A β-Thalassemia Cell Biobank: Updates, Further Validation in Genetic and Therapeutic Research and Opportunities During (and After) the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Isolation and Culture of Peripheral Blood Cells
2.3. Freezing, Cryopreservation, and Thawing
2.4. Quality Control Procedures
2.5. Treatment of Long-Storage Biobanked Cells with HbF Inducers
2.6. Treatment of Cells with the β039 CRISPR-Cas9 System
3. Results
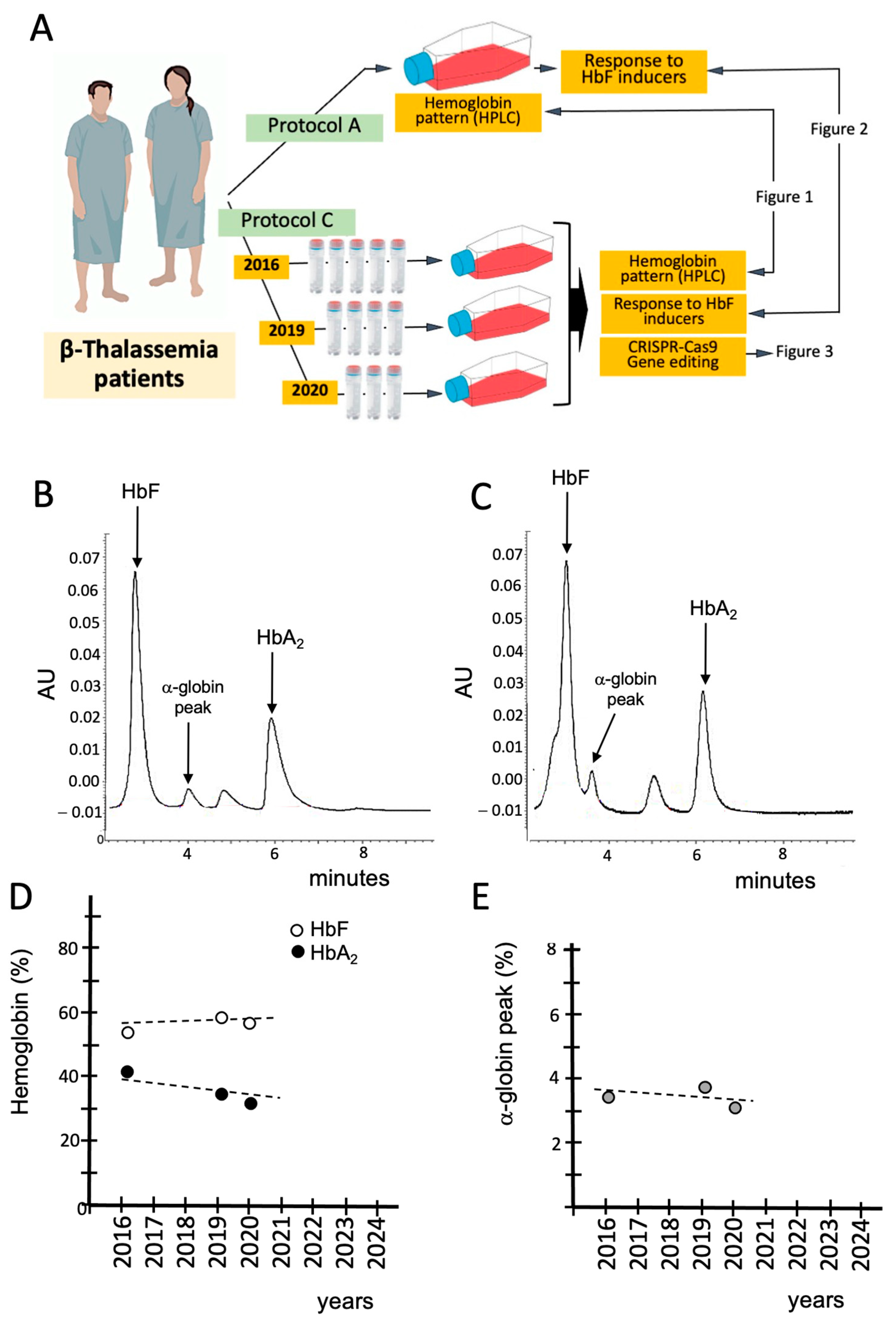
3.1. Characterization and Validation of the β-Thal Cell Biobank: Biobanked Samples of the Same β-Thalassemia Patient Maintain the Hemoglobin Pattern After Long-Time (6 Years) Storage
3.2. Validation of the β-Thal Cell Biobank: Use of Biobanked Samples in Pre-Clinical Studies
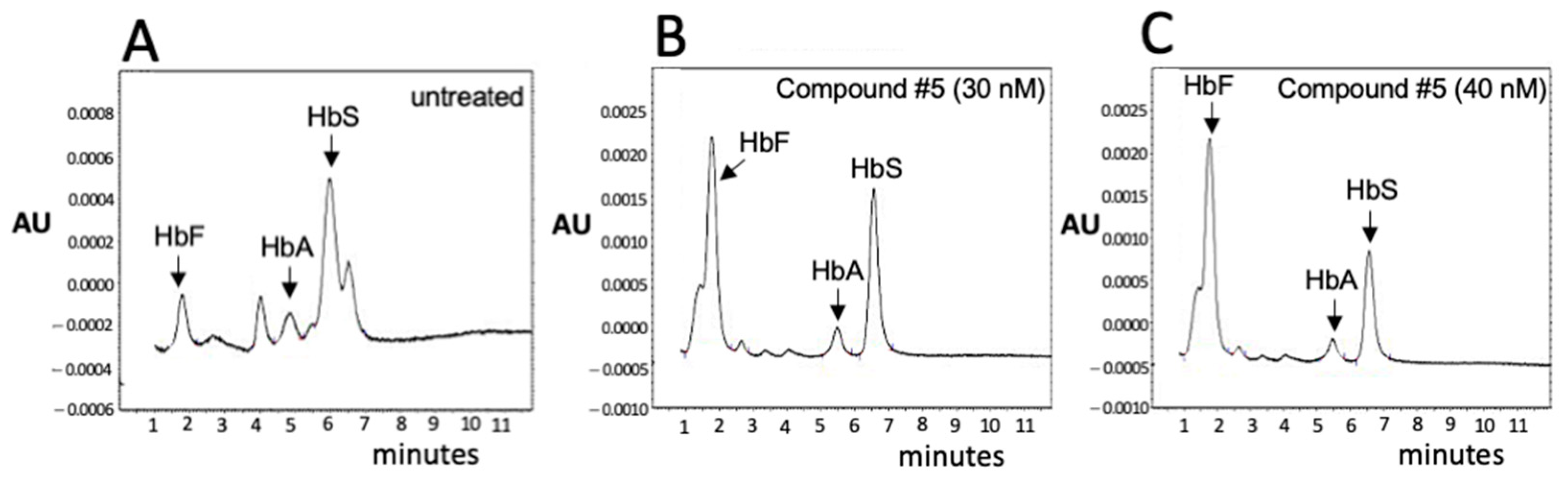
3.3. Validation of the β-Thal Cell Biobank: An Update on the Induction of Fetal Hemoglobin (HbF) Using Biobanked Samples
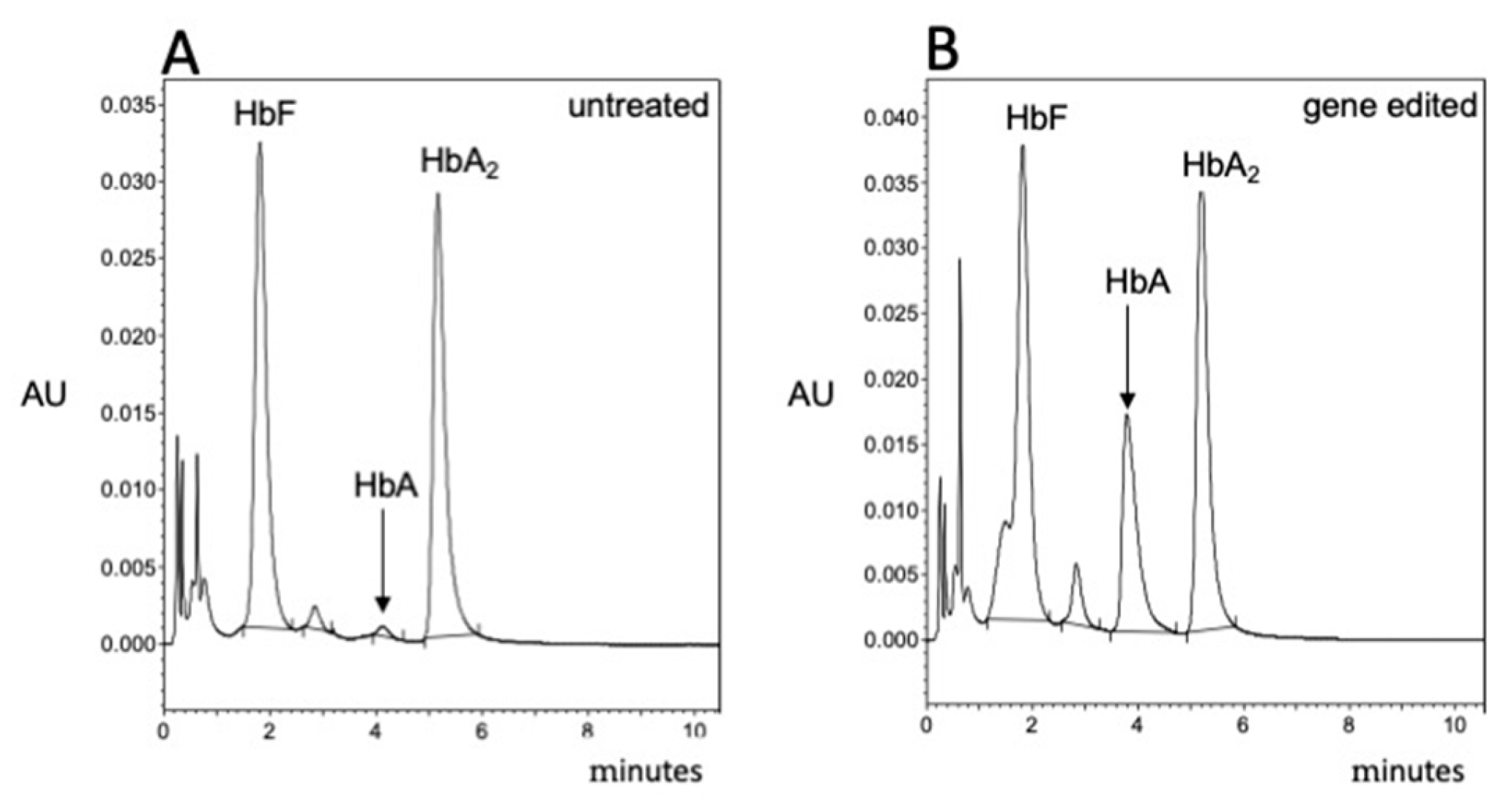
3.4. Validation of the β-Thal Cell Biobank: CRISPR-Cas9-Based Gene Editing Using Biobanked Samples
3.5. Updates on the Composition of the Cell β-Thal Cell Biobank
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al Diffalha, S.; Sexton, K.C.; Watson, P.H.; Grizzle, W.E. The importance of human tissue bioresources in advancing biomedical research. Biopreservation Biobanking 2019, 17, 209–212. [Google Scholar] [CrossRef]
- Paskal, W.; Paskal, A.M.; Dębski, T.; Gryziak, M.; Jaworowski, J. Aspects of modern biobank activity–comprehensive review. Pathol. Oncol. Res. 2018, 24, 771–785. [Google Scholar] [CrossRef]
- Compton, C.; Kelly, A. Biospecimen banking in the post-genome era. Genom. Pers. Med. 2013, 1, 229–236. [Google Scholar]
- Annaratone, L.; De Palma, G.; Bonizzi, G.; Sapino, A.; Botti, G.; Berrino, E.; Mannelli, C.; Arcella, P.; Di Martino, S.; Steffan, A.; et al. Basic principles of biobanking: From biological samples to precision medicine for patients. Virchows Arch. 2021, 479, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Han, C.; Yin, R.; Zhu, P.; Zhu, L.; Lu, Y.; Zheng, C. Quality Control of DNA Extracted from All-Cell Pellets After Cryopreservation for More Than 10 Years. Biopreservation Biobanking 2022, 20, 211–216. [Google Scholar] [CrossRef]
- Juurikka, K.; Åström, P.; Pekkala, T.; Öhman, H.; Sorsa, T.; Tervahartiala, T.; Salo, T.; Lehenkari, P.; Nyberg, P. Insights into Preservation of Blood Biomarkers in Biobank Samples. Biopreservation Biobanking 2022, 20, 297–301. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, S.; Dee, S.; Byrne, J.A.; Watson, P.H. How Many Health Research Biobanks Are There? Biopreservation Biobanking 2022, 20, 224–228. [Google Scholar] [CrossRef]
- Coppola, L.; Cianflone, A.; Grimaldi, A.M.; Incoronato, M.; Bevilacqua, P.; Messina, F.; Baselice, S.; Soricelli, A.; Mirabelli, P.; Salvatore, M. Biobanking in health care: Evolution and future directions. J. Transl. Med. 2019, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Catchpoole, D.R.; Reaiche-Miller, G.; Gilbert, T.; Ng, W.; Watson, P.H.; Byrne, J.A. What Do Biomedical Researchers Want from Biobanks? Results of an Online Survey. Biopreservation Biobanking 2022, 20, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.; Lucassen, A. Access to Biobanks: Responsibilities Within a Research Ecosystem. Biopreservation Biobanking 2023, 21, 275–281. [Google Scholar] [CrossRef]
- Gille, F.; Vayena, E.; Blasimme, A. Future-proofing biobanks’ governance. Eur. J. Hum. Genet. 2020, 28, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Roslan, F.F.; Yu, Y.; Ooi, G.C.; Then, K.L.; Then, K.Y.; Cheong, S.K.; Guo, Z.; Ab Patar, M.N.A.; Tan, J.J. From banked human cord blood to induced pluripotent stem cells: New opportunities and promise in induced pluripotent stem cell banking (Review). Int. J. Mol. Med. 2024, 54, 114. [Google Scholar] [CrossRef]
- Abraham, M.; Goel, S. Comprehensive characterisation and cryopreservation optimisation of buffalo (Bubalus bubalis) adipose tissue-derived mesenchymal stem cells. Cryobiology 2024, 115, 104896. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V. Human Schwann Cells in vitro II. Passaging, Purification, Banking, and Labeling of Established Cultures. Bio Protoc. 2023, 13, e4882. [Google Scholar] [CrossRef]
- D’Vaz, N.; Kidd, C.; Miller, S.; Amin, M.; Davis, J.A.; Talati, Z.; Silva, D.T.; Prescott, S.L. The ORIGINS Project Biobank: A Collaborative Bio Resource for Investigating the Developmental Origins of Health and Disease. Int. J. Environ. Res. Public Health 2023, 20, 6297. [Google Scholar] [CrossRef]
- Brophy, S.; Amet, R.; Foy-Stones, H.; Gardiner, N.; McElligott, A.M. Isolation and Cryopreservation of Mononuclear Cells from Peripheral Blood and Bone Marrow of Blood Cancer Patients. Methods Mol. Biol. 2023, 2645, 179–187. [Google Scholar]
- Degnin, M.; Agarwal, A.; Tarlock, K.; Meshinchi, S.; Druker, B.J.; Tognon, C.E. Novel Method Enabling the Use of Cryopreserved Primary Acute Myeloid Leukemia Cells in Functional Drug Screens. J. Pediatr. Hematol. Oncol. 2017, 39, e359–e366. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.R.; Burton, P.; Knoppers, B.M.; Lindpaintner, K.; Bledsoe, M.; Brookes, A.J.; Budin-Ljøsne, I.; Chisholm, R.; Cox, D.; Deschênes, M. Toward a roadmap in global biobanking for health. Eur. J. Hum. Genet. 2012, 20, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Zawati, M.H.; Tassé, A.M.; Mendy, M.; Caboux, E.; Lang, M.; on behalf of Biobank and Cohort Building Network Members. Barriers and opportunities in consent and access procedures in low- and middle-income country biobanks: Meeting notes from the BCNet training and general assembly. Biopreservation Biobanking 2018, 16, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Vodosin, P.; Jorgensen, A.K.; Mendy, M.; Kozlakidis, Z.; Caboux, E.; Zawati, M.H. A Review of Regulatory Frameworks Governing Biobanking in the Low and Middle Income Member Countries of BCNet. Biopreservation Biobanking 2021, 19, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Baldo, C.; Viotti, V.; Maioli, E.; Mogni, M.; Castagnetta, M.; Cavani, S.; Piombo, G.; Coviello, D. Galliera Genetic Bank: A DNA and cell line biobank from patients affected by genetic diseases. Open J. Bioresour. 2016, 3, e1–e5. [Google Scholar] [CrossRef]
- Bao, E.L.; Cheng, A.N.; Sankaran, V.G. The genetics of human hematopoiesis and its disruption in disease. EMBO Mol. Med. 2019, 11, e10316. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J. Phenotype-genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Genet. 2001, 2, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bunn, H.F.; Noguchi, C.T.; Hofrichter, J.; Schechter, G.P.; Schechter, A.N.; Eaton, W.A. Molecular and cellular pathogenesis of hemoglobin Sickle Cell disease. Proc. Natl. Acad. Sci. USA 1982, 79, 7527–7531. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.; Kumar Chandraker, S.; Misha Singh, M.; Kumar, R. Global distribution of β-thalassemia mutations: An update. Gene 2024, 896, 148022. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.W.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat. Commun. 2010, 1, 104. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.; Rice, M.; Haumschild, R.; Hoffer, D.; Morana, S.; Watkins, J. Optimizing the management of inherited blood disorders in a changing market: Findings from the AMCP Market Insights Program. J. Manag. Care Spec. Pharm. 2024, 30, S1–S12. [Google Scholar] [CrossRef] [PubMed]
- Musallam, K.M.; Viprakasit, V.; Lombard, L.; Gilroy, K.; Rane, A.; Vinals, L.; Tam, C.; Rizzo, M.; Coates, T.D. Systematic review and evidence gap assessment of the clinical, quality of life, and economic burden of alpha-thalassemia. EJHaem. 2024, 5, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Gamaleldin, M.; Abraham, I.; Meabed, M.; Elberry, A.; Abdelhalim, S.; Hussein, A.; Waggas, D.; Hussein, R. Cost-effectiveness analysis of Manuka honey-Omega-3 combination treatments in treating oxidative stress of pediatric β-thalassemia major. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 1144–1154. [Google Scholar] [PubMed]
- Cosenza, L.C.; Breda, L.; Breveglieri, G.; Zuccato, C.; Finotti, A.; Lampronti, I.; Borgatti, M.; Chiavilli, F.; Gamberini, M.R.; Satta, S.; et al. A validated cellular biobank for β-thalassemia. J. Transl. Med. 2016, 14, 255. [Google Scholar] [CrossRef]
- Gambari, R.; Waziri, A.D.; Goonasekera, H.; Peprah, E. Pharmacogenomics of Drugs Used in β-Thalassemia and Sickle-Cell Disease: From Basic Research to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 4263. [Google Scholar] [CrossRef]
- Sripichai, O.; Fucharoen, S. Fetal hemoglobin regulation in β-thalassemia: Heterogeneity, modifiers and therapeutic approaches. Expert. Rev. Hematol. 2016, 9, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, Z.; Ebrahimzadeh, M.A. Hemoglobin F (HbF) Inducers; History, Structure and Efficacies. Mini Rev. Med. Chem. 2022, 22, 52–68. [Google Scholar] [CrossRef]
- Langer, A.L.; Esrick, E.B. β-Thalassemia: Evolving treatment options beyond transfusion and iron chelation. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 600–606. [Google Scholar] [CrossRef]
- Bou-Fakhredin, R.; De Franceschi, L.; Motta, I.; Cappellini, M.D.; Taher, A.T. Pharmacological Induction of Fetal Hemoglobin in β-Thalassemia and Sickle Cell Disease: An Updated Perspective. Pharmaceuticals 2022, 15, 753. [Google Scholar] [CrossRef] [PubMed]
- Foong, W.C.; Loh, C.K.; Ho, J.J.; Lau, D.S. Foetal haemoglobin inducers for reducing blood transfusion in non-transfusion-dependent beta-thalassaemias. Cochrane Database Syst. Rev. 2023, 1, CD013767. [Google Scholar] [PubMed]
- CA22119 - Haemoglobinopathies in European Liaison of Medicine and Science (HELIOS). Available online: https://www.cost.eu/actions/CA22119/ (accessed on 9 December 2024).
- Kountouris, P.; Stephanou, C.; Archer, N.; Bonifazi, F.; Giannuzzi, V.; Kuo, K.H.M.; Maggio, A.; Makani, J.; Mañú-Pereira, M.D.M.; Michailidou, K.; et al. The International Hemoglobinopathy Research Network (INHERENT): An international initiative to study the role of genetic modifiers in hemoglobinopathies. Am. J. Hematol. 2021, 96, E416–E420. [Google Scholar] [CrossRef] [PubMed]
- Petelina, T.I.; Musikhina, N.A.; Avdeeva, K.S.; Shcherbinina, A.E.; Leonovich, S.V.; Zueva, E.V.; Garanina, V.D.; Gultiaeva, E.P.; Yaroslavskaya, E.I.; Kalyuzhnaya, E.N.; et al. Estimation of erythrocyte parameters of general blood analysis in patients with SARS-CoV-2 -associated pneumonia. Clin. Lab. Diagn. 2022, 67, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Delaye, J.B.; Alarcan, H.; Vallet, N.; Veyrat-Durebex, C.; Bernard, L.; Hérault, O.; Ropert, M.; Marlet, J.; Gyan, E.; Andres, C.; et al. Specific changes of erythroid regulators and hepcidin in patients infected by SARS-COV-2. J. Investig. Med. 2022, 70, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Elahi, S. Hematopoietic responses to SARS-CoV-2 infection. Cell Mol. Life Sci. 2022, 79, 187. [Google Scholar] [CrossRef] [PubMed]
- Allahyani, M.A.; Aljuaid, A.A.; Almehmadi, M.M.; Alghamdi, A.A.; Halawani, I.F.; Aldairi, A.F.; Alharbi, A.M.; Albshri, M.H.; Mutwalli, A.A.; Alhazmi, A.S. Detection of erythroid progenitors and erythrocytopathies in patients with severe COVID-19 disease. Saudi Med. J. 2022, 43, 899–906. [Google Scholar] [CrossRef]
- Saito, S.; Shahbaz, S.; Sligl, W.; Osman, M.; Tyrrell, D.L.; Elahi, S. Differential Impact of SARS-CoV-2 Isolates, Namely, the Wuhan Strain, Delta, and Omicron Variants on Erythropoiesis. Microbiol. Spectr. 2022, 10, e0173022. [Google Scholar] [CrossRef] [PubMed]
- Girón-Pérez, D.A.; Nava-Piedra, U.N.; Esquivel-Esparza, Z.E.; Benitez-Trinidad, A.B.; Barcelos-Garcia, R.G.; Vázquez-Pulido, E.Y.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Covantes-Rosales, C.E.; Barajas-Carrillo, V.W.; et al. Hematologic analysis of hospitalized patients and outpatients infected with SARS-CoV-2 and possible use as a prognostic biomarker. Exp. Hematol. 2023, 119–120, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.; Xu, L.; Osman, M.; Sligl, W.; Shields, J.; Joyce, M.; Tyrrell, D.L.; Oyegbami, O.; Elahi, S. Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Rep. 2021, 16, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Huerga Encabo, H.; Grey, W.; Garcia-Albornoz, M.; Wood, H.; Ulferts, R.; Aramburu, I.V.; Kulasekararaj, A.G.; Mufti, G.; Papayannopoulos, V.; Beale, R.; et al. Human Erythroid Progenitors Are Directly Infected by SARS-CoV-2: Implications for Emerging Erythropoiesis in Severe COVID-19 Patients. Stem Cell Rep. 2021, 16, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Balzanelli, M.G.; Distratis, P.; Dipalma, G.; Vimercati, L.; Inchingolo, A.D.; Lazzaro, R.; Aityan, S.K.; Maggiore, M.E.; Mancini, A.; Laforgia, R.; et al. Sars-CoV-2 Virus Infection May Interfere CD34+ Hematopoietic Stem Cells and Megakaryocyte-Erythroid Progenitors Differentiation Contributing to Platelet Defection towards Insurgence of Thrombocytopenia and Thrombophilia. Microorganisms 2021, 9, 1632. [Google Scholar] [CrossRef]
- Kronstein-Wiedemann, R.; Stadtmüller, M.; Traikov, S.; Georgi, M.; Teichert, M.; Yosef, H.; Wallenborn, J.; Karl, A.; Schütze, K.; Wagner, M.; et al. SARS-CoV-2 Infects Red Blood Cell Progenitors and Dysregulates Hemoglobin and Iron Metabolism. Stem Cell Rev. Rep. 2022, 18, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, J.P.; Mishra, N.; Tran, F.; Bahmer, T.; Best, L.; Blase, J.I.; Bordoni, D.; Franzenburg, J.; Geisen, U.; Josephs-Spaulding, J.; et al. Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity 2020, 53, 1296–1314.e9. [Google Scholar] [CrossRef]
- Eltobgy, M.; Johns, F.; Farkas, D.; Leuenberger, L.; Cohen, S.P.; Ho, K.; Karow, S.; Swoope, G.; Pannu, S.; Horowitz, J.C.; et al. Longitudinal transcriptomic analysis reveals persistent enrichment of iron homeostasis and erythrocyte function pathways in severe COVID-19 ARDS. Front. Immunol. 2024, 15, 1397629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shu, X.; Deng, R.; Yang, Z.; Shu, M.; Ou, X.; Zhang, X.; Wu, Z.; Zeng, H.; Shao, L. Transcriptome Changes of Hematopoietic Stem and Progenitor Cells in the Peripheral Blood of COVID-19 Patients by scRNA-seq. Int. J. Mol. Sci. 2023, 24, 10878. [Google Scholar] [CrossRef]
- Simeon-Dubach, D.; Zeisberger, S.M.; Hoerstrup, S.P. Quality Assurance in Biobanking for Pre-Clinical Research. Transfus. Med. Hemother. 2016, 43, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Grimaldi, A.M.; Sarnacchiaro, G.; di Fasano, M.S.; Smaldone, G.; Salvatore, M. An overview of Synlab SDN Biobank’s quality control system. Sci. Rep. 2024, 14, 19303. [Google Scholar] [CrossRef]
- Mouttham, L.; Garrison, S.J.; Archer, D.L.; Castelhano, M.G. A Biobank’s Journey: Implementation of a Quality Management System and Accreditation to ISO 20387. Biopreservation Biobanking 2021, 19, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Caixeiro, N.J.; Lai, K.; Lee, C.S. Quality assessment and preservation of RNA from biobank tissue specimens: A systematic review. J. Clin. Pathol. 2016, 69, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cosenza, L.C.; Tupini, C.; Finotti, A.; Sacchetti, G.; Simoni, D.; Gambari, R.; Lampronti, I. New Synthetic Isoxazole Derivatives Acting as Potent Inducers of Fetal Hemoglobin in Erythroid Precursor Cells Isolated from β-Thalassemic Patients. Molecules 2023, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Szuberski, J.; Oliveira, J.L.; Hoyer, J.D. A comprehensive analysis of hemoglobin variants by high-performance liquid chromatography (HPLC). Int. J. Lab. Hematol. 2012, 34, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Joutovsky, A.; Hadzi-Nesic, J.; Nardi, M.A. HPLC retention time as a diagnostic tool for hemoglobin variants and hemoglobinopathies: A study of 60000 samples in a clinical diagnostic laboratory. Clin Chem. 2004, 50, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cosenza, L.C.; Zurlo, M.; Gasparello, J.; Papi, C.; D’Aversa, E.; Breveglieri, G.; Lampronti, I.; Finotti, A.; Borgatti, M.; et al. Expression of γ-globin genes in β-thalassemia patients treated with sirolimus: Results from a pilot clinical trial (Sirthalaclin). Ther. Adv. Hematol. 2022, 13, 20406207221100648. [Google Scholar] [CrossRef]
- Cosenza, L.C.; Gasparello, J.; Romanini, N.; Zurlo, M.; Zuccato, C.; Gambari, R.; Finotti, A. Efficient CRISPR-Cas9-based genome editing of β-globin gene on erythroid cells from homozygous β039-thalassemia patients. Mol. Ther. Methods Clin. Dev. 2021, 21, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, L.C.; Zuccato, C.; Zurlo, M.; Gambari, R.; Finotti, A. Co-Treatment of Erythroid Cells from β-Thalassemia Patients with CRISPR-Cas9-Based β039-Globin Gene Editing and Induction of Fetal Hemoglobin. Genes 2022, 13, 1727. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, C.; Cosenza, L.C.; Zurlo, M.; Lampronti, I.; Borgatti, M.; Scapoli, C.; Gambari, R.; Finotti, A. Treatment of Erythroid Precursor Cells from β-Thalassemia Patients with Cinchona Alkaloids: Induction of Fetal Hemoglobin Production. Int. J. Mol. Sci. 2021, 22, 13433. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, L.C.; Marzaro, G.; Zurlo, M.; Gasparello, J.; Zuccato, C.; Finotti, A.; Gambari, R. Inhibitory effects of SARS-CoV-2 spike protein and BNT162b2 vaccine on erythropoietin-induced globin gene expression in erythroid precursor cells from patients with β-thalassemia. Exp. Hematol. 2024, 129, 104128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Peng, M. Outbreak of COVID-19: An emerging global pandemic threat. Biomed. Pharmacother. 2020, 129, 110499. [Google Scholar] [CrossRef]
- Gatto, M.; Bertuzzo, E.; Mari, L.; Miccoli, S.; Carraro, L.; Casagrandi, R.; Rinaldo, A. Spread and dynamics of the COVID-19 epidemic in Italy: Effects of emergency containment measures. Proc. Natl. Acad. Sci. USA 2020, 117, 10484–10491. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine "storm" and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Ropa, J.; Cooper, S.; Capitano, M.L.; Van’t Hof, W.; Broxmeyer, H.E. Human Hematopoietic Stem, Progenitor, and Immune Cells Respond Ex Vivo to SARS-CoV-2 Spike Protein. Stem Cell Rev. Rep. 2021, 17, 253–265. [Google Scholar] [CrossRef]
- Kucia, M.; Ratajczak, J.; Bujko, K.; Adamiak, M.; Ciechanowicz, A.; Chumak, V.; Brzezniakiewicz-Janus, K.; Ratajczak, M. An evidence that SARS-CoV-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia 2021, 35, 3026–3029. [Google Scholar] [CrossRef]
- Estep, B.K.; Kuhlmann, C.J.; Osuka, S.; Suryavanshi, G.W.; Nagaoka-Kamata, Y.; Samuel, C.N.; Blucas, M.T.; Jepson, C.E.; Goepfert, P.A.; Kamata, M. Skewed fate and hematopoiesis of CD34+ HSPCs in umbilical cord blood amid the COVID-19 pandemic. iScience 2022, 25, 105544. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M.A. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Zika, E.; Paci, D.; Schulte in den Bäumen, T.; Braun, A.; Rijkers-Defrasne, S.; Deschênes, M.; Fortier, I.; Laage-Hellman, J.; A Scerri, C.; Ibarreta Ruiz, D. Biobanks in Europe: Prospects for Harmonisation and Networking; JRC57831; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- Tzortzatou-Nanopoulou, O.; Akyüz, K.; Goisauf, M.; Kozera, Ł.; Mežinska, S.; Th Mayrhofer, M.; Slokenberga, S.; Reichel, J.; Croxton, T.; Ziaka, A.; et al. Ethical, legal, and social implications in research biobanking: A checklist for navigating complexity. Dev. World Bioeth. 2024, 24, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Gottweis, H.; Zatloukal, K. Biobank governance: Trends and perspectives. Pathobiology 2007, 74, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ducournau, P.; Strand, R. Trust, Distrust and Co-production: The Relationship Between Research Biobanks and Donors. In The Ethics of Research Biobanking; Solbakk, J., Holm, S., Hofmann, B., Eds.; Springer: Boston, MA, USA, 2009. [Google Scholar] [CrossRef]
- Hoeyer, K.; Olofsson, B.; Mjörndal, T.; Lynöe, N. The Ethics of Research Using Biobanks: Reason to Question the Importance Attributed to Informed Consent. Arch. Intern. Med. 2005, 165, 97–100. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.M.; Kolawole, O.I. Biobank donation in search of public benefits and the potential impact of intellectual property rights over access to health-technologies developed: A focus on the bioethical implications. Med. Law Rev. 2024, 32, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.; Liddicoat, J.; Liddell, K. An empirical study of large, human biobanks: Intellectual property policies and financial conditions for access. J. Law. Biosci. 2021, 8, lsab018. [Google Scholar] [CrossRef]
| THALAMOSS Project: Induction of HbF with LMW Drugs | Project: Induction of HbA with Readthrough-Based Molecules | Project: β039 CRISPR-Cas9 Genome Editing System | Project: Effects of SARS-CoV-2 Spike Protein on ErPCs | ||||
|---|---|---|---|---|---|---|---|
| Number of patients | Number of Vials | Number of patients | Number of Vials | Number of patients | Number of Vials | Number of patients | Number of Vials |
| 84 | 379 | 5 | 10 | 11 | 27 | 6 | 12 |
| Publications: Zuccato et al., 2021 [62]; Zuccato et al., 2022 [59]; Zuccato et al., 2023 [56] | Unpublished | Publications: Cosenza et al., 2021 [60]; Cosenza et al., 2022 [61] | Publications: Cosenza et al., 2024 [63] | ||||
| Cosenza et al., 2016 [26] | Present Composition of the Biobank | |||
|---|---|---|---|---|
| Genotype/Phenotype | Number of Patients | Number of Vials | Number of Patients | Number of Vials |
| β039/β039 | 29 | 260 | 103 | 421 |
| β+IVSI-110/β+IVSI-110 | 8 | 81 | 9 | 65 |
| β+IVSI-110/β039 | 17 | 191 | 25 | 51 |
| β+IVSI-6/β+IVSI-6 | 2 | 15 | 3 | 28 |
| β+IVSI-6/β039 | 4 | 67 | 5 | 24 |
| β+IVSI-110/β+IVSI-6 | 1 | 11 | 1 | 8 |
| β+IVSI-110/β+IVSI-1 | 1 | 7 | 1 | 7 |
| SCD/SCD | 2 | 13 | 3 | 17 |
| SCD/β039 | 1 | 7 | 2 | 10 |
| SCD/β+IVSI-110 | 1 | 5 | 2 | 8 |
| SCD/β+IVSI-6 | 1 | 7 | 1 | 7 |
| SCD/β+IVSI-1 | 2 | 20 | 2 | 15 |
| Others | 11 | 95 | 64 | 329 |
| Total | 80 | 779 | 221 | 990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambari, R.; Gamberini, M.R.; Cosenza, L.C.; Zuccato, C.; Finotti, A. A β-Thalassemia Cell Biobank: Updates, Further Validation in Genetic and Therapeutic Research and Opportunities During (and After) the COVID-19 Pandemic. J. Clin. Med. 2025, 14, 289. https://doi.org/10.3390/jcm14010289
Gambari R, Gamberini MR, Cosenza LC, Zuccato C, Finotti A. A β-Thalassemia Cell Biobank: Updates, Further Validation in Genetic and Therapeutic Research and Opportunities During (and After) the COVID-19 Pandemic. Journal of Clinical Medicine. 2025; 14(1):289. https://doi.org/10.3390/jcm14010289
Chicago/Turabian StyleGambari, Roberto, Maria Rita Gamberini, Lucia Carmela Cosenza, Cristina Zuccato, and Alessia Finotti. 2025. "A β-Thalassemia Cell Biobank: Updates, Further Validation in Genetic and Therapeutic Research and Opportunities During (and After) the COVID-19 Pandemic" Journal of Clinical Medicine 14, no. 1: 289. https://doi.org/10.3390/jcm14010289
APA StyleGambari, R., Gamberini, M. R., Cosenza, L. C., Zuccato, C., & Finotti, A. (2025). A β-Thalassemia Cell Biobank: Updates, Further Validation in Genetic and Therapeutic Research and Opportunities During (and After) the COVID-19 Pandemic. Journal of Clinical Medicine, 14(1), 289. https://doi.org/10.3390/jcm14010289







