Assessing the Diagnostic Performance of Automated Pituitary Gland Volume Measurement for Idiopathic Central Precocious Puberty
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Neuroimaging
2.3. GnRH Stimulation Test
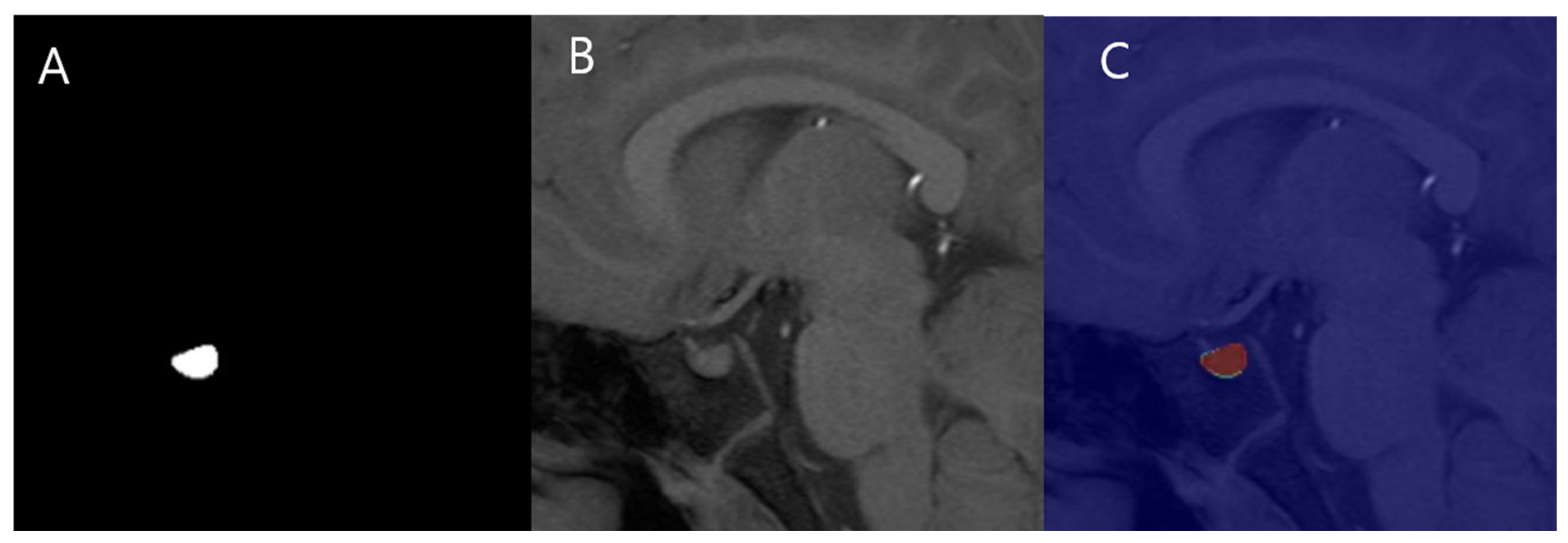
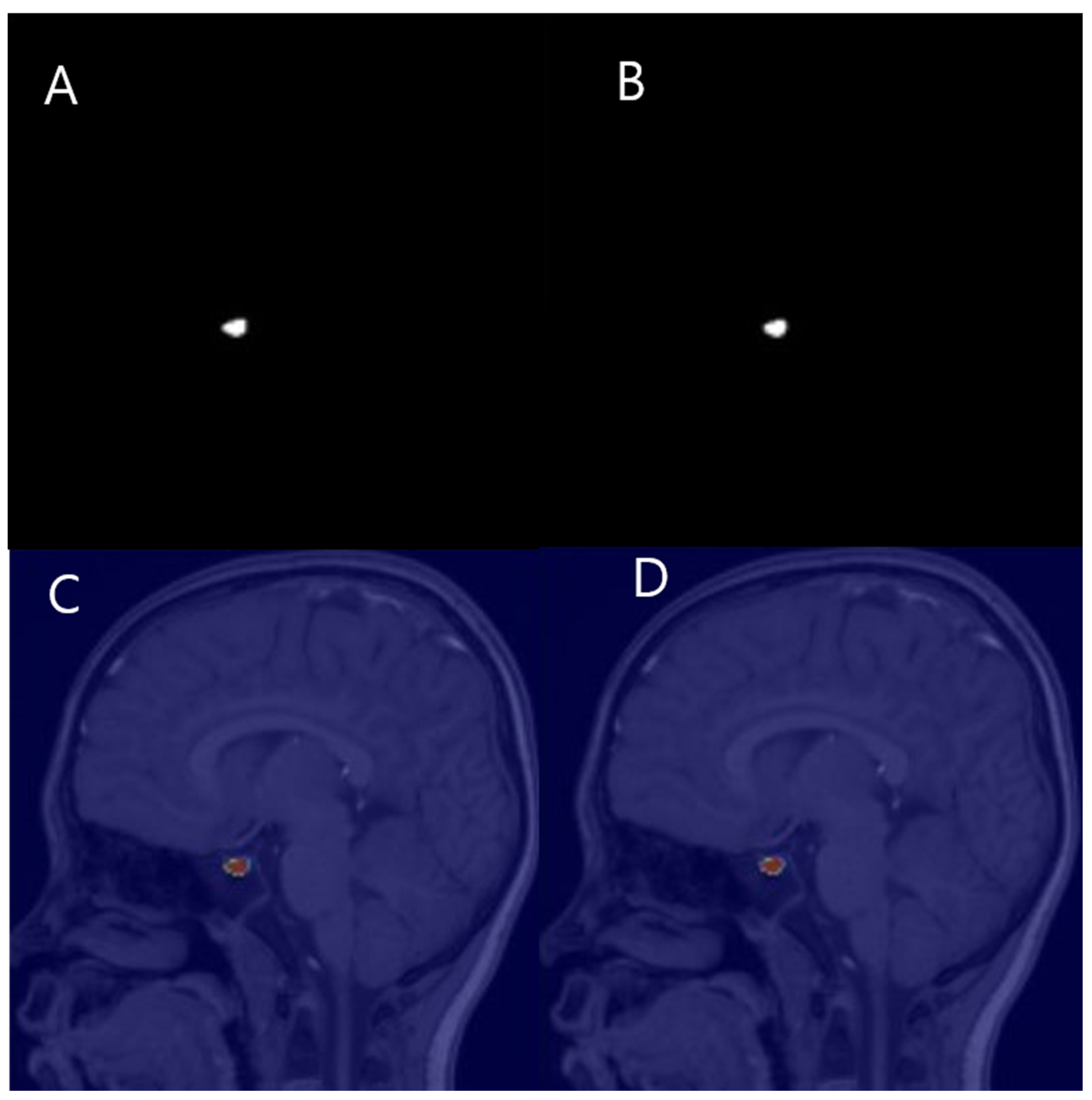
2.4. Manual PV Measurement
2.5. Automated PV Measurement
2.5.1. Preprocessing
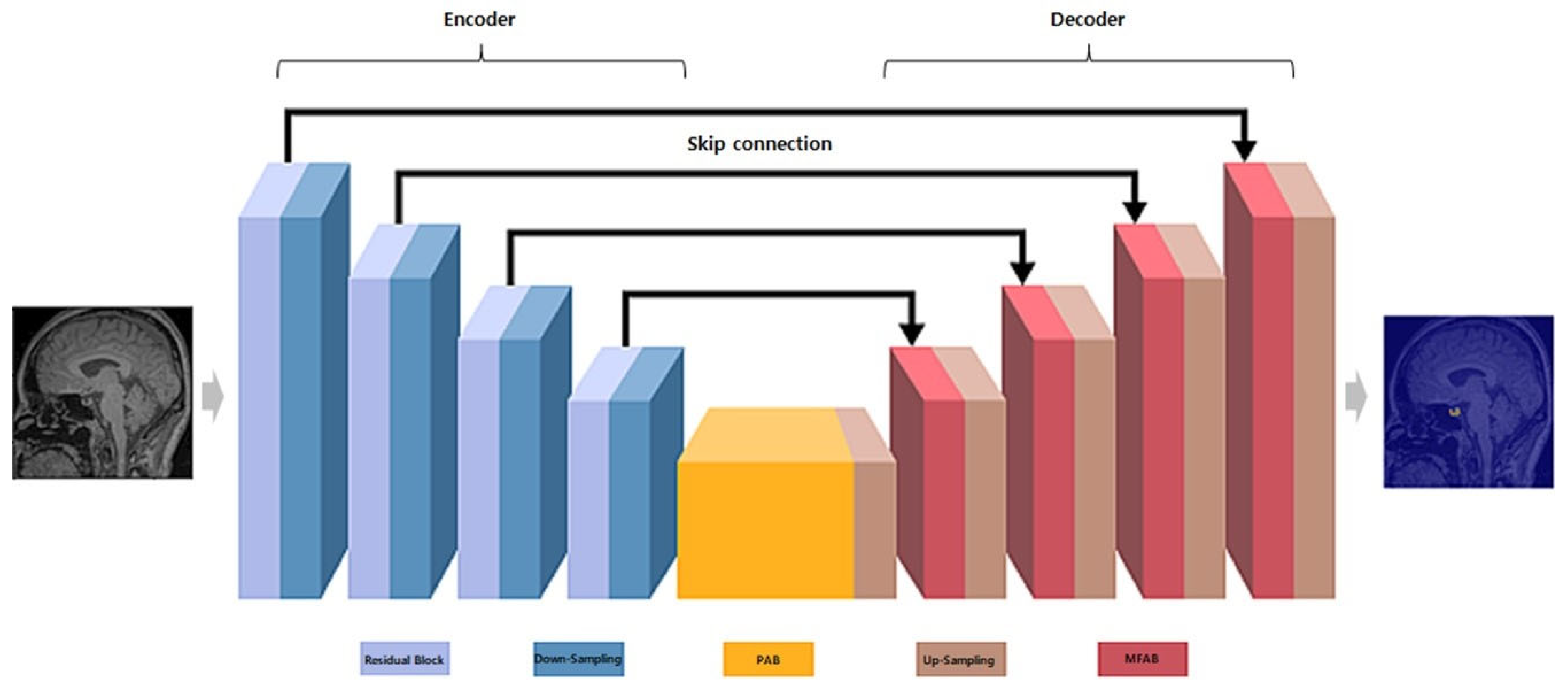
2.5.2. MANet Architecture
- Encoder: Utilizes a series of convolutional layers with varying kernel sizes to capture features at multiple scales, followed by pooling layers for down-sampling and reducing spatial dimensions [12].
- Decoder: Employs up-sampling layers and skip connections from the encoder to reconstruct the segmentation map, preserving spatial information and enabling precise edge detection [12].
- Attention block: Implements spatial and channel-wise attention mechanisms to emphasize relevant features and suppress irrelevant ones, thereby enhancing the capture of characteristics related to the pituitary gland.
2.5.3. Training
2.5.4. Inference
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GnRH | gonadotropin-releasing hormone |
| PV | pituitary gland volume |
| IPP | idiopathic central precocious puberty |
| PSM | propensity score matching |
References
- Wu, S.; Yang, Y.; Wang, Y. Diagnostic Value of Pituitary Volume in Girls with Precocious Puberty. BMC Pediatr. 2020, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Herman-Giddens, M.E.; Slora, E.J.; Wasserman, R.C.; Bourdony, C.J.; Bhapkar, M.V.; Koch, G.G.; Hasemeier, C.M. Secondary Sexual Characteristics and Menses in Young Girls Seen in Office Practice: A Study from the Pediatric Research in Office Settings Network. Pediatrics 1997, 99, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Q.; Li, G.M.; Li, Y. Advanced Bone Age as an Indicator Facilitates the Diagnosis of Precocious Puberty. J. Pediatr. (Rio J.) 2018, 94, 69–75. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, X.; Wang, M.; Feng, Q.; Lin, C. Noninvasive Radiomics-Based Method for Evaluating Idiopathic Central Precocious Puberty in Girls. J. Int. Med. Res. 2021, 49, 300060521991023. [Google Scholar] [CrossRef] [PubMed]
- Sharafuddin, M.J.; Luisiri, A.; Garibaldi, L.R. MR Imaging Diagnosis of Central Precocious Puberty: Importance of Changes in the Shape and Size of the Pituitary Gland. AJR Am. J. Roentgenol. 1994, 162, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Peper, J.S.; Brouwer, R.M.; van Leeuwen, M. HPG-Axis Hormones During Puberty: A Study on the Association with Hypothalamic and Pituitary Volumes. Psychoneuroendocrinology 2010, 35, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tien, R.D.; Kucharczyk, J.; Bessette, J. MR Imaging of the Pituitary Gland in Infants and Children: Changes in Size, Shape, and MR Signal with Growth and Development. AJR Am. J. Roentgenol. 1992, 158, 1151–1154. [Google Scholar] [CrossRef][Green Version]
- Kim, M.S.; Sung, K.J. MR Measurement of Normal Pituitary Gland Height on Midsagittal Section: Age and Sex Differentiation. J. Korean Radiol. Soc. 1992, 28, 523–526. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, H.V.; Summers, R.M. Challenges and Opportunities in Medical Image Analysis Using Deep Learning. Annu. Rev. Biomed. Eng. 2016, 18, 221–248. [Google Scholar]
- Rubin, C.B. Manual vs. Automated Segmentation Methods in Medical Imaging. J. Digit. Imaging 2012, 25, 545–552. [Google Scholar]
- He, L.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Fan, T.; Li, Y.; Wang, H. MA-Net: A Multi-Scale Attention Network for Liver and Tumor Segmentation. IEEE Access 2020, 8, 179656–179665. [Google Scholar] [CrossRef]
- Qubvel. Segmentation Models Pytorch. Available online: https://github.com/qubvel-org/segmentation_models.pytorch (accessed on 1 May 2023).
- Muneuchi, J.; Nagatomo, Y.; Okada, S. Increased Pituitary Volumes in Children After Fontan Operation: Congestion in the Other Portal Circulation. J. Pediatr. 2018, 193, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Sari, E.; Akgun, V. Measures of Pituitary Gland and Stalk: From Neonate to Adolescence. J. Pediatr. Endocrinol. Metab. 2014, 27, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.M.; Vidmar, S.; Kumbla, S. Age-Related Pituitary Volumes in Prepubertal Children with Normal Endocrine Function: Volumetric Magnetic Resonance Data. J. Clin. Endocrinol. Metab. 2005, 90, 3274–3278. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xiu, J.; Huang, Z. Three-Dimensional Magnetic Resonance Volumetry of the Pituitary Gland is Effective in Detecting Short Stature in Children. Exp. Ther. Med. 2014, 8, 551–556. [Google Scholar] [CrossRef]
- Fehrenbach, U.; Jadan, A.; Auer, T.A. Obesity and Pituitary Gland Volume: A Correlation Study Using Three-Dimensional Magnetic Resonance Imaging. Neuroradiol. J. 2020, 33, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, E.M.; Haukedal, M.D.; Haberg, A.K. Normative Data for Pituitary Size and Volume in the General Population Between 50 and 66 Years. Pituitary 2021, 24, 737–745. [Google Scholar] [CrossRef]
- Yadav, P.; Chauhan, S.; Harit, S. MRI Evaluation of Size and Shape of Normal Pituitary Gland: Age and Sex Related Changes. J. Clin. Diagn. Res. 2017, 11, 1–4. [Google Scholar] [CrossRef]
- Naik, D.; Reddy, P.D.; Srinath, M.G. Pituitary Gland Assessment by MR Volumetry in the Normal Indian Adolescent Population. Int. J. Med. Imaging 2015, 3, 105–109. [Google Scholar] [CrossRef][Green Version]
- Ertekin, T.; Acer, N.; Turgut, A.T. Comparison of Three Methods for the Estimation of the Pituitary Gland Volume Using Magnetic Resonance Imaging: A Stereological Study. Pituitary 2011, 14, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Okur Akşan, İ. The Assessment of the Pituitary Volume in Normal Prepubertal Children with Three-Dimensional MRI. Cukurova Med. J. 2021, 46, 912–919. [Google Scholar] [CrossRef]
- Carel, J.C.; Lahlou, N.; Roger, M.; Chaussain, J.L. Precocious Puberty and Statural Growth. Hum. Reprod. Update 2004, 10, 135–147. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Zhang, Y.; Zhu, H. Medical Image Analysis Using Deep Learning Algorithms. Front. Public Health 2023, 11, 1273253. [Google Scholar] [CrossRef] [PubMed]

| Precontrast T1 Sagittal Image | ||
|---|---|---|
| SL, mm | 1 | 3 |
| TR, ms | 1690 | 271 |
| TE, ms | 3.1 | 2.9 |
| Matrix | 352 × 246 | 512 × 264 |
| FOV, mm | 180 | 2 |
| Section number | 1 | 2 |
| Flip angle | 150 | 75 |
| Control | IPP | Total | |
|---|---|---|---|
| Number of patients | 52 | 226 | 278 |
| Sex (female) | 15/52 | 109/226 | 124/278 |
| Age, year (range) a | 8 (7–9) | 8 (8–9) | 8 (8–9) |
| Height (cm) a,* | 124 (119–136) | 138 (132–142) | 136 (128–141) |
| Weight (kg) a,* | 27 (22–37) | 35 (29–45) | 35 (27–44) |
| ICC | CI 95% | Actual Value 0 for F-test | |||||
|---|---|---|---|---|---|---|---|
| LLCI | HLCI | F | df1 | df2 | p-Value | ||
| Single measure | 0.993 | 0.988 | 0.996 | 285.787 | 56 | 56 | <0.001 * |
| Average measure | 0.997 | 0.994 | 0.998 | 285.787 | 56 | 56 | <0.001 * |
| Control | IPP | |
|---|---|---|
| Pituitary Gland Volume | ||
| Height | 0.439 (0.001) ** | 0.005 (0.93) |
| Weight | 0.251 (0.07) | 0.140 (0.03) * |
| Age | 0.347 (0.01) * | 0.174 (0.009) ** |
| Control | IPP | Total | |
|---|---|---|---|
| PV a,* | 380 (301–460) | 432 (352–493) | 427 (347–488) |
| Method | Description | Advantages | Limitations |
|---|---|---|---|
| Evaluation of Pituitary Shape | Assesses pituitary shape based on the surface outline in the midline plane, divided into 5 stages: 1. Distinct concave; 2. Slightly concave; 3. Flat; 4. Slightly convex; 5. Marked convex | Simple and quick to evaluate by visual grading | Subjective method with potential for observer bias |
| Height Measurement Method | Measures the longest vertical distance between the base and apex of the pituitary gland in the midline sagittal plane using T1-weighted images | Simple to perform; provides numerical values | May not accurately reflect total PV |
| Elliptic Formula Method | Measures the maximum height and length in the mid-sagittal plane and maximum width in the coronal plane. PV is calculated as follows: height × length × width/2 | Effective for ellipsoid-shaped glands | Provides an estimated value; may not account for all shape variability |
| Planimetry Method | Uses a 3D polygonal ROI tool to manually draw the pituitary’s entire volume, layer by layer, on sagittal slices. PV is calculated using the ROI and section thickness | Accurate and considers entire gland structure | Time-consuming and requires manual effort |
| Point Counting Method | Quantifies pituitary volume through manual measurement and point counting on MRI images | Objective and numerical | Labor-intensive and repetitive; not efficient for large datasets |
| Automated Measurement Methods | Utilizes automated techniques to calculate pituitary volume from imaging | Saves time and effort; reduces manual workload | May require advanced software and expertise to implement effectively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Yu, I. Assessing the Diagnostic Performance of Automated Pituitary Gland Volume Measurement for Idiopathic Central Precocious Puberty. J. Clin. Med. 2025, 14, 15. https://doi.org/10.3390/jcm14010015
Kim H, Yu I. Assessing the Diagnostic Performance of Automated Pituitary Gland Volume Measurement for Idiopathic Central Precocious Puberty. Journal of Clinical Medicine. 2025; 14(1):15. https://doi.org/10.3390/jcm14010015
Chicago/Turabian StyleKim, Hayoun, and Inkyu Yu. 2025. "Assessing the Diagnostic Performance of Automated Pituitary Gland Volume Measurement for Idiopathic Central Precocious Puberty" Journal of Clinical Medicine 14, no. 1: 15. https://doi.org/10.3390/jcm14010015
APA StyleKim, H., & Yu, I. (2025). Assessing the Diagnostic Performance of Automated Pituitary Gland Volume Measurement for Idiopathic Central Precocious Puberty. Journal of Clinical Medicine, 14(1), 15. https://doi.org/10.3390/jcm14010015






