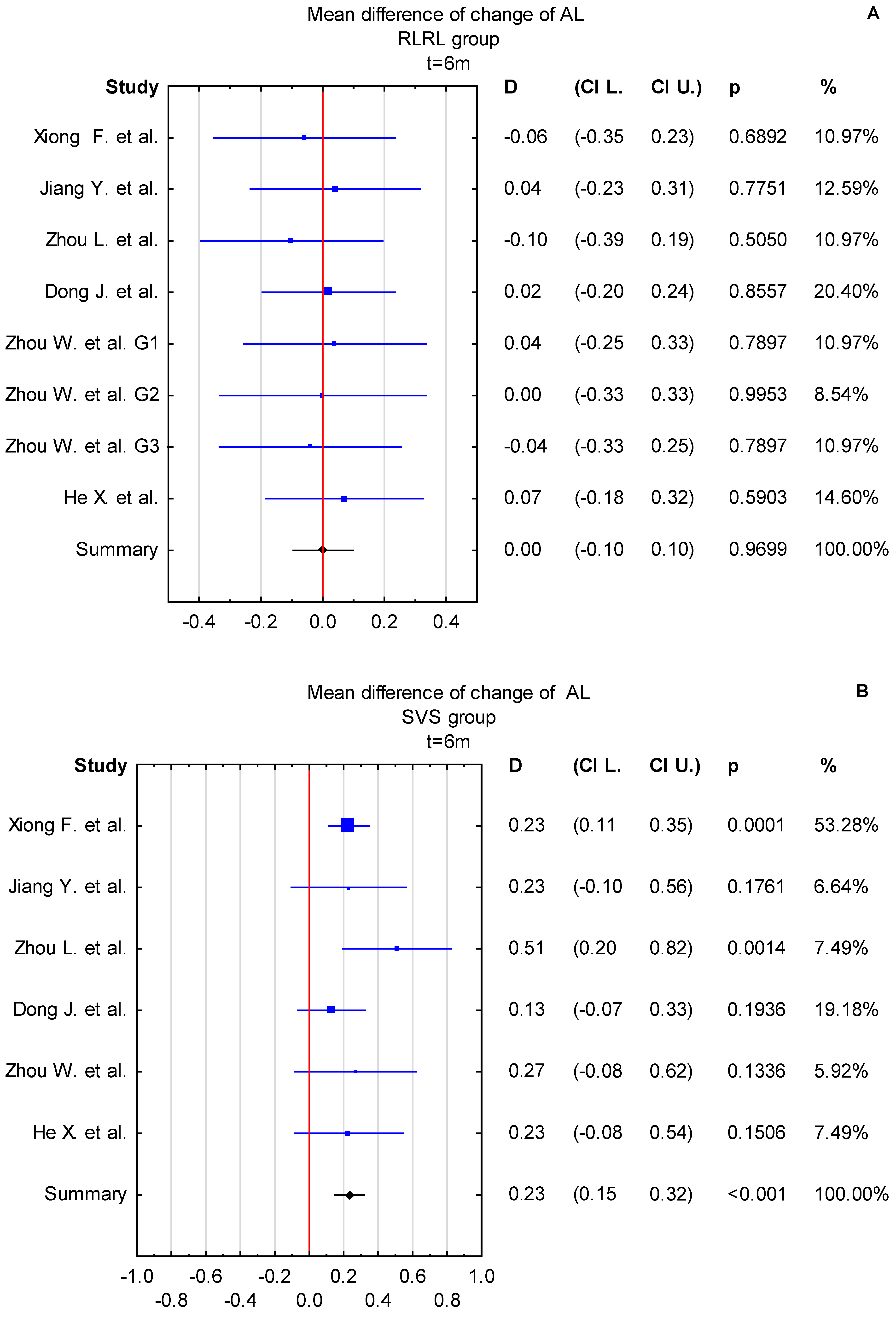
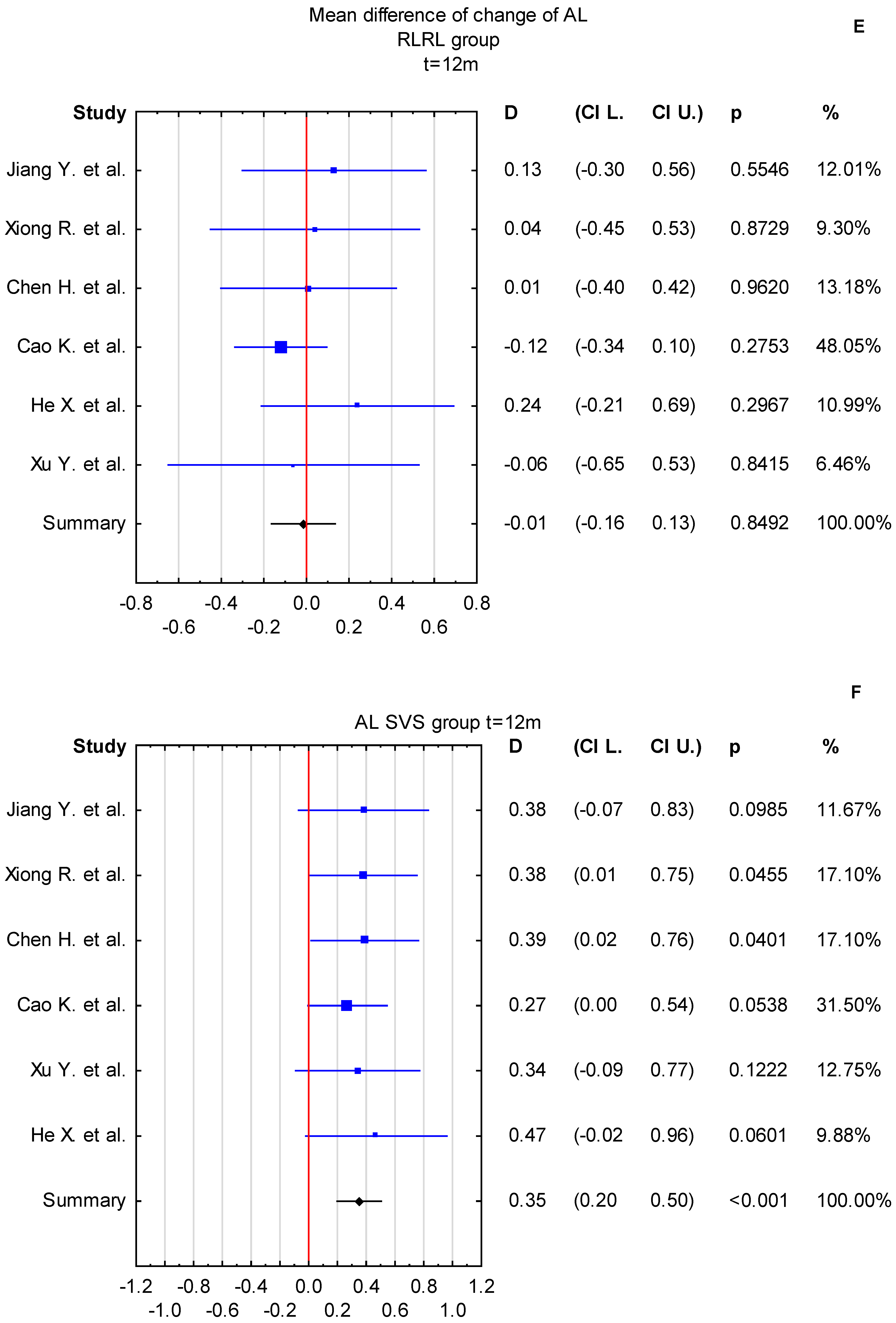
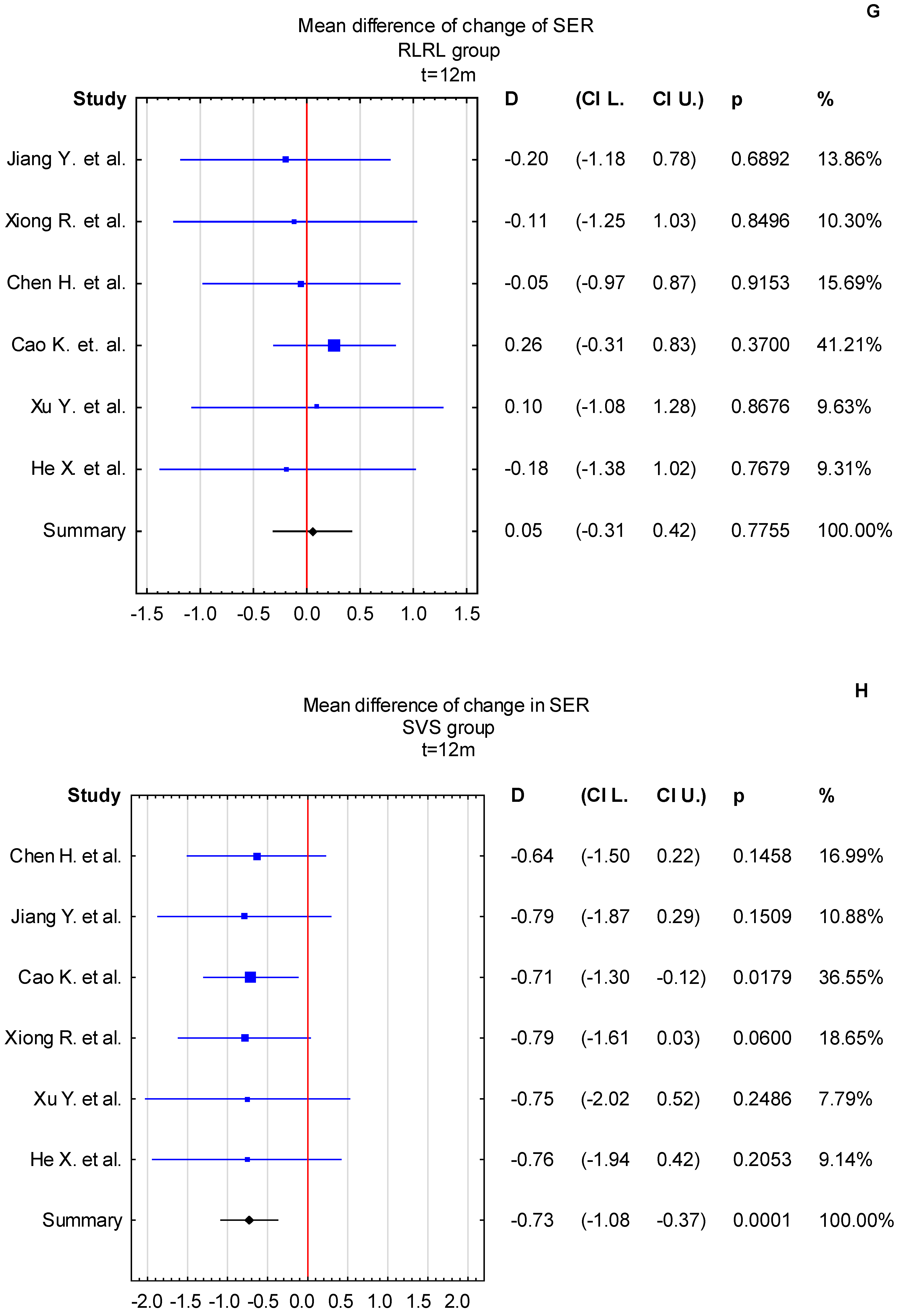
3.4. Time Points: t = 6 Months and t = 12 Months
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baird, P.N.; Saw, S.-M.; Lanca, C.; Guggenheim, J.A.; Smith, E.L., III; Zhou, X.; Matsui, K.-O.; Wu, P.-C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Kobia-Acquah, E.; Flitcroft, D.I.; Akowuah, P.K.; Lingham, G.; Loughman, J. Regional Variations and Temporal Trends of Childhood Myopia Prevalence in Africa: A Systematic Review and Meta-Analysis. Ophthalmic Physiol. Opt. 2022, 42, 1232–1252. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Gao, Z.; Leng, L.; Guo, Z. Temporal and Spatial Characterization of Myopia in China. Front. Public Health 2022, 10, 896926. [Google Scholar] [CrossRef] [PubMed]
- Fricke, T.R.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Naduvilath, T.J.; Ho, S.M.; Wong, T.Y.; Resnikoff, S. Global Prevalence of Visual Impairment Associated with Myopic Macular Degeneration and Temporal Trends from 2000 through 2050: Systematic Review, Meta-Analysis and Modelling. Br. J. Ophthalmol. 2018, 102, 855–862. [Google Scholar] [CrossRef]
- Haarman, A.E.G.; Enthoven, C.A.; Tideman, J.W.L.; Tedja, M.S.; Verhoeven, V.J.M.; Klaver, C.C.W. The Complications of Myopia: A Review and Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 49. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Wu, P.-C.; Yamashiro, K.; Vutipongsatorn, K.; Fang, Y.; Cheung, C.M.G.; Lai, T.Y.Y.; Ikuno, Y.; Cohen, S.Y.; Gaudric, A.; et al. IMI Pathologic Myopia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Choquet, H.; Khawaja, A.P.; Jiang, C.; Yin, J.; Melles, R.B.; Glymour, M.M.; Hysi, P.G.; Jorgenson, E. Association Between Myopic Refractive Error and Primary Open-Angle Glaucoma: A 2-Sample Mendelian Randomization Study. JAMA Ophthalmol. 2022, 140, 864–871. [Google Scholar] [CrossRef]
- Wang, Y.X.; Yang, H.; Wei, C.C.; Xu, L.; Wei, W.B.; Jonas, J.B. High Myopia as Risk Factor for the 10-Year Incidence of Open-Angle Glaucoma in the Beijing Eye Study. Br. J. Ophthalmol. 2023, 107, 935–940. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Ting, D.; Wu, X.; Huang, J.; Zhu, Y.; Chen, C.; Lin, B.; Li, S.; Zhang, X.; et al. The Associations of High Academic Performance with Childhood Ametropia Prevalence and Myopia Development in China. Ann. Transl. Med. 2021, 9, 745. [Google Scholar] [CrossRef]
- Cooper, J.; Tkatchenko, A.V. A Review of Current Concepts of the Etiology and Treatment of Myopia. Eye Contact Lens 2018, 44, 231. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.L.; Xu, L.; Sabanayagam, C.; Htoon, H.M.; Ang, M.; Zhang, J.; Ohno-Matsui, K.; Cheng, C.Y.; Hoang, Q.V.; Tan, C.-S.; et al. Predictors of Myopic Macular Degeneration in a 12-Year Longitudinal Study of Singapore Adults with Myopia. Br. J. Ophthalmol. 2023, 107, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, C.; Chen, Y.; He, M. Myopia Prediction: A Systematic Review. Eye 2022, 36, 921–929. [Google Scholar] [CrossRef]
- Vera-Diaz, F.A.; Jnawali, A.; Panorgias, A.; Bex, P.J.; Kerber, K.L. Baseline Metrics That May Predict Future Myopia in Young Children. Ophthalmic Physiol. Opt. 2023, 43, 466–481. [Google Scholar] [CrossRef]
- Gong, Q.; Janowski, M.; Luo, M.; Wei, H.; Chen, B.; Yang, G.; Liu, L. Efficacy and Adverse Effects of Atropine in Childhood Myopia: A Meta-Analysis. JAMA Ophthalmol. 2017, 135, 624–630. [Google Scholar] [CrossRef]
- Yam, J.C.; Jiang, Y.; Tang, S.M.; Law, A.K.P.; Chan, J.J.; Wong, E.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control. Ophthalmology 2019, 126, 113–124. [Google Scholar] [CrossRef]
- Ha, A.; Kim, S.J.; Shim, S.R.; Kim, Y.K.; Jung, J.H. Efficacy and Safety of 8 Atropine Concentrations for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology 2022, 129, 322–333. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, J. The Effect of 0.01% Atropine and Orthokeratology on Ocular Axial Elongation for Myopia Children: A Meta-Analysis (a PRISMA-Compliant Article). Medicine 2022, 101, e29191. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Wang, J.-H.; Chiu, C.-J. Effect of Orthokeratology on Myopia Progression: Twelve-Year Results of a Retrospective Cohort Study. BMC Ophthalmol. 2017, 17, 243. [Google Scholar] [CrossRef]
- Hiraoka, T. Myopia Control With Orthokeratology: A Review. Eye Contact Lens 2022, 48, 100. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Zhang, H.Y.; Lee, P.H.; Tse, D.Y.Y.; Qi, H.; Vlasak, N.; To, C.H. Long-Term Myopia Control Effect and Safety in Children Wearing DIMS Spectacle Lenses for 6 Years. Sci. Rep. 2023, 13, 5475. [Google Scholar] [CrossRef] [PubMed]
- Nucci, P.; Lembo, A.; Schiavetti, I.; Shah, R.; Edgar, D.F.; Evans, B.J.W. A Comparison of Myopia Control in European Children and Adolescents with Defocus Incorporated Multiple Segments (DIMS) Spectacles, Atropine, and Combined DIMS/Atropine. PLoS ONE 2023, 18, e0281816. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.-Y.; Tang, Y.Y.; To, C.H. Defocus Incorporated Soft Contact (DISC) Lens Slows Myopia Progression in Hong Kong Chinese Schoolchildren: A 2-Year Randomised Clinical Trial. Br. J. Ophthalmol. 2014, 98, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Brennan, N.A.; Toubouti, Y.; Greenaway, N.L. Safety of Soft Contact Lenses in Children: Retrospective Review of Six Randomized Controlled Trials of Myopia Control. Acta Ophthalmol. 2020, 98, e346–e351. [Google Scholar] [CrossRef]
- Garcia-del valle, A.M.; Blázquez, V.; Gros-otero, J.; Infante, M.; Culebras, A.; Verdejo, A.; Sebastián, J.; García, M.; Bueno, S.; Piñero, D.P. Efficacy and Safety of a Soft Contact Lens to Control Myopia Progression. Clin. Exp. Optom. 2021, 104, 14–21. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, J.; Brennan, N.A. Randomized Trial of Soft Contact Lenses with Novel Ring Focus for Controlling Myopia Progression. Ophthalmol. Sci. 2023, 3, 100232. [Google Scholar] [CrossRef]
- Brennan, N.A.; Toubouti, Y.M.; Cheng, X.; Bullimore, M.A. Efficacy in Myopia Control. Prog. Retin. Eye Res. 2021, 83, 100923. [Google Scholar] [CrossRef]
- Huang, J.; Wen, D.; Wang, Q.; McAlinden, C.; Flitcroft, I.; Chen, H.; Saw, S.M.; Chen, H.; Bao, F.; Zhao, Y.; et al. Efficacy Comparison of 16 Interventions for Myopia Control in Children: A Network Meta-Analysis. Ophthalmology 2016, 123, 697–708. [Google Scholar] [CrossRef]
- Xiong, S.; Sankaridurg, P.; Naduvilath, T.; Zang, J.; Zou, H.; Zhu, J.; Lv, M.; He, X.; Xu, X. Time Spent in Outdoor Activities in Relation to Myopia Prevention and Control: A Meta-Analysis and Systematic Review. Acta Ophthalmol. 2017, 95, 551–566. [Google Scholar] [CrossRef]
- Xiong, F.; Mao, T.; Liao, H.; Hu, X.; Shang, L.; Yu, L.; Lin, N.; Huang, L.; Yi, Y.; Zhou, R.; et al. Orthokeratology and Low-Intensity Laser Therapy for Slowing the Progression of Myopia in Children. BioMed Res. Int. 2021, 2021, 8915867. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, Z.; Tan, X.; Kong, X.; Zhong, H.; Zhang, J.; Xiong, R.; Yuan, Y.; Zeng, J.; Morgan, I.G.; et al. Effect of Repeated Low-Level Red-Light Therapy for Myopia Control in Children: A Multicenter Randomized Controlled Trial. Ophthalmology 2022, 129, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xing, C.; Quiang, W.; Hua, C.; Tong, L. Low-intensity, Long-wavelength Red Light Slows the Progression of Myopia in Children: An Eastern China-based Cohort. Ophthalmic Physiol. Opt. 2022, 42, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhu, Z.; Xu, H.; He, M. Myopia Control Effect of Repeated Low-Level Red-Light Therapy in Chinese Children: A Randomized, Double-Blind, Controlled Clinical Trial. Ophthalmology 2023, 130, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Kong, X.; Zhang, J.; Wang, W.; Kiburg, K.; Yuan, Y.; Chen, Y.; Zhang, S.; et al. Sustained and Rebound Effect of Repeated Low-Level Red-Light Therapy on Myopia Control: A 2-Year Post-Trial Follow-up Study. Clin. Experiment. Ophthalmol. 2022, 50, 1013–1024. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Liao, Y.; Zhou, W.; Li, Q.; Wang, J.; Tang, J.; Pei, Y.; Wang, X. Low-Intensity Red-Light Therapy in Slowing Myopic Progression and the Rebound Effect after Its Cessation in Chinese Children: A Randomized Controlled Trial. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 575–584. [Google Scholar] [CrossRef]
- Cao, K.; Tian, L.; Ma, D.-L.; Zhao, S.-Q.; Li, A.; Jin, Z.-B.; Jie, Y. Daily Low-Level Red Light for Spherical Equivalent Error and Axial Length in Children With Myopia: A Randomized Clinical Trial. JAMA Ophthalmol. 2024, 142, 560–567. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, L.; Kong, M.; Li, Q.; Feng, X.; Feng, K.; Zhu, H.; Cui, H.; Shi, C.; Zhang, J.; et al. Repeated Low-Level Red Light Therapy for Myopia Control in High Myopia Children and Adolescents: A Randomized Clinical Trial. Ophthalmology 2024, 131, 1314–1323. [Google Scholar] [CrossRef]
- Zhou, W.; Liao, Y.; Wang, W.; Sun, Y.; Li, Q.; Liu, S.; Tang, J.; Li, L.; Wang, X. Efficacy of Different Powers of Low-Level Red Light in Children for Myopia Control. Ophthalmology 2024, 131, 48–57. [Google Scholar] [CrossRef]
- He, X.; Wang, J.; Zhu, Z.; Xiang, K.; Zhang, X.; Zhang, B.; Chen, J.; Yang, J.; Du, L.; Niu, C.; et al. Effect of Repeated Low-Level Red Light on Myopia Prevention Among Children in China With Premyopia: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e239612. [Google Scholar] [CrossRef]
- Huang, Z.; He, T.; Zhang, J.; Du, C. Red Light Irradiation as an Intervention for Myopia. Indian J. Ophthalmol. 2022, 70, 3198. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, R.; Chen, X.; Zhang, J.; Bulloch, G.; Lin, X.; Wu, X.; Li, J. Efficacy Comparison of Repeated Low-Level Red Light and Low-Dose Atropine for Myopia Control: A Randomized Controlled Trial. Transl. Vis. Sci. Technol. 2022, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Liu, F.; Yu, X.; Xie, L.; Tan, S.; Liu, J.; Liu, Y.; Huang, J.; Zhang, S.; et al. Effects of Repeated Low-Level Red-Light Therapy on Macular Retinal Thickness and Microvascular System in Children with Myopia. Photodiagnosis Photodyn. Ther. 2024, 45, 103938. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, Y.; Fang, D.; Li, M.; Fu, T.; Yao, K.; Wang, P.; Sun, X.; Xiang, Y. Safety of Repeated Low-Level Red-Light Therapy for Children with Myopia. Photodiagnosis Photodyn. Ther. 2024, 47, 104198. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Dai, X.; Tian, J.; Sun, J. Efficacy of Repeated Low-Level Red-Light Therapy Combined with Optical Lenses for Myopia Control in Children and Adolescents. Am. J. Transl. Res. 2024, 16, 4903–4911. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, W.; Tang, X.; He, M.; Hu, Y.; Zhang, J.; Du, B.; Jiang, Y.; Zhu, Z.; Chen, Y.; et al. Myopia Control Effect of Repeated Low-Level Red-Light Therapy Combined with Orthokeratology: A Multicenter Randomized Controlled Trial. Ophthalmology 2024, 131, 1304–1313. [Google Scholar] [CrossRef]
- Austin, E.; Huang, A.; Wang, J.Y.; Cohen, M.; Heilman, E.; Maverakis, E.; Michl, J.; Jagdeo, J. Red Light Phototherapy Using Light-Emitting Diodes Inhibits Melanoma Proliferation and Alters Tumor Microenvironments. Front. Oncol. 2022, 12, 928484. [Google Scholar] [CrossRef]
- Ostrin, L.A.; Schill, A.W. Red Light Instruments for Myopia Exceed Safety Limits. Ophthalmic Physiol. Opt. 2024, 44, 241–248. [Google Scholar] [CrossRef]
- Schaeffel, F.; Wildsoet, C.F. Red Light Therapy for Myopia: Merits, Risks and Questions. Ophthalmic Physiol. Opt. 2024, 44, 801–807. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, R.; Yang, S.; Zhu, Z.; Li, H.; Xiang, K.; Congdon, N.; Wang, W.; He, M. Safety of Repeated Low-Level Red-Light Therapy for Myopia: A Systematic Review. Asia-Pac. J. Ophthalmol. 2024, 13, 100124. [Google Scholar] [CrossRef]
- University of California, San Francisco. Efficacy of Repeated Low-Level Red-Light Therapy in Myopia Control: A Randomized Controlled Trial; Clinicaltrials.gov: Bethesda, MA, USA, 2024.
- University of Houston. Effects of Narrowband and Broadband Light on Choroidal Thickness and Melanopsin-Driven Pupil Response; Clinicaltrials.gov: Bethesda, MA, USA, 2023.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Zhu, Z.; Jiang, Y.; Wang, W.; Zhang, J.; Chen, Y.; Bulloch, G.; Yuan, Y.; Zhang, S.; Xuan, M.; et al. Longitudinal Changes and Predictive Value of Choroidal Thickness for Myopia Control after Repeated Low-Level Red-Light Therapy. Ophthalmology 2023, 130, 286–296. [Google Scholar] [CrossRef]
- Xun, X.; Mingguang, H. Expert Consensus on Repeated Low-Level Red-Light as an Alternative Treatment for Childhood Myopia (2022). CJEO J. 2022, 40, 599–603. [Google Scholar] [CrossRef]
- Knight, O.J.; Girkin, C.A.; Budenz, D.L.; Durbin, M.K.; Feuer, W.J.; Cirrus OCT Normative Database Study Group. Effect of Race, Age, and Axial Length on Optic Nerve Head Parameters and Retinal Nerve Fiber Layer Thickness Measured by Cirrus HD-OCT. Arch. Ophthalmol. 2012, 130, 312–318. [Google Scholar] [CrossRef]
- Zouache, M.A.; Silvestri, G.; Amoaku, W.M.; Silvestri, V.; Hubbard, W.C.; Pappas, C.; Akafo, S.; Lartey, S.; Mastey, R.R.; Carroll, J.; et al. Comparison of the Morphology of the Foveal Pit Between African and Caucasian Populations. Transl. Vis. Sci. Technol. 2020, 9, 24. [Google Scholar] [CrossRef]





| Time | SVS | SVS | SVS | SVS Age | RLRL | RLRL | RLRL | RLRL Age | Study Design | |
|---|---|---|---|---|---|---|---|---|---|---|
| [Months] | AL Change ± SD or (95% CI) [mm] | SER ± SD or (95% CI) [D] | Number of Subjects | Mean ± SD Range [Years] | AL Change ± SD or (95% CI) [mm] | SER ± SD or (95% CI) [D] | Number of Subjects | Mean ± SD Range [Years] | ||
| Xiong F. et al. [30] | 1 | 0.02 ± 0.02 | −0.07 ± 0.11 | 74 | 10.33 ± 2.03 (7−14) | −0.05 ± 0.07 | 0.11 ± 0.17 | 74 | 10.22 ± 2.38 (7–15) | randomized controlled trial |
| 3 | 0.10 ± 0.04 | −0.24 ± 0.16 | −0.07 ± 0.12 | 0.22 ± 0.32 | ||||||
| 6 | 0.23 ± 0.06 | −0.50 ± 0.24 | −0.06 ± 0.15 | 0.21 ± 0.34 | ||||||
| Jiang Y. et al. [31] CI | 1 | 0.02 (0.01 to 0.03) | −0.01 (−0.06 to 0.03) | 129 | 10.5 (8−13) | −0.004 (−0.05 to −0.03) | 0.08 (0.04 to 0.13) | 117 | 10.4 (8–13) | randomized, parallel-group, single-blind clinical trial |
| 3 | 0.10 (0.09 to 0.12) | −0.18 (−0.27 to −0.13) | −0.01 (−0.03 to 0.00) | 0.07 (0.02 to 0.12) | ||||||
| 6 | 0.23 (0.20 to 0.26) | −0.38 (−0.47 to −0.30) | 0.04 (0.02 to 0.07) | −0.03 (−0.11 to 0.05) | ||||||
| 12 | 0.38 (0.34 to 0.42) | −0.79 (−0.88 to −0.69) | 0.13 (0.09 to 0.17) | −0.20 (−0.29 to −0.11) | ||||||
| Zhou L. et al. [32] | 3 | 0.33 ± 0.12 | −0.47 ± 0.09 | 8−14 | −0.08 ± 0.11 | 0.07 ± 0.14 | 8–14 | a retrospective case series | ||
| 6 | 0.51 ± 0.16 | −0.65 ± 0.16 | 76 | −0.10 ± 0.15 | 0.19 ± 0.25 | 76 | ||||
| 9 | 0.61 ± 0.20 | −0.94 ± 0.13 | −0.06 ± 0.18 | 0.22 ± 0.17 | ||||||
| Dong J. et al. [33] | 6 | 0.13 ± 0.10 | −0.11 ± 0.33 | 55 | 9.86 ± 1.41 (7–12) | 0.02 ± 0.11 | 0.06 ± 0.30 | 56 | 10.3 ± 2.07 (7–12) | randomized, double-blind, controlled clinical trial |
| Xiong R. et al. [34] | 12 | 0.38 ± 0.19 | −0.79 ± 0.42 | 41 | 10.79 ± 1.55 | 0.04 ± 0.25 | −0.11 ± 0.58 | 11 | 11.18 ± 1.67 | multicenter randomized controlled trial |
| 24 | 0.64 ± 0.29 | −1.24 ± 0.63 | 0.16 ± 037 | −0.31 ± 0.79 | ||||||
| Chen H. et al. [35] CI | 12 | 0.39 (0.33 to 0.45) | −0.64 (−0.78 to −0.51) | 40 | 8.98 ± 1.92 (6−13) | 0.01 (−0.05 to 0.07) | −0.05 (−0.08 to 0.19) | 46 | 9.0 ± 1.9 (6−13) | prospective, randomized, controlled study |
| 12 | −0.19 (−0.24 to −0.14) | −0.03 (−0.01 to 0.08) | −0.22 (−0.45 to −0.05) | −0.60 (−0.71 to−0.48) | ||||||
| Cao K. et al. [36] | 12 | 0.27 ± 0.14 | −0.71 ± 0.30 | 112 | 9.0 ± 1.9 | −0.12 ± 0.11 | 0.26 ± 0.29 | 112 | 9.0 ± 2.0 | single-masked, single-center, randomized clinical trial |
| Yan X. et al. [37] CI | 12 | 0.34 (0.30 to 0.39) | −0.75 (−0.88 to −0.62) | 95 | 11.2 ± 2.1 | −0.06 (−0.1 to 0.02) | 0.1 (−0.02 to 0.22) | 96 | 10.4 ± 2.4 | a prospective, single-blind, parallel-group, multicenter, randomized clinical trial |
| Zhou W. et al. [38] CI | 6 | 0.27 (−0.50 to 0.30) | −0.22 (−0.50 to 0.30) | 43 | 8.83 ± 1.53 (6–15) | 0.04 (−0.01 to 0.08) | 0.01 (−0.12 to 0.15) | 43 | 8.51 ± 1.51 | single-center, randomized, parallel-group clinical trial |
| G1 (0.37 mW) | ||||||||||
| G2 (0.60 mW) | 0.00 (−0.05 to 0.05) | −0.05 (−0.18 to 0.07) | 47 | 8.77 ± 1.43 | ||||||
| G3 (1.20 mW) | −0.04 (−0.08 to 0.01) | 0.16 (0.03 to 0.30) | 44 | 8.68 ± 1.39 | ||||||
| He X. et al. [39] | 6 | 0.23 ± 0.16 | 120 | 8.3 ± 1.1 | 0.07 ± 0.13 | 0.18 ± 0.35 | 139 | 8.3 ± 1.1 | single-blinded, randomized clinical trial | |
| 12 | 0.47 ± 0.25 | 0.24 ± 0.23 | −0.18 ± 0.61 | 32 |
| Manuscript | Power | Wavelength | Manufacturer/Model |
|---|---|---|---|
| Xiong F. et al. [30] | 2 ± 0.5 mW | 650 nm | Ya Kun Optoelectronic Co. (Wuhan, China) |
| Jiang Y. et al. [31] | 0.29 mW for 4 mm pupil | 650 ± 10 nm | Eyerising International—Suzhou Xuanjia Optoelectronics Technology (South Yarra, Australia) |
| Zhou L. et al. [32] | 0.4 mW | 635 ± 1 nm | Optoelectronic Co. (Changchun, China) |
| Dong J. et al. [33] | 0.29 mW for 4 mm pupil | n/a | Eyerising International—Suzhou Xuanjia Optoelectronics Technology (South Yarra, Australia) |
| Xiong R. et al. [34] | Class 1 device for 4 mm pupil | 650 ± 10 nm | Eyerising International —Suzhou Xuanjia Optoelectronics Technology (South Yarra, Australia) |
| Chen H. et al. [35] | 0.35 ± 0.02 mW | 635 nm | Jilin Londa Optoelectronics Technology (Jilin City, China)/LD-A |
| Cao K. et al. [36] | n/a | 650 nm | Hunan EnVan Technology Co., Ltd. (China)/YF020A |
| Yan X. et al. [37] | 0.29 mW for 4 mm pupil | 650 ± 10 nm | Eyerising International —Suzhou Xuanjia Optoelectronics Technology (South Yarra, Australia) |
| Wen Z. et al. [38] | 0.37 ± 0.2 mW (group 1) 0.60 ± 0.2 mW (group 2) 1.2 ± 0.2 mW (group 3) | 650 nm | Beijing Ming Ren Shi Kang Science & Technology Co. (Beijing, China)/sky-n1201 |
| He X. et al. [39] | n/a | 650 ± 10 nm | Eyerising International —Suzhou Xuanjia Optoelectronics Technology (South Yarra, Australia) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobol, M.; Pniewski, J. Efficacy of Repeated Low-Level Red Light (RLRL) Therapy in Managing Childhood Myopia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 83. https://doi.org/10.3390/jcm14010083
Sobol M, Pniewski J. Efficacy of Repeated Low-Level Red Light (RLRL) Therapy in Managing Childhood Myopia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(1):83. https://doi.org/10.3390/jcm14010083
Chicago/Turabian StyleSobol, Maria, and Jacek Pniewski. 2025. "Efficacy of Repeated Low-Level Red Light (RLRL) Therapy in Managing Childhood Myopia: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 1: 83. https://doi.org/10.3390/jcm14010083
APA StyleSobol, M., & Pniewski, J. (2025). Efficacy of Repeated Low-Level Red Light (RLRL) Therapy in Managing Childhood Myopia: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(1), 83. https://doi.org/10.3390/jcm14010083







