Abstract
Background/Objectives: The characterization of patients with inflammatory bowel disease (IBD) and type 2 diabetes mellitus (T2DM) as a new group has not been well detailed. This study aimed to evaluate the impact of T2DM on IBD progression and analyze the prevalence of steatotic liver disease and liver damage in these patients. Methods: Through a retrospective case–control study, we compared severe IBD occurrence in patients with both IBD-T2DM (cases) versus those with IBD alone (controls). Among 1047 medical records, 79 IBD-T2DM patients were selected and compared to 308 controls in a 1:4 ratio. Severe IBD was defined by variables such as surgery, target therapy, corticosteroid use, and hospitalization. Liver damage was assessed using Fib-4 (>1.3), and hepatic steatosis was evaluated by imaging. Results: There was no significant difference in severe disease rates (59.5% vs. 59.7%; p = 0.97). IBD-T2DM patients had higher rates of hepatic steatosis (62.9% vs. 27.2%; p < 0.0001) and liver damage (55.4% vs. 26.6%; p < 0.0001). IBD-T2DM patients used more corticosteroids (p < 0.0001) and fewer anti-TNF-alpha drugs (p = 0.007). The median age at diagnosis was higher in IBD-T2DM patients (48 vs. 32; p < 0.0001). In Crohn’s disease, 24.3% of IBD-T2DM patients had exclusive colonic involvement compared to 5% in the IBD-only group (p = 0.003). Conclusions: T2DM was not associated with worse IBD progression, but was linked to increased liver steatosis and damage. Differences such as age of onset, colonic involvement, and liver damage suggest that IBD-T2DM patients could configure a special population worthy of further studies.
1. Introduction
Both inflammatory bowel disease (IBD) and type 2 diabetes mellitus (T2DM) are chronic, multifactorial diseases influenced by environmental factors acting on genetic predisposition.
A diet rich in fats, particularly animal fats, and polyunsaturated fatty acids has been associated with an increased incidence of Crohn’s disease (CD) and ulcerative colitis (UC), as well as type 2 diabetes mellitus (T2DM), due to its influence of increasing the systemic inflammatory status [1]. Certain dietary components can indeed affect the structure and function of the microbiota, with downstream effects on immune activity [2]. A factor implicated in both diseases is intestinal dysbiosis; both diseases share an increase in the presence of microbial species with pathogenic potential, such as Ruminococcus gnavus, which utilizes mucin and dietary glycans and produces bacteriocins and adhesins. These compounds elicit a pro-inflammatory host response and modulate host metabolism of bile acids and tryptophan metabolic pathways [3,4]. Conversely, there is a decrease in Akkermansia muciniphila, a bacterium that plays a beneficial role in maintaining intestinal mucosal integrity and reducing the production of pro-inflammatory substances [5].
The growing interest in the management of comorbidities, within an increasingly elderly population, has prompted several research groups to investigate the relationship between these diseases.
In a cohort study conducted in Denmark on 6,028,844 people, a higher number of diabetic subjects was observed in the cohort of IBD patients compared to the general population, resulting in a 50% higher risk of IBD patients developing T2DM [6]. In another cohort study conducted in South Korea, an increased relative risk (RR) of 1.09 was observed in IBD patients for developing T2DM: 1.41 in CD patients and 1.04 in UC patients [7]. Additionally, in a cohort study conducted in China, involving 454,804 individuals, CD patients showed an increased risk of T2DM compared to the general population, as well as UC patients [8].
Corticosteroid therapy is one of the main assumptions underlying the link between IBD and T2DM, as steroids are currently a first-line therapy to induce remission in IBD exacerbations [9,10]. The effect of systemic corticosteroids (but also, partially, topical corticosteroids) of raising blood glucose has been widely demonstrated [11].
In mouse models, hyperglycemia has been observed to worsen the clinical outcomes of IBD activity, presumably acting on the integrity of the colonic epithelial barrier [12]. However, the role of T2DM in the course of IBD in clinical trials is controversial. For example, in Uwagbale et al.’s study, patients with IBD and T2DM were found to have a lower risk of IBD-related complications and a lower risk of undergoing IBD-related surgery, compared to patients with isolated IBD; on the other hand, an increase in septic complications was highlighted [13]. An increase in septic complications was also described by Kumar et al., together with an increase in hospitalizations, IBD-related complications, and disease flare-ups, despite a non-significant difference in the incidence of IBD-related surgeries [14]. Fuschillo et al. conducted a meta-analysis, where it emerged that T2DM in patients with IBD did not worsen the course of the disease in terms of IBD-related complications, IBD-related surgery, and mortality, despite representing an independent risk factor for IBD-related hospitalizations and sepsis [15].
The characterization of the new category of patients with IBD and T2DM has not been well detailed yet: studies on the effects of diabetes on inflammatory disease have yielded conflicting results. Among the possible explanations, the heterogeneity of IBD clinical presentations stands out, along with the variability in the criteria for endpoint attribution and the description of results [15]. Therefore, to assess how T2DM might influence the course of intestinal disease, we selected the definition of severe IBD as the primary objective of our study, independent of the increased comorbidity risk associated with T2DM.
Furthermore, both IBD and T2DM patients are at an elevated risk for liver disease compared to the general population [16,17,18], yet the extent of these complications in patients with both conditions has been poorly investigated. Consequently, as a secondary endpoint, we examined the prevalence of steatotic liver disease (SLD) and liver damage in patients with IBD and T2DM.
2. Materials and Methods
We conducted a retrospective case–control study to compare patients with both IBD and T2DM to patients who had IBD without T2DM. We reviewed the medical records of patients followed at the IBD Clinical Center of the ‘San Giovanni Antica Sede’ Hospital, AOU Città della Salute e della Scienza in Turin, Italy.
2.1. Inclusion and Exclusion Criteria
Inclusion in cases:
- -
- Minimum follow-up period of 1 year;
- -
- Confirmed diagnosis of IBD according to the 2019 ECCO-ESGAR guidelines [19];
- -
- History of T2DM at the time of their last visit.
Exclusion in cases:
- -
- Inability to determine severe disease according to a composite outcome that considers IBD manifestations and complications described by Yarur et al. (see Section 2.3) [20].
Inclusion in controls:
- -
- Minimum follow-up period of 1 year;
- -
- Confirmed diagnosis of IBD according to the 2019 ECCO-ESGAR guidelines [19].
Exclusion in controls:
- -
- Inability to determine severe disease according to a composite outcome that considers IBD manifestations and complications described by Yarur et al. (see Section 2.3) [20];
- -
- Medical history of T2DM or iatrogenic diabetes;
- -
- Past/current therapy with hypoglycemic drugs.
Figure 1 presents the patient qualification flow chart.
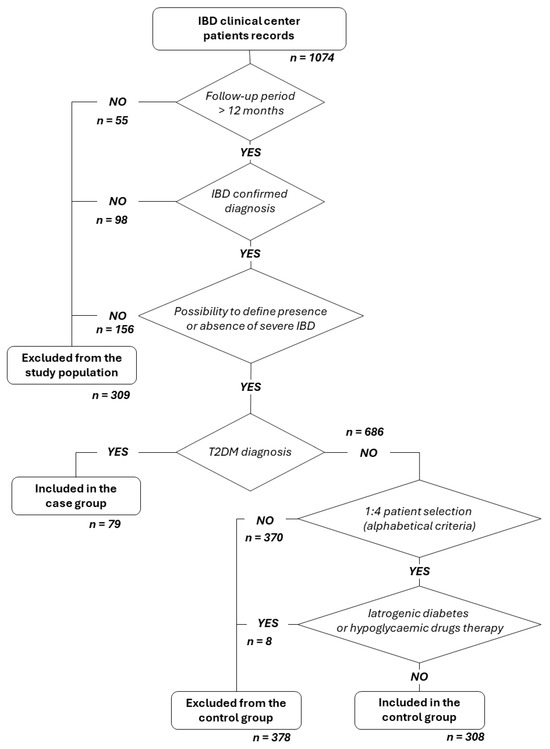
Figure 1.
Patient qualification flow chart.
The control group was randomly selected from selected IBD patients (in alphabetical order) at an approximate ratio of 1:4 between cases and controls.
2.2. Type and Source of Data
In Table 1 type and source of data are listed.

Table 1.
Type and source of data.
2.3. Endpoint Definition
The primary endpoint of this study was to determine the prevalence of severe IBD in IBD-T2DM patients compared to a control group of IBD patients without T2DM. For this purpose, we used a composite outcome that considers IBD manifestations and complications described by Yarur et al. in a systematic review aimed at establishing predictors of aggressive IBD [20]. Thus, patients were considered to have severe IBD if at least one of the following conditions occurred during the entire follow-up period:
Need for IBD-related surgery; need for immunosuppressive drugs/biologics/small molecules as maintenance therapy; more than two courses of systemic corticosteroids during the entire duration of follow-up; need for hospitalization for flare-ups or complications after initial diagnosis; at least one of the following: presence of persistent diarrhea for more than 12 months with nocturnal awakenings or urgency, intestinal stricture with symptoms.
Moreover, we chose the presence of liver damage and the presence of hepatic steatosis as secondary endpoints.
We defined liver damage through the use of the FIB4 score, calculated as age in years × AST in U/L/platelets in ×10⁹/L × √ALT in U/L [21]; in this study, a result higher than 1.3 determined the presence of liver damage. This endpoint was evaluated only in patients whose records reported AST, ALT, and platelet count values at least once. Where multiple measurements were reported, the most recent ones were taken into consideration.
We took into consideration the presence of SLD if steatosis was described in medical history, and if examinations by abdominal ultrasound or FibroScan® were suggestive of it. Patients who had never undergone ultrasound/Fibroscan were not evaluated. The absence of steatosis was defined for those patients who underwent at least one of the previous imaging investigations and in whom hepatic steatosis was not described in their medical history, the liver was normal, or the CAP values were normal.
2.4. Statistical Analysis
Continuous variables were reported as mean (standard deviation (SD)) or median (interquartile range (IQR)) based on the distribution of values. Normality was verified using the D’Agostino–Pearson test. Categorical variables were reported as numbers and percentages.
Comparison between independent categorical variables was performed using the Chi-square test. Comparison between continuous variables was performed using the independent samples t-test or the Mann–Whitney test based on the distribution of values.
The association between variables was tested using the proportional hazard model or Cox regression; therefore, the strength of the association was reported as a hazard ratio (HR) with a 95% confidence interval (95% CI).
All statistical analyses were performed using MedCalc® v. 20.104 (MedCalc Software Ltd., Ostend, Belgium), and a p value ≤ 0.05 was considered statistically significant.
3. Results
We analyzed 1047 medical records, from which we selected 79 patients with IBD-T2DM (confirmed diagnosis of both diseases), comparing the selected population with 308 patients with IBD alone, randomly chosen in a 1:4 ratio. Baseline characteristics of the entire study population (n = 387) are reported in Table 2.

Table 2.
Baseline characteristics of the study population.
In Table 3, a comparison of baseline characteristics in cases and controls is shown.

Table 3.
Comparison of baseline characteristics between the two groups.
We observed a statistically significant difference in the disease localization of CD in the two groups (Figure 2).
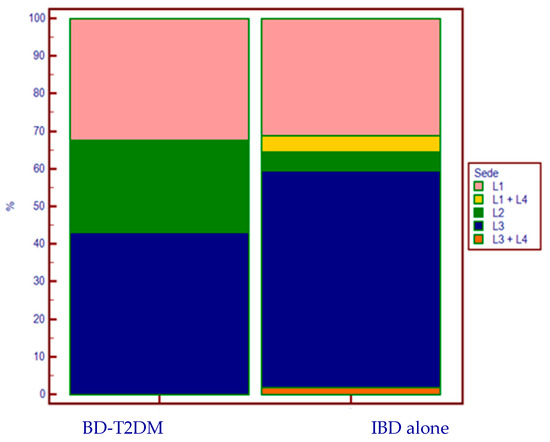
Figure 2.
CD’s localization according to the presence (or absence) of T2DM.
As regards the specific therapy for intestinal disease (Table 3), there was a greater current and past use of systemic corticosteroids (CSs) in the T2DM group compared to the group with IBD alone (p < 0.0001) (Figure 3).
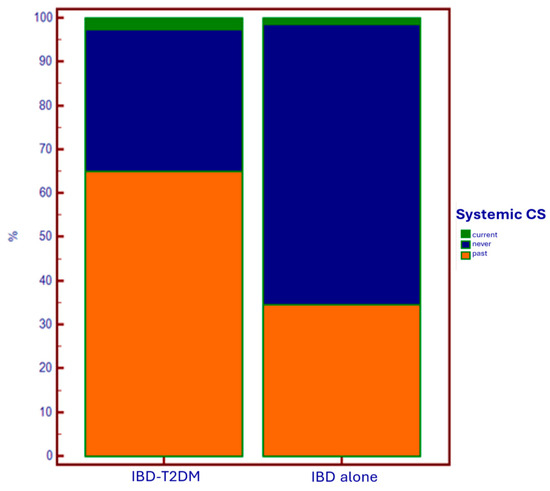
Figure 3.
Use of systemic corticosteroids based on the presence of T2DM.
Lower use of anti-TNF-alpha drugs was observed in the T2DM group, both in current therapy and in the past compared to controls (p = 0.007) (Figure 4).
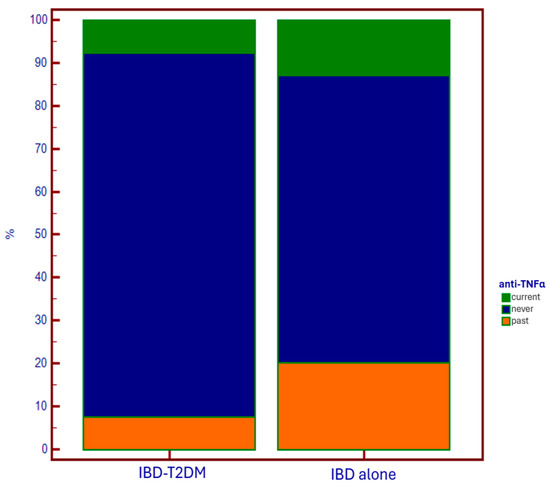
Figure 4.
Use of anti-TNF-alpha drugs based on the presence of T2DM.
3.1. T2DM Patients
We were able to exactly determine when the diagnosis of T2DM occurred in 35 out of 79 patients. The average age at diagnosis was 56.2 years (±12.6). Among them, 25 patients were diagnosed after IBD diagnosis, with a mean IBD-T2DM latency of 18.0 years (±11.1). There were also 10 patients who developed diabetes before bowel disease, with a median latency of −3.0 years (IQR: −8.0 to −1.0).
As regards specific therapies for diabetes, the absolute and relative frequencies by pharmacological class found in the study group are reported in Supplementary Table S1.
3.2. Metabolic Parameters
For all the preset metabolic parameters, except for alcohol intake, we observed a significant difference between the two groups (see Table 4).

Table 4.
Metabolic parameters in participants.
3.3. Severe IBD
In Table 5, the comparison of baseline characteristics based on the presence or absence of severe IBD is reported.

Table 5.
Characteristics regarding the presence or absence of severe IBD.
We performed a univariate analysis (in Table 6) for those variables which, using the Chi-square test and the T-test, showed a statistically significant association for the outcome “severe IBD”.

Table 6.
Univariate analysis based on the presence of severe IBD for the duration of follow-up.
The multivariate analysis regarding predictors of severe IBD is reported in Table 7.

Table 7.
Multivariate analysis based on the presence of severe IBD for the duration of follow-up.
3.4. Liver Damage
Table 8 describes the comparison of baseline characteristics based on the presence or absence of liver damage defined by a Fib 4 score > 1.3.

Table 8.
Characteristics based on the presence or absence of Fib 4 > 1.3.
Then, we performed a univariate analysis for the variables that, in the Chi-square test and the T-test, showed a statistically significant association with the outcome Fib-4 > 1.3. As reported in Table 9, three dichotomous variables and one continuous variable were identified as significant in increasing the risk of liver damage during the duration of follow-up.

Table 9.
Univariate analysis of predictors of Fib-4 > 1.3 for duration of follow-up.
In Table 10, the multivariate analysis of predictors for Fib-4 > 1.3 is described.

Table 10.
Multivariate analysis of predictors of Fib-4 > 1.3.
4. Discussion
Median age at IBD diagnosis was significantly higher in cases (48.0), while in controls (32.0; p < 0.0001), the median age fell within the range reported in epidemiology for this pathology (25–35 years) [22]. This result has not been described in the literature to date.
In the sub-analysis in patients with CD, there was a notable difference in the disease location of activity between cases and controls: 24.3% of the CD-DMT2 group had a disease localized exclusively at the colonic level (L2), compared to 5% in the group without T2DM (p = 0.003). Furthermore, no upper-intestinal localization (L4) was found in any case, whereas it was present in 10 patients in the control group (6.2%). This distribution does not reflect the percentages described in the literature [22,23], and could suggest another distinct characteristic of this special subpopulation.
Regarding specific therapy for IBD, in diabetic patients, we observed a wider (current and especially past) administration of systemic corticosteroids, and a lower administration of anti-TNF-alpha drugs. The first data could support an iatrogenic explanation of the epidemiological relationship between IBD and T2DM. On the other hand, in patients using anti-TNF-alpha as maintenance therapy for disease control, there is a lower need for steroids. This results in a reduced need to undergo steroid courses due to disease flare-ups, which has a favorable impact on reducing the risk of developing diabetes associated with steroid administration.
The reason why anti-TNF-alpha agents are underutilized in patients with diabetes is likely due to the increased fragility of these patients, both because of the immune system deficiency that makes them more susceptible to infections (to which anti-TNF-alpha agents could further expose them), and due to the older age of this population, which is associated with the presence of multiple comorbidities, including those in which anti-TNF-alpha agents are contraindicated (e.g., heart failure). In this context, the use of anti-integrin and anti-IL12/23 agents would certainly be appropriate options, thanks to the lower immunosuppressive effect and the better safety profile [24,25]. Conversely, the use of anti-JAK molecules would not be a good choice for these patients, considering the EMA alert on their administration in populations with increased metabolic/thrombotic risk, and suggesting that they be considered only in the absence of other alternatives [26]. Anyway, it is clear how in IBD-T2DM patients, it is fundamental to identify the best therapeutic strategy that allows for a reduction in steroid administration, through optimal disease control. In fact, the usage of steroids in patients who already have a diagnosis of diabetes is implicated in the occurrence of diabetes decompensation and, consequently, the development of multiple complications [27].
Among patients with IBD and T2DM, 67.1% were hypertensive, while 59.7% were dyslipidemic (vs. 18.6% and 21.2% in controls). However, these percentages were comparable to those found in diabetics without intestinal comorbidity [28].
Regarding the presence of severe IBD, as the primary endpoint, no significant difference emerged from the comparison between the IBD + T2DM group and the controls, with a prevalence of 59.5 and 59.7%, respectively. These data partly differ from the literature, where an increase in IBD-related hospitalizations (included in the composite endpoint) in IBD-T2DM patients is reported [14,15], despite very discordant data among studies [13,15]. Then, our study confirmed a lack of increased need for IBD surgery in IBD-T2DM patients [13,14,15].
In our cohort, 69.3% of patients with severe IBD were diagnosed with CD, whereas only 41.7% of patients who did not have severe IBD were diagnosed with CD (p < 0.0001). However, in the univariate analysis, which accounts for the different follow-up lengths for each patient, the presence of CD was not associated with an increased risk of a severe IBD course (HR 1.25; 95% CI 0.94–1.65).
In our study, being naïve to SGLT2 inhibitors, although significant in the χ2 test (27.7% vs. 12.5%; p = 0.0383), was not protective against severe disease in the univariate analysis. Instead, being naïve to GLP-1 ra was significantly protective for severe IBD in the univariate analysis, but this significance was not confirmed in the multivariate analysis. Currently, in the literature, there are no clinical studies on the prescription of SGLT2i in the specific population of IBD-DMT2. As regards GLP-1 ra, one study assumed that these drugs could improve the course of IBD [29], but a similar result was not observed in our study.
The only significant predictor for severe disease in uni- and multivariable analysis was the age at last follow-up: increasing age decreases the risk of severe IBD (HR: 0.98; 95% CI: 0.97 to 0.99; p < 0.0001). This finding may be explained by the fact that IBD in elderly patients has a more indolent presentation [30], with symptoms and complications that do not fit our definition of serious disease.
Regarding secondary endpoints, the comparison between the IBD-T2DM group and controls revealed a more than doubled prevalence of hepatic steatosis in cases (62.9% vs. 27.2%; p < 0.0001). However, the prevalence of steatosis in IBD-T2DM patients did not differ from the prevalence reported in the general diabetic population [31]; thus, we presume that the association of the two pathologies does not provide a multiplicative risk factor for steatosis.
The assessment of liver damage, using the Fib-4 score, differs significantly between cases and controls (55.4% vs. 26.6%; p < 0.0001). However, it is likely that the difference in average age (higher in diabetic patients of 19.2y) has negatively influenced score parameters (ALT, AST, and no. of platelets) [32,33]. Evaluating the predictors of Fib-4 score > 1.3, DMT2 increased the risk for liver damage (HR: 1.57) with a trend toward statistical significance in the univariate analysis (95% CI 0.99 to 2.51; p = 0.06).
In the sub-analysis evaluating the characteristics of the population regarding the presence of liver damage, the association of hypertension (HR: 1.61; p = 0.047) and dyslipidemia (HR: 1.68; p = 0.03) was statistically significant in the univariate analysis. This aligns with the numerous studies describing metabolic syndrome and its effects on the liver [16,31,34]. Moreover, in the univariate analysis, we observed that being naïve to thiopurine constituted another risk factor for liver damage (HR: 1.81; p = 0.02), even though the contribution of the covariates hypertension, dyslipidemia, and being naïve to thiopurines was not significant in the multivariate analysis. The contribution of being naïve to anti-TNF-alpha drugs was not significant.
In the multivariable analysis on predictors of liver damage, age was the only covariate resulting in a significant increase in risk (HR 1.03; 95% CI 1.01–1.05; p = 0.01). This result, although relevant from a statistical point of view, raises doubts about the clinical usefulness of a non-specific predictor such as Fib-4 in the older population due to the presence of age in the numerator of the score, as already highlighted by van Kleef et al. [33].
No association between liver damage and alcohol consumption was observed, although alcohol is one of the most important causes of liver fibrosis and an increase in hepatic cytolysis indices. However, it is possible that this result was conditioned by the data collection method, since the definition of alcohol consumption included the occasional drink, so it was not possible to define the alcohol units consumed.
5. Chapter Limitation and Future Directions
Regarding the criticisms of our study, one of the main limitations is its retrospective nature, which does not allow us to establish a causal link between the associations found. Furthermore, the analysis of metabolic characteristics between cases and controls revealed several variables that could potentially introduce biases, since conditions such as T2DM, hepatic steatosis, hypertension, dyslipidemia, and obesity share many common causative factors. However, the effect of these potential biases was mitigated by multivariate analyses. Another limitation derives from the monocentric design of this study.
It would certainly be useful for future studies to conduct prospective research with well-defined endpoints in order to reduce the biases typical of retrospective studies.
6. Conclusions
In our study, the presence of T2DM was not associated with a worse clinical course of IBD.
In patients with IBD, T2DM has been associated with a higher presence of steatotic liver disease and liver damage, but the IBD-T2DM association is not a multiplicative risk factor for these outcomes.
Regarding specific therapies for IBD, in patients with T2DM, we observed a greater administration (current and especially past) of systemic corticosteroids in clinical practice and a lower usage of anti-TNF-alpha drugs.
The differences in age at onset of the pathology, the greater prevalence of colonic involvement, and the greater presence of liver damage suggest that patients with IBD and T2DM could represent a special population worthy of further studies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14010143/s1: Table S1: Antidiabetic therapy in the IBD + T2DM group.
Author Contributions
Conceptualization, A.A., G.P.C., F.B., G.B., and D.G.R.; methodology, G.B. and D.G.R.; software, G.P.C.; validation, D.G.R.; formal analysis G.P.C.; investigation, B.G. and M.B.; resources, F.B.; data curation, G.P.C.; writing—original draft preparation, B.Z.; writing—review and editing, D.G.R.; visualization, M.V.; supervision, F.B.; project administration, F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of AOU CITTA’ DELLA SALUTE E DELLA SCIENZA DI TORINO (protocol code 0116726, 9 October 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wark, G.; Samocha-Bonet, D.; Ghaly, S.; Danta, M. The Role of Diet in the Pathogenesis and Management of Inflammatory Bowel Disease: A Review. Nutrients 2021, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota-Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fu, T.; Liu, W.; Du, Y.; Bu, J.; Wei, G.; Yu, M.; Lin, Y.; Min, C.; Lin, D. An Update on the Role and Potential Molecules in Relation to Ruminococcus Gnavus in Inflammatory Bowel Disease, Obesity and Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2024, 17, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Juge, N. Microbe Profile: Ruminococcus Gnavus: The Yin and Yang of Human Gut Symbionts. Microbiology 2023, 169, 001383. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Carlos, D. Akkermansia Muciniphila and Gut Immune System: A Good Friendship That Attenuates Inflammatory Bowel Disease, Obesity, and Diabetes. Front. Immunol. 2022, 13, 934695. [Google Scholar] [CrossRef]
- Jess, T.; Jensen, B.W.; Andersson, M.; Villumsen, M.; Allin, K.H. Inflammatory Bowel Diseases Increase Risk of Type 2 Diabetes in a Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2020, 18, 881–888.e1. [Google Scholar] [CrossRef]
- Kang, E.A.; Han, K.; Chun, J.; Soh, H.; Park, S.; Im, J.P.; Kim, J.S. Increased Risk of Diabetes in Inflammatory Bowel Disease Patients: A Nationwide Population-Based Study in Korea. J. Clin. Med. 2019, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jia, Y.; Li, F.-R.; Li, Y.; Chen, L.-H.; Yang, H.-H.; Guo, D.; Sun, L.; Shi, M.; Wang, T.; et al. Inflammatory Bowel Disease and Risk of Global Cardiovascular Diseases and Type 2 Diabetes. Inflamm. Bowel Dis. 2024, 30, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Farmacologia, 4th ed.; Rossi, F., Cuomo, V., Riccardi, C., AA, V., Eds.; Minerva Medica: Torino, Italy, 2019; Available online: https://www.hoepli.it/libro/farmacologia-4ed/9788877119957.html (accessed on 16 November 2024).
- Francis, K.L.; Alonge, K.M.; Pacheco, M.C.; Hu, S.J.; Krutzsch, C.A.; Morton, G.J.; Schwartz, M.W.; Scarlett, J.M. Diabetes exacerbates inflammatory bowel disease in mice with diet-induced obesity. World J. Gastroenterol. 2023, 29, 4991. [Google Scholar] [CrossRef] [PubMed]
- Uwagbale, E.; Adeniran, O.G.; Adeniran, O.A.; Onukogu, I.; Agbroko, S.; Sonpal, N.; Uwagbale, E.; Adeniran, O.G.; Adeniran, O.A.; Onukogu, I.; et al. In-Hospital Outcomes of Inflammatory Bowel Diseases in Patients With Diabetes Mellitus: A Propensity Score Matching Analysis. Cureus 2021, 13, e16566. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Teslova, T.; Taub, E.; Miller, J.D.; Lukin, D.J. Comorbid Diabetes in Inflammatory Bowel Disease Predicts Adverse Disease-Related Outcomes and Infectious Complications. Dig. Dis. Sci. 2021, 66, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Fuschillo, G.; Celentano, V.; Rottoli, M.; Sciaudone, G.; Gravina, A.G.; Pellegrino, R.; Marfella, R.; Romano, M.; Selvaggi, F.; Pellino, G. Influence of Diabetes Mellitus on Inflammatory Bowel Disease Course and Treatment Outcomes. A Systematic Review with Meta-Analysis. Dig. Liver Dis. 2023, 55, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Duque, J.C.; Calleja, J.L.; Iruzubieta, P.; Hernández-Conde, M.; Rivas-Rivas, C.; Vera, M.I.; Garcia, M.J.; Pascual, M.; Castro, B.; García-Blanco, A.; et al. Increased Risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin. Gastroenterol. Hepatol. 2023, 21, 406–414.e7. [Google Scholar] [CrossRef]
- Zamani, M.; Alizadeh-Tabari, S.; Singh, S.; Loomba, R. Meta-Analysis: Prevalence of, and Risk Factors for, Non-Alcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2022, 55, 894–907. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohns Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef]
- Yarur, A.J.; Strobel, S.G.; Deshpande, A.R.; Abreu, M.T. Predictors of Aggressive Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2011, 7, 652–659. [Google Scholar]
- Kjaergaard, M.; Lindvig, K.P.; Thorhauge, K.H.; Andersen, P.; Hansen, J.K.; Kastrup, N.; Jensen, J.M.; Hansen, C.D.; Johansen, S.; Israelsen, M.; et al. Using the ELF Test, FIB-4 and NAFLD Fibrosis Score to Screen the Population for Liver Disease. J. Hepatol. 2023, 79, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Cheifetz, A.S. Management of Active Crohn Disease. JAMA 2013, 309, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Irving, P.M.; Samaan, M.A. JAK Inhibitors for the Treatment of Inflammatory Bowel Disease: Results of an International Survey of Perceptions, Attitudes, and Clinical Practice. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2016, 66, 241–255. [Google Scholar] [CrossRef]
- CDC. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 17 November 2024).
- Villumsen, M.; Schelde, A.B.; Jimenez-Solem, E.; Jess, T.; Allin, K.H. GLP-1 Based Therapies and Disease Course of Inflammatory Bowel Disease. eClinicalMedicine 2021, 37, 100979. [Google Scholar] [CrossRef]
- Andres, P.G.; Friedman, L.S. Epidemiology and the Natural Course of Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 1999, 28, 255–281, vii. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The Global Epidemiology of NAFLD and NASH in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Montenont, E.; Rondina, M.T.; Campbell, R.A. Altered Functions of Platelets during Aging. Curr. Opin. Hematol. 2019, 26, 336–342. [Google Scholar] [CrossRef]
- van Kleef, L.A.; Sonneveld, M.J.; de Man, R.A.; de Knegt, R.J. Poor Performance of FIB-4 in Elderly Individuals at Risk for Chronic Liver Disease—Implications for the Clinical Utility of the EASL NIT Guideline. J. Hepatol. 2022, 76, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Suwała, S.; Junik, R. Assessment of the Liver Steatosis and Fibrosis Risk in Metabolic Syndrome and Its Individual Components, Considering the Varying Definitions Used in Clinical Practice throughout Time: A Retrospective Cross-Sectional Study. Biomedicines 2024, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).