Macular Pigment Changes and Visual Recovery Following Successful Full-Thickness Macular Hole Closure Using the Inverted Flap Technique
Abstract
1. Introduction
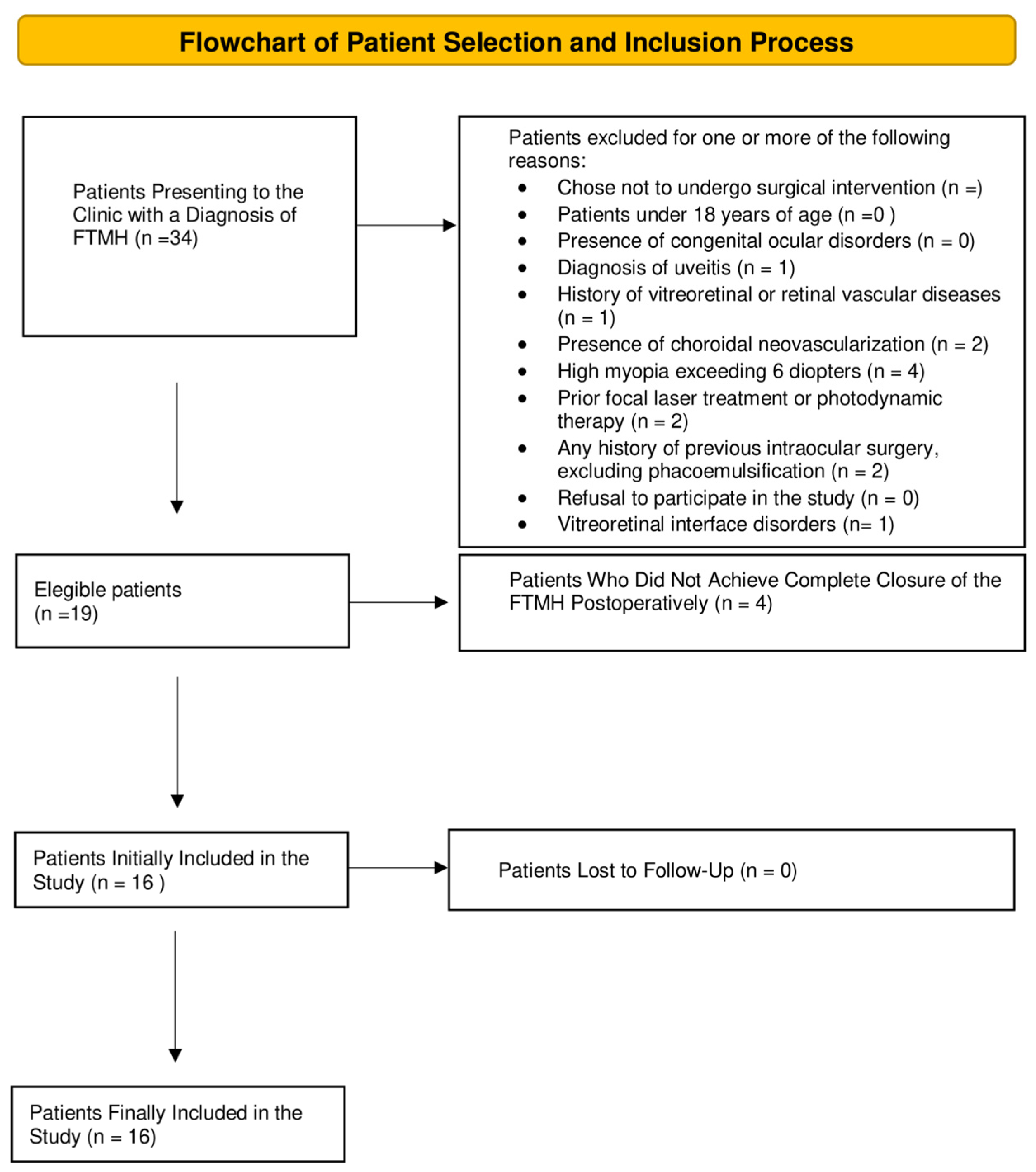
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Surgical Technique
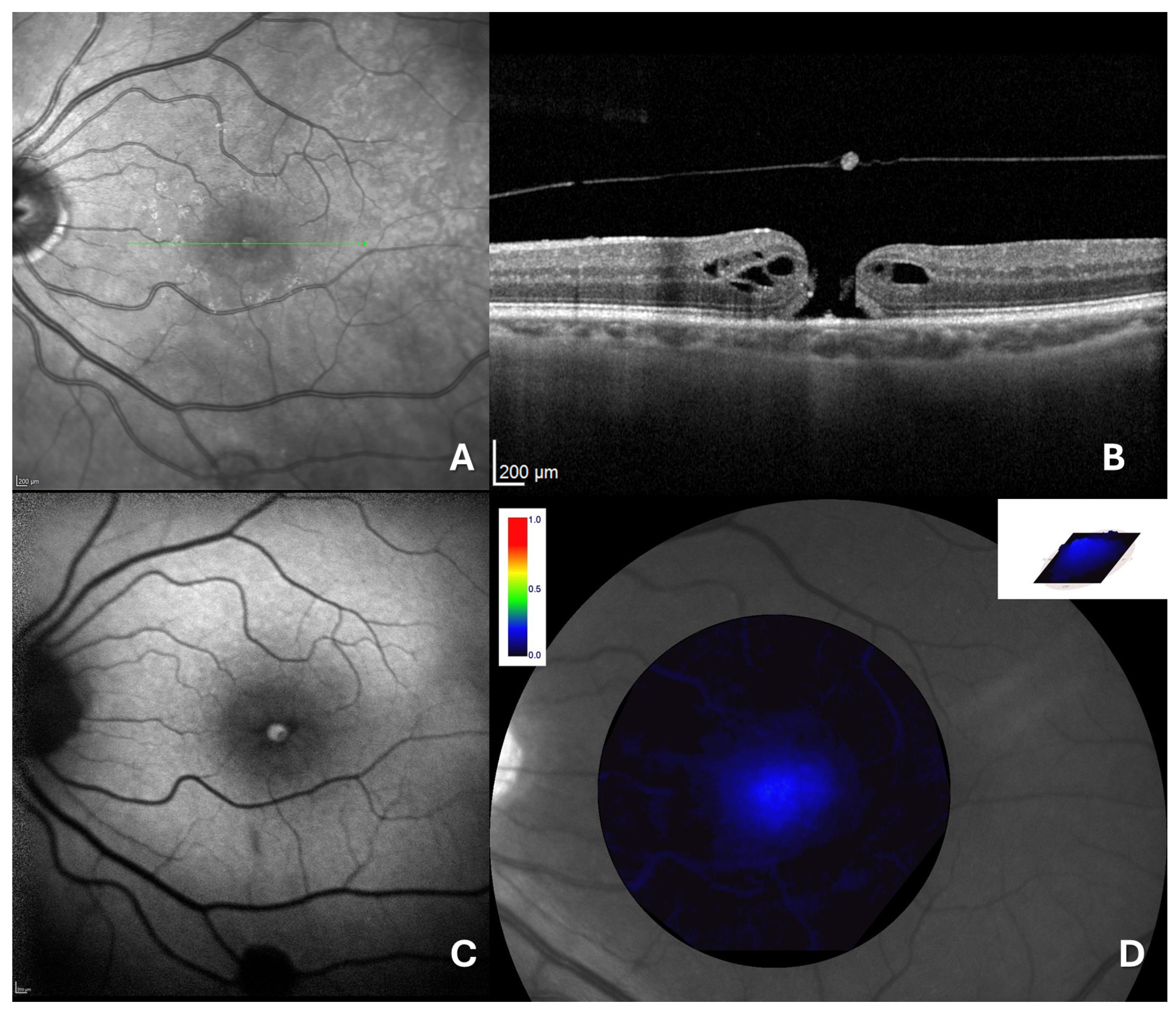
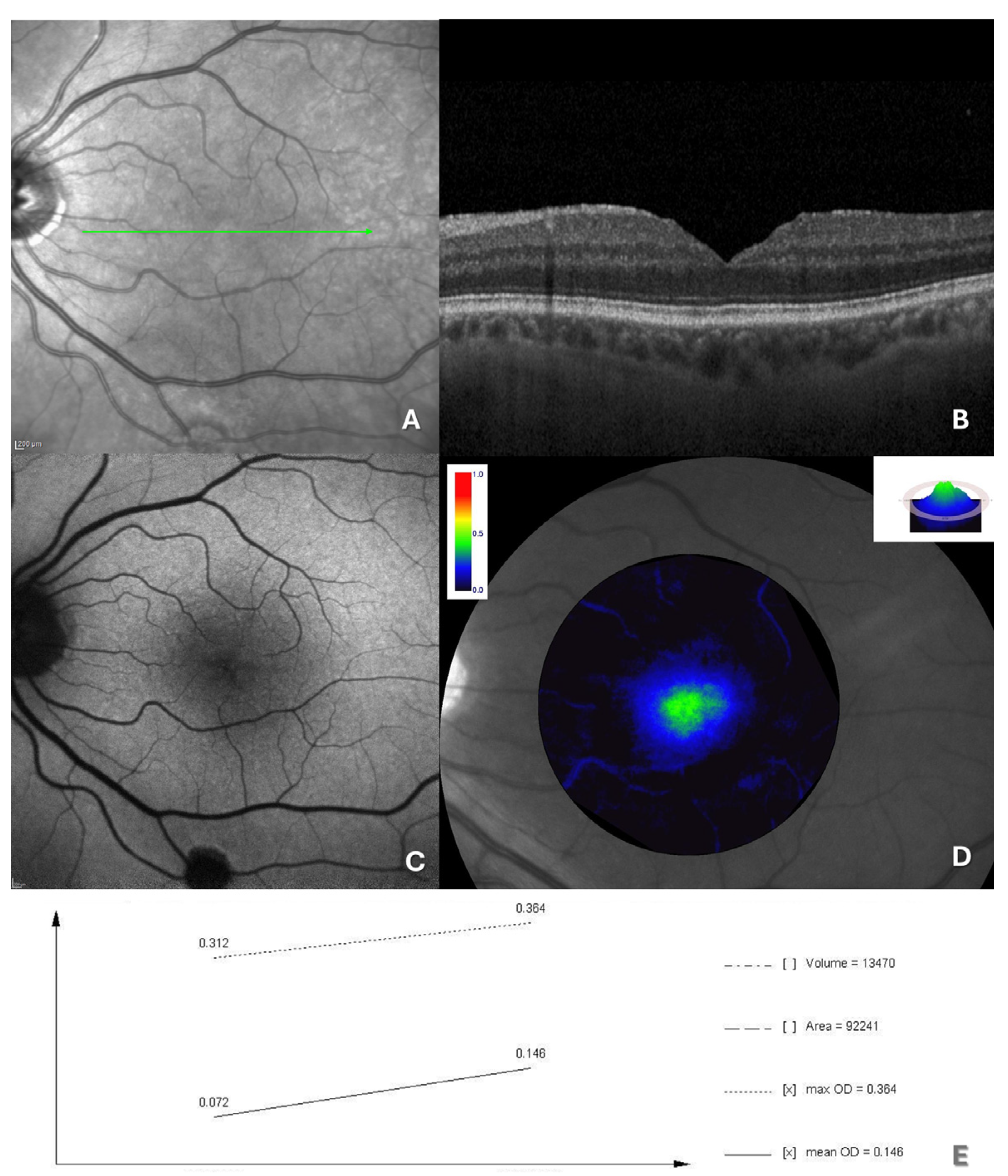
2.4. Retinal Imaging and Data Measurement
2.5. Macular Pigment Assessment and Analysis
2.6. Variables and Outcomes
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, P.J.; Steel, D.H.; Hirneiß, C.; Brazier, J.; Aly, A.; Lescrauwaet, B. Patient-reported prevalence of metamorphopsia and predictors of vision-related quality of life in vitreomacular traction: A prospective, multi-centre study. Eye 2019, 33, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, L.; Jonas, J.B. Prevalence of full-thickness macular holes in urban and rural adult Chinese: The Beijing Eye Study. Am. J. Ophthalmol. 2006, 141, 589–591. [Google Scholar] [CrossRef]
- Sen, P.; Bhargava, A.; Vijaya, L.; George, R. Prevalence of idiopathic macular hole in adult rural and urban south Indian population. Clin. Exp. Ophthalmol. 2008, 36, 257–260. [Google Scholar] [CrossRef] [PubMed]
- McCannel, C.A.; Ensminger, J.L.; Diehl, N.N.; Hodge, D.N. Population-based Incidence of Macular Holes. Ophthalmology 2009, 116, 1366–1369. [Google Scholar] [CrossRef]
- Chehaibou, I.; Tadayoni, R.; Hubschman, J.P.; Bottoni, F.; Caputo, G.; Chang, S.; Dell’Omo, R.; Figueroa, M.S.; Gaudric, A.; Haritoglou, C.; et al. Natural History and Surgical Outcomes of Lamellar Macular Holes. Ophthalmol. Retina 2024, 8, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Fujiwara, M.; Sakaguchi, H.; Kusaka, S.; Oshima, Y. Foveal microstructure and visual acuity in surgically closed macular holes: Spectral-domain optical coherence tomographic analysis. Ophthalmology 2010, 117, 1815–1824. [Google Scholar] [CrossRef]
- Gellermann, W.; Bernstein, P.S. Noninvasive detection of macular pigments in the human eye. J. Biomed. Opt. 2004, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.A.; Landrum, J.T.; Fernandez, L.; Tarsis, S.L. Analysis of the macular pigment by HPLC: Retinal distribution and age study. Investig. Ophthalmol. Vis. Sci. 1988, 29, 843–849. [Google Scholar]
- zu Westrup, V.M.; Dietzel, M.; Pauleikhoff, D.; Hense, H.W. The association of retinal structure and macular pigment distribution. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1169–1175. [Google Scholar] [CrossRef][Green Version]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef]
- Trieschmann, M.; Van Kuijk, F.J.; Alexander, R.; Hermans, P.; Luthert, P.; Bird, A.C.; Pauleikhoff, D. Macular pigment in the human retina: Histological evaluation of localization and distribution. Eye 2008, 22, 132–137. [Google Scholar] [CrossRef]
- Howells, O.; Eperjesi, F.; Bartlett, H. Measuring macular pigment optical density in vivo: A review of techniques. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 315–347. [Google Scholar] [CrossRef]
- Obana, A.; Nakazawa, R.; Noma, S.; Sasano, H.; Gohto, Y. Macular pigment in eyes with macular hole formation and its change after surgery. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; O’Gorman, N.; Nolan, J.; O’Donovan, O.; Au Eong, K.G.; Beatty, S. Macular pigment levels following successful macular hole surgery. Br. J. Ophthalmol. 2005, 89, 1105–1108. [Google Scholar] [CrossRef]
- Rinaldi, M.; Cennamo, G.; Passaro, M.L.; Costagliola, C. Changes in macular pigment optical density after full-thickness macular hole closure using inverted flap technique. Photodiagn. Photodyn. Ther. 2024, 45, 103950. [Google Scholar] [CrossRef]
- Sano, M.; Shimoda, Y.; Hashimoto, H.; Kishi, S. Restored Photoreceptor Outer Segment and Visual Recovery After Macular Hole Closure. Am. J. Ophthalmol. 2009, 147, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Shimozono, M.; Oishi, A.; Hata, M.; Kurimoto, Y. Restoration of the photoreceptor outer segment and visual outcomes after macular hole closure: Spectral-domain optical coherence tomography analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1469–1476. [Google Scholar] [CrossRef]
- Shiragami, C.; Shiraga, F.; Nitta, E.; Fukuda, K.; Yamaji, H. Correlation of increased fundus autofluorescence signals at closed macula with visual prognosis after successful macular hole surgery. Retina 2012, 32, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Cennamo, G.; Grassi, P.; Sparnelli, F.; Allegrini, D.; Cennamo, G. Changes in macular pigment optical density after membrane peeling. PLoS ONE 2018, 13, e0197034. [Google Scholar] [CrossRef]
- Bottoni, F.; Zanzottera, E.; Carini, E.; Cereda, M.; Cigada, M.; Staurenghi, G. Re-accumulation of macular pigment after successful macular hole surgery. Br. J. Ophthalmol. 2016, 100, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Jentsch, S.; Augsten, R.; Strobel, J.; Dawczynski, J. Untersuchungen zum zeitlichen Verlauf der Makulapigmentdichte bei Patienten mit idiopathischem Makulaforamen—Klinischer Verlauf und Einfluss der Operation. Klin. Monbl Augenheilkd. 2012, 229, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Yamamoto, S.; Arai, M.; Arai, E.; Sugawara, T.; Mitamura, Y.; Mizunoya, S. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina 2008, 28, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Duker, J.S.; Kaiser, P.K.; Binder, S.; de Smet, M.D.; Gaudric, A.; Reichel, E.; Sadda, S.R.; Sebag, J.; Spaide, R.F.; Stalmans, P. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 2013, 120, 2611–2619. [Google Scholar] [CrossRef]
- Wolf-Schnurrbusch, U.E.K.; Röösli, N.; Weyermann, E.; Heldner, M.R.; Höhne, K.; Wolf, S. Ethnic differences in macular pigment density and distribution. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.G.; Lievens, C.; Amonoo-Monney, S. Differences in macular pigment optical density across four ethnicities: A comparative study. Ther. Adv. Ophthalmol. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Desmarchelier, C.; Nowicki, M.; Bott, R.; Morange, S.; Lesavre, N. Interindividual variability of lutein bioavailability in healthy men: Characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am. J. Clin. Nutr. 2014, 100, 168–175. [Google Scholar] [CrossRef] [PubMed]
| Control (16 Eyes) | FTMH (16 Eyes) | p Value | |
|---|---|---|---|
| Age | 62.6 (7.4) | 61.5 (8.7) | 0.97 |
| BCVA (logMAR) | 0.05 (0.1) | 1.4 (0.5) | <0.001 |
| MPOD mean (d.u.) | 0.15 (0.02) | 0.1 (0.02) | <0.0001 |
| MPOD max (d.u.) | 0.47 (0.06) | 0.4 (0.08) | 0.002 |
| MPOD area (pixel) | 80,628.8 (8282.6) | 63,597.1 (16,739.2) | 0.03 |
| MPOD volume (d.u. × pixel) | 16,338.3 (2822) | 10,812.6 (3329.7) | <0.001 |
| FTMH | |||
|---|---|---|---|
| Preoperative | Postoperative | p-Value | |
| BCVA (logMAR) | 1.5 (0.5) | 0.7 (0.4) | 0.0011 |
| MPOD mean (d.u.) | 0.1(0.02) | 0.1 (0.01) | >0.9 |
| MPOD max (d.u.) | 0.4 (0.08) | 0.4 (0.03) | >0.9 |
| MPOD area (pixel) | 63,597.1 (16,739.2) | 79,982.5 (25,703.0) | 0.2 |
| MPOD volume (d.u. × pixel) | 10,812.6 (3329.7) | 14,569.1 (4764.6) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, M.; Galantuomo, N.; Passaro, M.L.; Cennamo, G.; Chiosi, F.; Costagliola, C. Macular Pigment Changes and Visual Recovery Following Successful Full-Thickness Macular Hole Closure Using the Inverted Flap Technique. J. Clin. Med. 2025, 14, 290. https://doi.org/10.3390/jcm14010290
Rinaldi M, Galantuomo N, Passaro ML, Cennamo G, Chiosi F, Costagliola C. Macular Pigment Changes and Visual Recovery Following Successful Full-Thickness Macular Hole Closure Using the Inverted Flap Technique. Journal of Clinical Medicine. 2025; 14(1):290. https://doi.org/10.3390/jcm14010290
Chicago/Turabian StyleRinaldi, Michele, Nicola Galantuomo, Maria Laura Passaro, Gilda Cennamo, Flavia Chiosi, and Ciro Costagliola. 2025. "Macular Pigment Changes and Visual Recovery Following Successful Full-Thickness Macular Hole Closure Using the Inverted Flap Technique" Journal of Clinical Medicine 14, no. 1: 290. https://doi.org/10.3390/jcm14010290
APA StyleRinaldi, M., Galantuomo, N., Passaro, M. L., Cennamo, G., Chiosi, F., & Costagliola, C. (2025). Macular Pigment Changes and Visual Recovery Following Successful Full-Thickness Macular Hole Closure Using the Inverted Flap Technique. Journal of Clinical Medicine, 14(1), 290. https://doi.org/10.3390/jcm14010290







