Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Statistical Analysis
3. Results
3.1. Patient Population
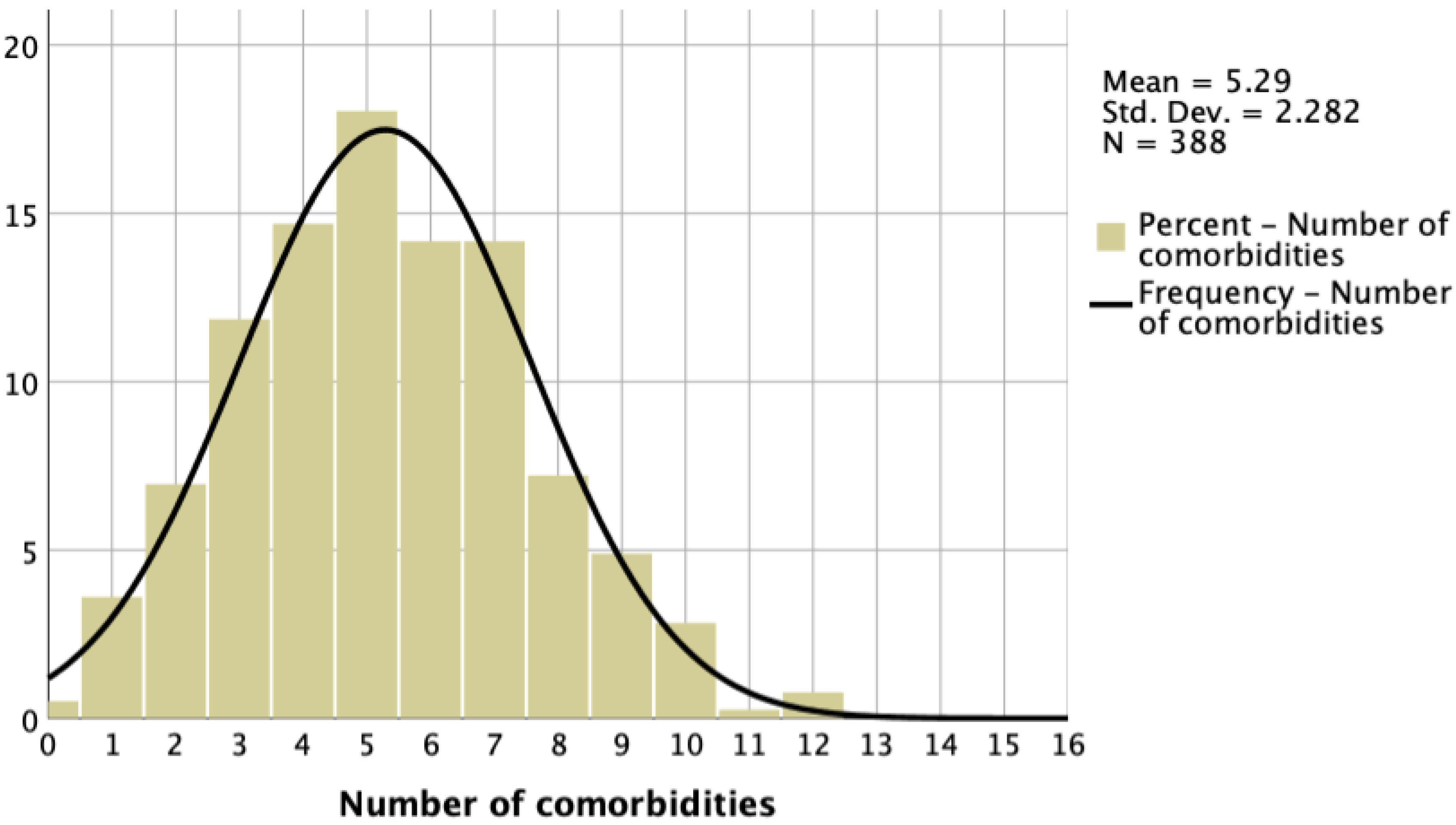
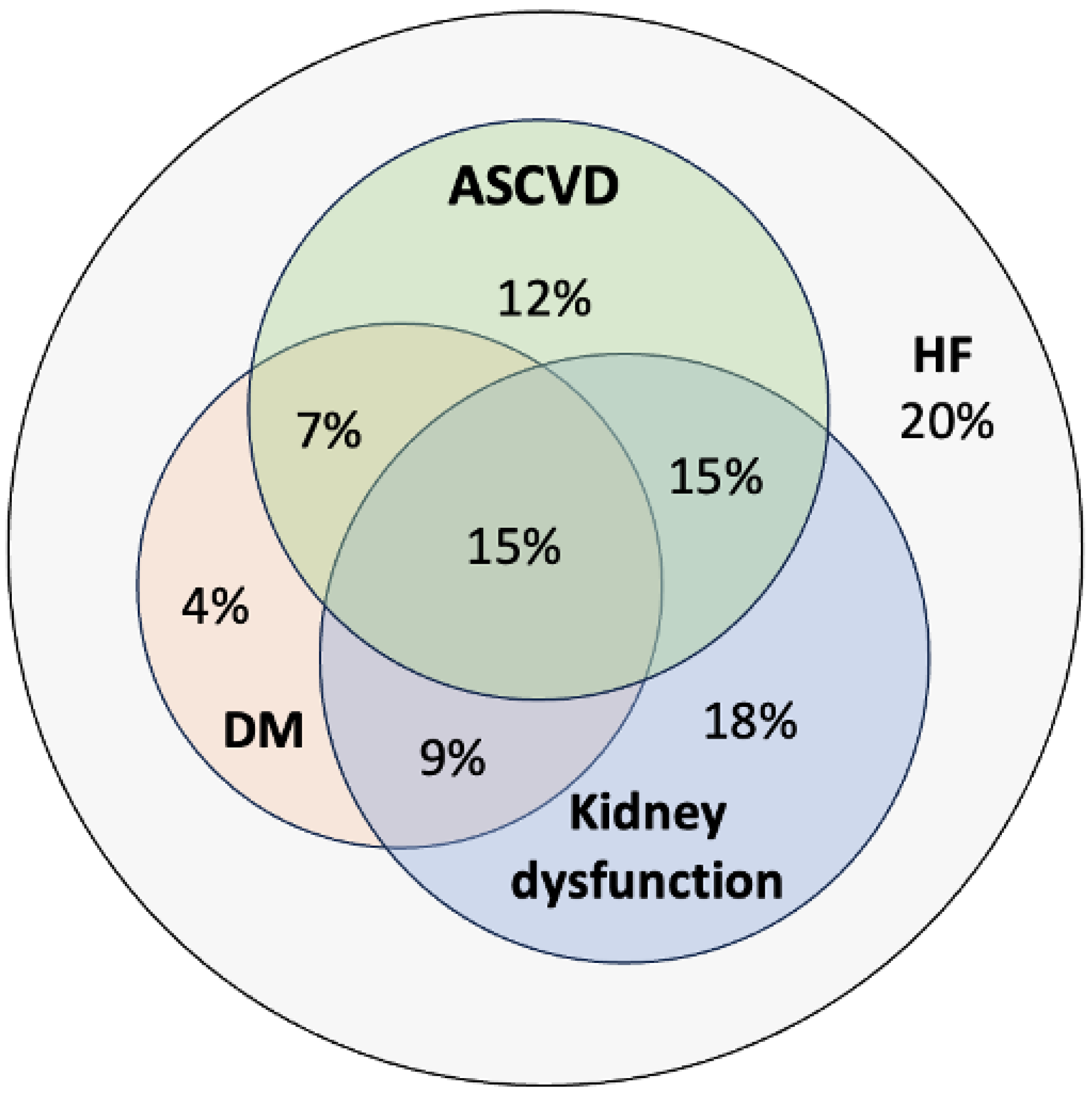
3.2. Multimorbidity in HF
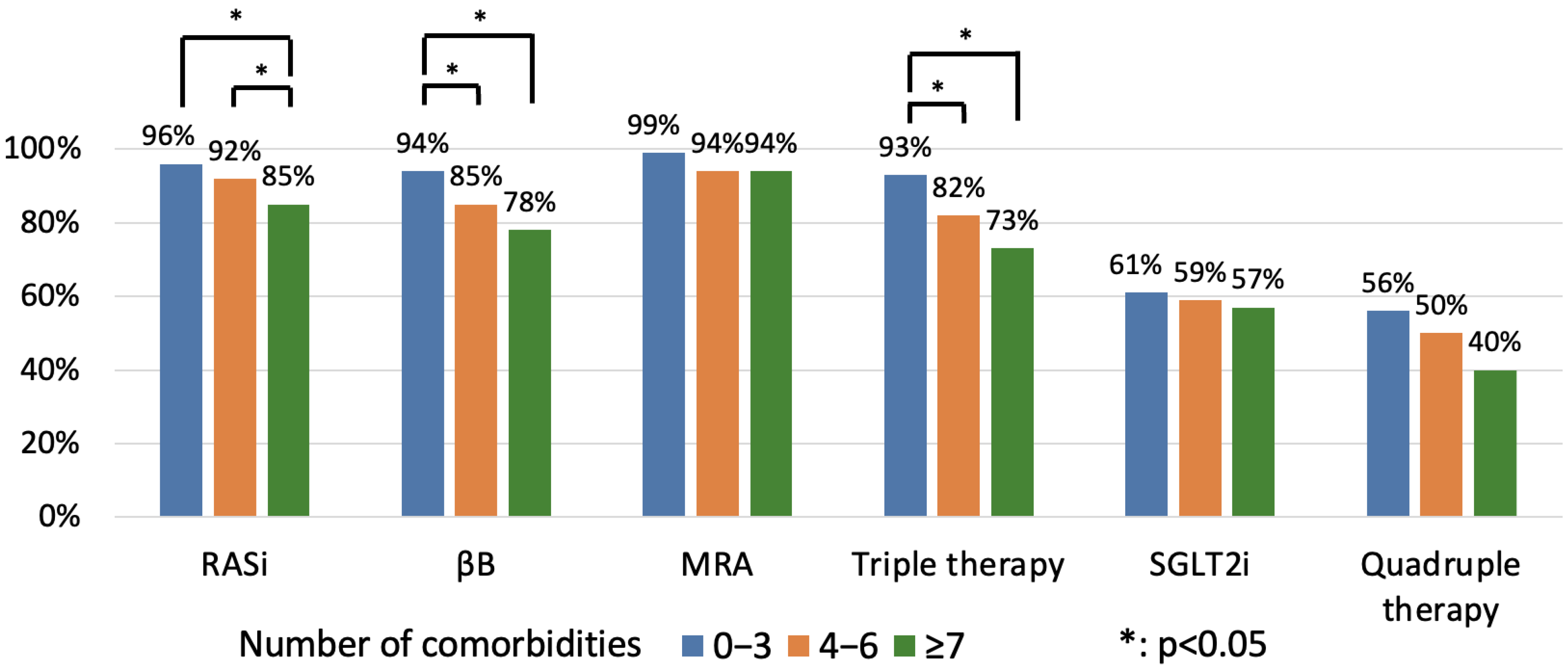
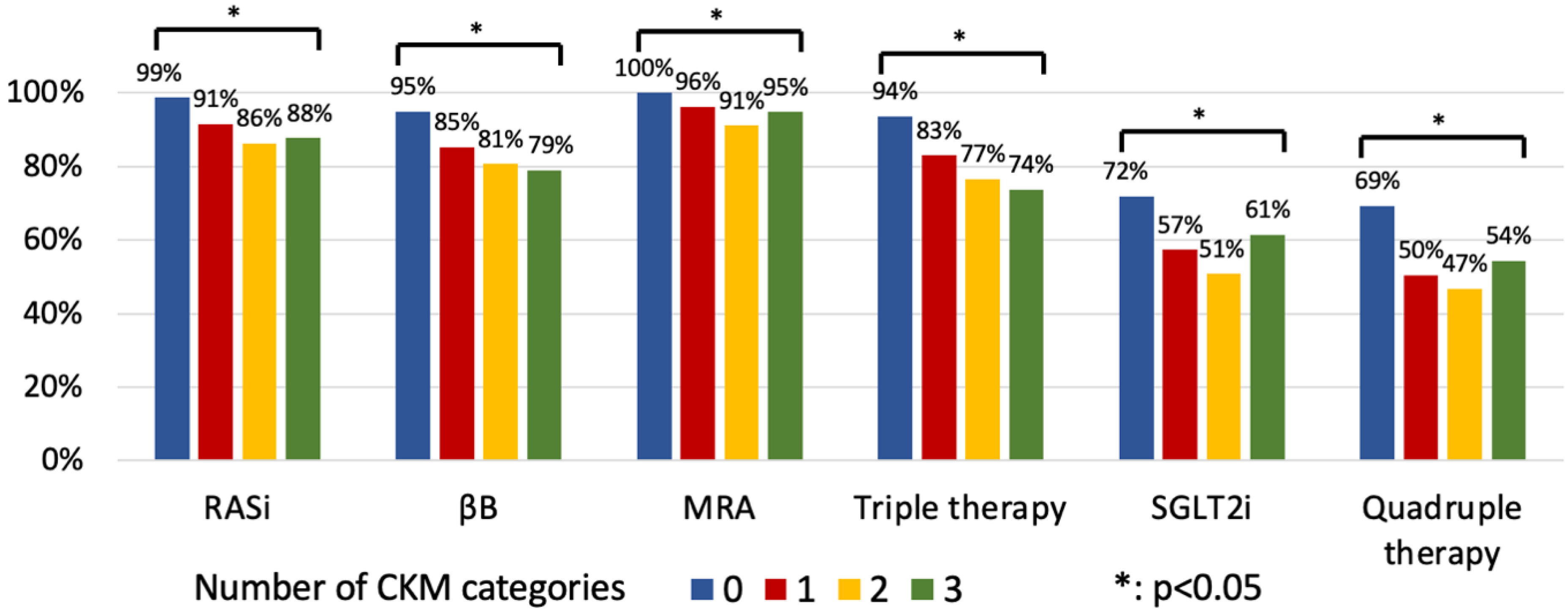
3.3. The Effect of Multimorbidity on GDMT Implementation
3.4. Effect of Multimorbidity on Prognosis and Predictors of Mortality
4. Discussion
4.1. Main Findings
4.2. Multimorbidity in HF
4.3. The Effect of Multimorbidity on GDMT Implementation
| ESC HF Long-Term Registry (2023) [51] | REPORT-HF (2023) [17] | GTWG-HF (2018) [31] | ASIAN-HF (2019) [65] | ASCEND-HF (2020) [2] | STRONG-HF (2022) [44] | SwedeHF (2023) [3] | Current Analysis (2024) | |
|---|---|---|---|---|---|---|---|---|
| Number of CMs | not documented | 0: 5.4% 1: 13.0% 2: 18.6% 3: 21.2% 4: 17.8% ≥5: 24.0% | not documented | median [IQR]: 3 [2–4] | not documented | not reported | 0: 1.9% 1: 7.2% 2: 13.0% 3: 16.8% 4:18.1% 5: 16.4% 6: 12.5 ≥7: 14.1% | median [IQR]: 5 [4–7] 0: 1% 1: 4% 2: 7% 3: 12% 4: 15% 5: 18% 6: 14% 7:14% ≥8: 15% |
| Number of non-CV/non-cardiac CMs | 0: 20.5% 1: 28.7% 2: 23% 3: 15.4% ≥4: 12.5% | not reported | 0: 18% 1: 30% 2: 27% ≥3: 25% | not reported | mean ± SD: 2.2 ± 1.37 0: 8.9% 1: 25.3% 2: 30.0% 3: 20.1% ≥4: 15.6% | 0: 24.3% 1: 39.8% 2: 24.5% ≥3: 11.4% | 0: 14.8% 1: 26.4% 2: 26.1% 3: 18.3% 4: 23% ≥5: 14.4% | median [IQR]: 3 [2–4] 0: 2% 1: 10% 2: 21% 3: 21% 4: 23% ≥5: 23% |
| RASi | total cohort: not documented 0 non-CV CMs: 86% ≥4 non-CV CMs: 66.3% | total cohort: not reported 0 CMs: 78% * ≥4 CMs: 62% * * not exact values, estimated based on published diagram | not documented | 73.7% | total cohort: 60.6% | “High-intensity care” group at day 180: 97.2% | 85.4% | 91% |
| βB | total cohort: not documented 0 non-CV CMs: 83.1% ≥4 non-CV CMs: 63.4% | total cohort: not reported 0 CMs: 72% * ≥4 CMs: 56% * * not exact values, estimated based on published diagram | not documented | 75.7% | total cohort: 58.1% | “High-intensity care” group at day 180: 95.7% | 88.4% | 85% |
| MRA | total cohort: not documented 0 non-CV CMs: 62.3% ≥4 non-CV CMs: 41.2% | total cohort: not reported 0 CMs: 80% * ≥4 CMs: 80% * * not exact values, estimated based on published diagram | not documented | 52.1% | total cohort: 27.8% | “High-intensity care” group at day 180: 95.7% | 35.2% | 95% |
| TT | not documented | not reported | not documented | not reported | not documented | not reported | not documented | 82% |
| SGLT2i | not documented | not reported | not documented | not reported | not documented | not reported | not documented | 59% |
| QT | not documented | not reported | not documented | not reported | not documented | not reported | not documented | 54% |
4.4. The Effect of Multimorbidity on Prognosis and the Predictors of Mortality
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Correale, M.; Paolillo, S.; Mercurio, V.; Ruocco, G.; Tocchetti, C.G.; Palazzuoli, A. Non-cardiovascular comorbidities in heart failure patients and their impact on prognosis. Kardiol. Pol. 2021, 79, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.S.; Ambrosy, A.P.; Dunning, A.; DeVore, A.D.; Butler, J.; Reed, S.; Voors, A.; Starling, R.; Armstrong, P.W.; Ezekowitz, J.A.; et al. The burden of non-cardiac comorbidities and association with clinical outcomes in an acute heart failure trial—Insights from ASCEND-HF. Eur. J. Heart Fail. 2020, 22, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Vitale, C.; Guidetti, F.; Benson, L.; Braunschweig, F.; Dahlström, U.; Melin, M.; Rosano, G.M.C.; Lund, L.H.; Metra, M.; et al. The role of multimorbidity in patients with heart failure across the left ventricular ejection fraction spectrum: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2024, 26, 854–868. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Vaduganathan, M.; Fonarow, G.C.; Bonow, R.O. Rehospitalization for heart failure: Problems and perspectives. J. Am. Coll. Cardiol. 2013, 61, 391–403. [Google Scholar] [CrossRef]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar] [CrossRef]
- Takeuchi, S.; Kohno, T.; Goda, A.; Shiraishi, Y.; Kawana, M.; Saji, M.; Nagatomo, Y.; Nishihata, Y.; Takei, M.; Nakano, S.; et al. Multimorbidity, guideline-directed medical therapies, and associated outcomes among hospitalized heart failure patients. ESC Heart Fail. 2022, 9, 2500–2510. [Google Scholar] [CrossRef]
- Kovács, L.; Bozzay, K.; Laczkó, K.; Sztankó, P.; Ladányi, E.; Wittmann, I.; Laczy, B. The online representation of diabetes mellitus and chronic kidney disease and comparison with other public health diseases. Orv. Hetil. 2024, 165, 510–518. [Google Scholar] [CrossRef]
- Muk, B.; Vámos, M.; Bógyi, P.; Szabó, B.; Dékány, M.; Vágány, D.; Majoros, Z.; Borsányi, T.; Duray, G.Z.; Kiss, R.G.; et al. The impact of serum concentration-guided digoxin therapy on mortality of heart failure patients: A long-term follow-up, propensity-matched cohort study. Clin. Cardiol. 2020, 43, 1641–1648. [Google Scholar] [CrossRef]
- Meekers, E.; Dhont, S.; Mullens, W. Diuretic therapy in acute decompensated heart failure. Cardiol. Hung. 2024, 54, 193–199. [Google Scholar] [CrossRef]
- Vamos, M.; Oldgren, J.; Nam, G.B.; Lip, G.Y.H.; Calkins, H.; Zhu, J.; Ueng, K.C.; Ludwigs, U.; Wieloch, M.; Stewart, J.; et al. Dronedarone vs. placebo in patients with atrial fibrillation or atrial flutter across a range of renal function: A post hoc analysis of the ATHENA trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Bánfi-Bacsárdi, F.; Muk, B.; Pilecky, D.; Duray, G.Z.; Kiss, R.G.; Nyolczas, N. The Optimization of Guideline-Directed Medical Therapy during Hospitalization among Patients with Heart Failure with Reduced Ejection Fraction in Daily Clinical Practice. Cardiology 2023, 148, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Muk, B.; Bánfi-Bacsárdi, F.; Vámos, M.; Pilecky, D.; Majoros, Z.; Török, G.M.; Vágány, D.; Polgár, B.; Solymossi, B.; Borsányi, T.D.; et al. The Impact of Specialised Heart Failure Outpatient Care on the Long-Term Application of Guideline-Directed Medical Therapy and on Prognosis in Heart Failure with Reduced Ejection Fraction. Diagnostics 2024, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Gerhardt, T.; Gerhardt, L.M.S.; Ouwerkerk, W.; Roth, G.A.; Dickstein, K.; Collins, S.P.; Cleland, J.G.F.; Dahlstrom, U.; Tay, W.T.; Ertl, G.; et al. Multimorbidity in patients with acute heart failure across world regions and country income levels (REPORT-HF): A prospective, multicentre, global cohort study. Lancet Glob. Health 2023, 11, e1874–e1884. [Google Scholar] [CrossRef]
- Khan, M.S.; Samman Tahhan, A.; Vaduganathan, M.; Greene, S.J.; Alrohaibani, A.; Anker, S.D.; Vardeny, O.; Fonarow, G.C.; Butler, J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur. J. Heart Fail. 2020, 22, 1032–1042. [Google Scholar] [CrossRef]
- Aimo, A.; Barison, A.; Castiglione, V.; Emdin, M. The unbearable underreporting of comorbidities in heart failure clinical trials. Eur. J. Heart Fail. 2020, 22, 1043–1044. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. 2017, 8, 148–151. [Google Scholar] [CrossRef]
- Cittadini, A.; Bossone, E.; Ventura, H.O. Emerging Comorbidities in Heart Failure. Cardiol. Clin. 2022, 40, xi–xiv. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Lautsch, D.; Pulungan, Z.; Kim, S.; Hilkert, R.; Teigland, C.; Yang, M.; Djatche, L. Medication Trajectory and Treatment Patterns in Medicare Patients With Heart Failure and Reduced Ejection Fraction. J. Card. Fail. 2021, 28, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Bánfi-Bacsárdi, F.; Boldizsár, E.M.; Gergely, T.G.; Forrai, Z.; Kazay, Á.; Füzesi, T.; Hanuska, L.F.; Schäffer, P.P.; Pilecky, D.; Vámos, M.; et al. The role of complex patient education program in heart failure care. Orv. Hetil. 2024, 165, 1461–1471. [Google Scholar] [CrossRef]
- Gergely, G.T.; Bánfi-Bacsárdi, F.; Komáromi, A.; Pilecky, D.; Boldizsár, E.M.; Flegler, D.; Kazay, Á.; Füzesi, T.; Forrai, Z.; Vértes, V.; et al. Rapid up-titration of guide-directed medical therapy after a heart failure hospitalisation. Orv. Hetil. 2024, 165, 1197–1205. [Google Scholar] [CrossRef]
- Janse, R.J.; Fu, E.L.; Dahlström, U.; Benson, L.; Lindholm, B.; van Diepen, M.; Dekker, F.W.; Lund, L.H.; Carrero, J.J.; Savarese, G. Use of guideline-recommended medical therapy in patients with heart failure and chronic kidney disease: From physician’s prescriptions to patient’s dispensations, medication adherence and persistence. Eur. J. Heart Fail. 2022, 24, 2185–2195. [Google Scholar] [CrossRef]
- Patel, R.B.; Fonarow, G.C.; Greene, S.J.; Zhang, S.; Alhanti, B.; DeVore, A.D.; Butler, J.; Heidenreich, P.A.; Huang, J.C.; Kittleson, M.M.; et al. Kidney Function and Outcomes in Patients Hospitalized With Heart Failure. J. Am. Coll. Cardiol. 2021, 78, 330–343. [Google Scholar] [CrossRef]
- Cobo Marcos, M.; de la Espriella, R.; Gayán Ordás, J.; Llàcer, P.; Pomares, A.; Fort, A.; Ponz de Antonio, I.; Méndez, A.; Blázquez-Bermejo, Z.; Caravaca Pérez, P.; et al. Prevalence and clinical profile of kidney disease in patients with chronic heart failure. Insights from the Spanish cardiorenal registry. Rev. Esp. Cardiol. 2024, 77, 50–59. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Pilecky, D.; Vámos, M.; Majoros, Z.; Török, G.M.; Borsányi, T.D.; Dékány, M.; Solymossi, B.; Andréka, P.; Duray, G.Z.; et al. The effect of kidney function on guideline-directed medical therapy implementation and prognosis in heart failure with reduced ejection fraction. Clin. Cardiol. 2024, 47, e24244. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef]
- Sharma, A.; Zhao, X.; Hammill, B.G.; Hernandez, A.F.; Fonarow, G.C.; Felker, G.M.; Yancy, C.W.; Heidenreich, P.A.; Ezekowitz, J.A.; DeVore, A.D. Trends in Noncardiovascular Comorbidities Among Patients Hospitalized for Heart Failure: Insights From the Get With The Guidelines-Heart Failure Registry. Circ. Heart Fail. 2018, 11, e004646. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Sjöström, C.D.; Jongs, N.; Chertow, G.M.; Kosiborod, M.; Hou, F.F.; McMurray, J.J.V.; Rossing, P.; Correa-Rotter, R.; Kurlyandskaya, R.; et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: A pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur. Heart J. 2021, 42, 1216–1227. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Damman, K.; Gori, M.; Claggett, B.; Jhund, P.S.; Senni, M.; Lefkowitz, M.P.; Prescott, M.F.; Shi, V.C.; Rouleau, J.L.; Swedberg, K.; et al. Renal Effects and Associated Outcomes During Angiotensin-Neprilysin Inhibition in Heart Failure. JACC Heart Fail. 2018, 6, 489–498. [Google Scholar] [CrossRef]
- Chatur, S.; Claggett, B.L.; McCausland, F.R.; Rouleau, J.; Zile, M.R.; Packer, M.; Pfeffer, M.A.; Lefkowitz, M.; McMurray, J.J.V.; Solomon, S.D.; et al. Variation in Renal Function Following Transition to Sacubitril/Valsartan in Patients With Heart Failure. J. Am. Coll. Cardiol. 2023, 81, 1443–1455. [Google Scholar] [CrossRef]
- Bánfi-Bacsárdi, F.; Ormos, F.; Muk, B. The importance of chronic kidney disease in the management of patients with heart failure. Cardiol. Hung. 2024, 54, 206–211. [Google Scholar] [CrossRef]
- Screever, E.M.; van der Wal, M.H.L.; van Veldhuisen, D.J.; Jaarsma, T.; Koops, A.; van Dijk, K.S.; Warink-Riemersma, J.; Coster, J.E.; Westenbrink, B.D.; van der Meer, P.; et al. Comorbidities complicating heart failure: Changes over the last 15 years. Clin. Res. Cardiol. 2023, 112, 123–133. [Google Scholar] [CrossRef]
- Tomcsányi, J.; Arányi, P.; Kántor, Z.; Csizmadia, Z. Chronic renal failure in patients hospitalized for heart failure. Cardiol. Hung. 2024, 54, 24–26. [Google Scholar] [CrossRef]
- Flores-Le Roux, J.A.; Comin, J.; Pedro-Botet, J.; Benaiges, D.; Puig-de Dou, J.; Chillarón, J.J.; Goday, A.; Bruguera, J.; Cano-Perez, J.F. Seven-year mortality in heart failure patients with undiagnosed diabetes: An observational study. Cardiovasc. Diabetol. 2011, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Kodur, N.; Tang, W.H.W. Non-cardiac comorbidities in heart failure: An update on diagnostic and management strategies. Minerva Med. 2024, 115, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, O.; Davison, B.; Adamo, M.; Antohi, L.E.; Arrigo, M.; Barros, M.; Biegus, J.; Čerlinskaitė-Bajorė, K.; Celutkiene, J.; Cohen-Solal, A.; et al. Non-cardiac comorbidities and intensive up-titration of oral treatment in patients recently hospitalized for heart failure: Insights from the STRONG-HF trial. Eur. J. Heart Fail. 2023, 25, 1994–2006. [Google Scholar] [CrossRef]
- Lindberg, F.; Lund, L.H.; Benson, L.; Linde, C.; Orsini, N.; Carrero, J.J.; Savarese, G. Iron deficiency in heart failure: Screening, prevalence, incidence and outcome data from the Swedish Heart Failure Registry and the Stockholm CREAtinine Measurements collaborative project. Eur. J. Heart Fail. 2023, 25, 1270–1280. [Google Scholar] [CrossRef]
- Rocha, B.M.L.; Cunha, G.J.L.; Menezes Falcão, L.F. The Burden of Iron Deficiency in Heart Failure: Therapeutic Approach. J. Am. Coll. Cardiol. 2018, 71, 782–793. [Google Scholar] [CrossRef]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef]
- Nyolczas, N.; Heltai, K.; Borbély, A.; Habon, T.; Járai, Z.; Sziliczei, E.; Stadler, P.; Faludi, R.; Herczeg, B.; Papp, E.; et al. Magyar Szívelégtelenség Regiszter 2015–2016. Kezdeti eredmények. Orvosi Hetilap 2017, 158, 94–100. [Google Scholar] [CrossRef]
- Ryan, D.K.; Banerjee, D.; Jouhra, F. Management of Heart Failure in Patients with Chronic Kidney Disease. Eur. Cardiol. 2022, 17, e17. [Google Scholar] [CrossRef]
- McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: Impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ. Heart Fail. 2012, 5, 309–314. [Google Scholar] [CrossRef]
- Chioncel, O.; Benson, L.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.S.; Filippatos, G.; McDonagh, T.; Margineanu, C.; Mebazaa, A.; Metra, M.; et al. Comprehensive characterization of non-cardiac comorbidities in acute heart failure: An analysis of ESC-HFA EURObservational Research Programme Heart Failure Long-Term Registry. Eur. J. Prev. Cardiol. 2023, 30, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.; Ferreira, J.P.; Butt, J.H.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.; Packer, M.; Rouleau, J.L.; et al. Impact of multimorbidity on mortality in heart failure with reduced ejection fraction: Which comorbidities matter most? An analysis of PARADIGM-HF and ATMOSPHERE. Eur. J. Heart Fail. 2023, 25, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ostrominski, J.W.; Claggett, B.L.; Miao, Z.M.; Mc Causland, F.R.; Anand, I.S.; Desai, A.S.; Jhund, P.S.; Lam, C.S.P.; Pfeffer, M.A.; Pitt, B.; et al. Cardiovascular-Kidney-Metabolic Overlap in Heart Failure With Mildly Reduced or Preserved Ejection Fraction: A Trial-Level Analysis. J. Am. Coll. Cardiol. 2024, 84, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.; Mentz, R.J.; Greene, S.J. Update on the Impact of Comorbidities on the Efficacy and Safety of Heart Failure Medications. Curr. Heart Fail. Rep. 2021, 18, 132–143. [Google Scholar] [CrossRef]
- Greene, S.J.; Ayodele, I.; Pierce, J.B.; Khan, M.S.; Lewsey, S.C.; Yancy, C.W.; Alhanti, B.; Spall, H.G.C.V.; Allen, L.A.; Fonarow, G.C. Eligibility and Projected Benefits of Rapid Initiation of Quadruple Medical Therapy for Newly Diagnosed Heart Failure. JACC Heart Fail. 2024, 12, 1365–1377. [Google Scholar] [CrossRef]
- Zheng, J.; Sandhu, A.T.; Bhatt, A.S.; Collins, S.P.; Flint, K.M.; Fonarow, G.C.; Fudim, M.; Greene, S.J.; Heidenreich, P.A.; Lala, A.; et al. Inpatient Use of Guideline-Directed Medical Therapy During Heart Failure Hospitalizations Among Community-Based Health Systems. JACC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Stolfo, D.; Lund, L.H.; Benson, L.; Lindberg, F.; Ferrannini, G.; Dahlström, U.; Sinagra, G.; Rosano, G.M.C.; Savarese, G. Real-world use of sodium–glucose cotransporter 2 inhibitors in patients with heart failure and reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2023, 25, 1648–1658. [Google Scholar] [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Pitt, B.; Remme, W.; Zannad, F.; Neaton, J.; Martinez, F.; Roniker, B.; Bittman, R.; Hurley, S.; Kleiman, J.; Gatlin, M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003, 348, 1309–1321. [Google Scholar] [CrossRef]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef]
- Mullens, W.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Fudim, M.; Iacoviello, M.; Franke, J.; Flammer, A.J.; et al. Renal effects of guideline-directed medical therapies in heart failure: A consensus document from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 603–619. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Tromp, J.; Tay, W.T.; Ouwerkerk, W.; Teng, T.K.; Yap, J.; MacDonald, M.R.; Leineweber, K.; McMurray, J.J.V.; Zile, M.R.; Anand, I.S.; et al. Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry. PLoS Med. 2018, 15, e1002541. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Neeland, I.J.; Tuttle, K.R.; Chow, S.L.; Mathew, R.O.; Khan, S.S.; Coresh, J.; Baker-Smith, C.M.; Carnethon, M.R.; Després, J.P.; et al. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1636–1664. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Mayne, K.J.; Sardell, R.J.; Staplin, N.; Judge, P.K.; Zhu, D.; Sammons, E.; Cherney, D.Z.; Cheung, A.K.; Maggioni, A.P.; Nangaku, M.; et al. Frailty, multimorbidity and polypharmacy: Exploratory analyses of the effects of empagliflozin from the EMPA-KIDNEY trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 1119–1129. [Google Scholar] [CrossRef]
- Docherty, K.F.; McMurray, J.J.V.; Kalra, P.R.; Cleland, J.G.F.; Lang, N.N.; Petrie, M.C.; Robertson, M.; Ford, I. Intravenous iron and SGLT2 inhibitors in iron-deficient patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2024, 11, 1875–1879. [Google Scholar] [CrossRef]
- Tziastoudi, M.; Pissas, G.; Golfinopoulos, S.; Filippidis, G.; Dousdampanis, P.; Eleftheriadis, T.; Stefanidis, I. Sodium-Glucose Transporter 2 (SGLT2) Inhibitors and Iron Deficiency in Heart Failure and Chronic Kidney Disease: A Literature Review. Life 2023, 13, 2338. [Google Scholar] [CrossRef]
- Lavalle, C.; Mariani, M.V.; Severino, P.; Palombi, M.; Trivigno, S.; D’Amato, A.; Silvetti, G.; Pierucci, N.; Di Lullo, L.; Chimenti, C.; et al. Efficacy of Modern Therapies for Heart Failure with Reduced Ejection Fraction in Specific Population Subgroups: A Systematic Review and Network Meta-Analysis. Cardiorenal Med. 2024, 14, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Krum, H.; Roecker, E.B.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Coats, A.J.; Katus, H.A.; Fowler, M.B.; Packer, M. Effects of initiating carvedilol in patients with severe chronic heart failure: Results from the COPERNICUS Study. JAMA 2003, 289, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.H.; Packer, M.; Fonarow, G.C.; Faselis, C.; Allman, R.M.; Morgan, C.J.; Singh, S.N.; Pitt, B.; Ahmed, A. Early Effects of Starting Doses of Enalapril in Patients with Chronic Heart Failure in the SOLVD Treatment Trial. Am. J. Med. 2020, 133, e25–e31. [Google Scholar] [CrossRef]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients With Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef]
- Berg, D.D.; Jhund, P.S.; Docherty, K.F.; Murphy, S.A.; Verma, S.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 499–507. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef]
- Neuen, B.L.; Claggett, B.L.; Perkovic, V.; Jardine, M.; Heerspink, H.J.L.; Mahaffey, K.W.; McMurray, J.J.V.; Solomon, S.D.; Vaduganathan, M. Timing of Cardio-Kidney Protection With SGLT2 Inhibitors: Insights From 4 Large-Scale Placebo-Controlled Outcome Trials. Circulation 2024, 150, 343–345. [Google Scholar] [CrossRef]
- Rangaswami, J.; Tuttle, K.; Vaduganathan, M. Cardio-Renal-Metabolic Care Models: Toward Achieving Effective Interdisciplinary Care. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e007264. [Google Scholar] [CrossRef]
- Muk, B.; Pilecky, D.; Bánfi-Bacsárdi, F.; Füzesi, T.; Gergely, G.T.; Komáromi, A.; Papp, E.; Szőnyi, M.D.; Forrai, Z.; Kazay, Á.; et al. The changes in the pharmacotherapy of heart failure with reduced ejection fraction and its effect on prognosis: Experience in the Hungarian clinical practice. Orv. Hetil. 2024, 165, 698–710. [Google Scholar] [CrossRef]
- Tromp, J.; Ouwerkerk, W.; van Veldhuisen, D.J.; Hillege, H.L.; Richards, A.M.; van der Meer, P.; Anand, I.S.; Lam, C.S.P.; Voors, A.A. A Systematic Review and Network Meta-Analysis of Pharmacological Treatment of Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2022, 10, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Claggett, B.L.; Jhund, P.S.; Cunningham, J.W.; Pedro Ferreira, J.; Zannad, F.; Packer, M.; Fonarow, G.C.; McMurray, J.J.V.; Solomon, S.D. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 2020, 396, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Wang, D.; Pfeffer, M.A.; Yusuf, S.; McMurray, J.J.V.; Swedberg, K.B.; Östergren, J.; Michelson, E.L.; Pieper, K.S.; Granger, C.B.; et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2005, 27, 65–75. [Google Scholar] [CrossRef] [PubMed]
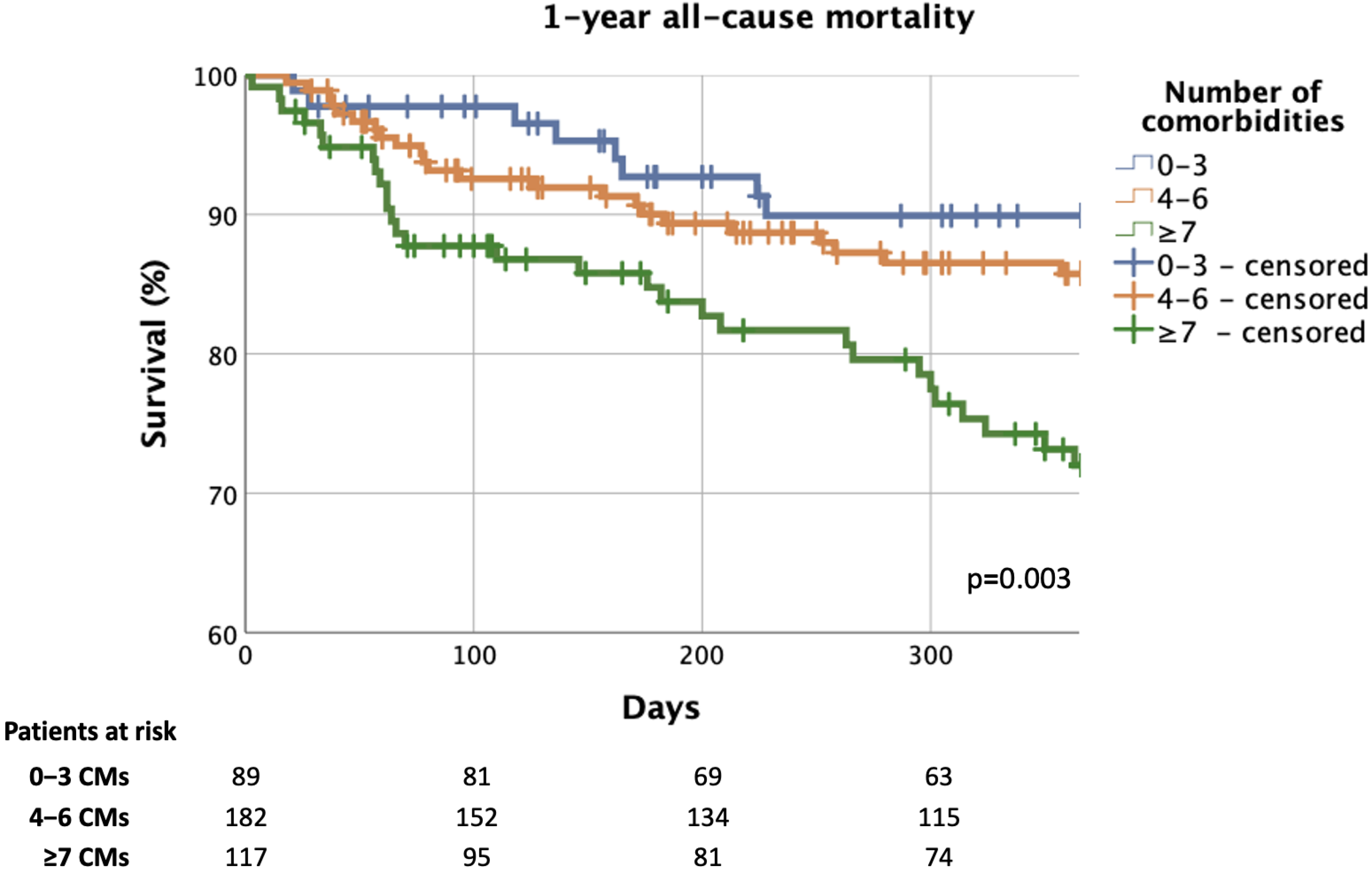
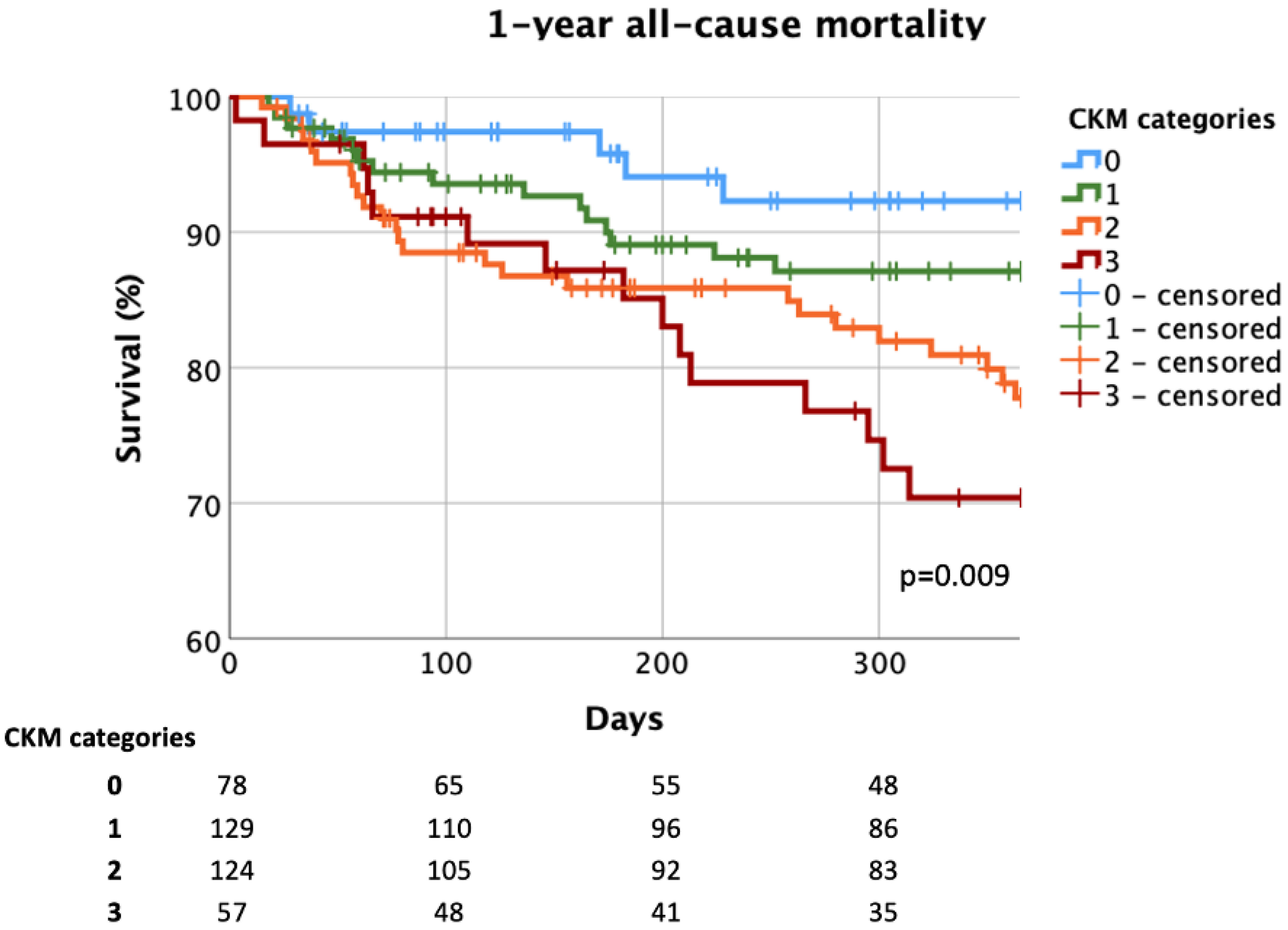
| Parameters | Total Cohort n = 388 | 0–3 CMs n = 89 | 4–6 CMs n = 182 | ≥7 CMs n = 117 | p-Value |
|---|---|---|---|---|---|
| Male gender (%) | 76 | 73 | 81 | 68 | 0.033 |
| Age, median [IQR], years | 61 [50–70] | 52 [44–63] | 60 [50–71] | 68 [60–75] | <0.001 |
| Previous hospitalisation primarily due to HF (%) | 41 | 29 | 36 | 60 | <0.001 |
| De novo HF (%) | 39 | 51 | 41 | 19 | <0.001 |
| LVEF, median [IQR], % | 25 [20–30] | 24 [19–29] | 25 [20–30] | 26 [20–31] | 0.474 |
| Heart rate, median [IQR], min−1 | 90 [76–108] | 92 [76–100] | 95 [79–112] | 84 [73–102] | 0.017 |
| Systolic blood pressure, median [IQR], mmHg | 120 [110–139] | 120 [110–133] | 124 [110–143] | 116 [104–137] | 0.092 |
| Laboratory parameters at admission | |||||
| creatinine, median [IQR], μmol/L | 113 [89–136] | 91 [77–104] | 108 [86–130] | 134 [115–177] | <0.001 |
| eGFR, median [IQR], mL/min/1.73 m2 | 57 [46–74] | 72 [60–86] | 59 [51–75] | 44 [33–54] | <0.001 |
| potassium, median [IQR], mmol/L | 4.1 [3.9–4.5] | 4.1 [3.9–4.4] | 4.2 [3.9–4.5] | 4.1 [3.9–4.6] | 0.482 |
| sodium, median [IQR], mmol/L | 138 [135–140] | 138 [137–140] | 137 [135–140] | 138 [135–140] | 0.135 |
| haemoglobin, median [IQR], g/L | 140 [124–152] | 146 [137–158] | 140 [127–154] | 130 [114–143] | <0.001 |
| NT-proBNP, median [IQR], pg/mL | 5286 [2570–9923] | 4209 [1912–9058] | 4986 [2507–9365] | 6477 [3600–14,736] | 0.004 |
| Parameters | Total Cohort n = 388 |
|---|---|
| CV CMs | |
| Hypertension (%) | 65 |
| Atrial fibrillation/flutter (%) | 44 |
| CAD (%) | 42 |
| VHD (%) | 20 |
| Stroke (%) | 10 |
| PAD (%) | 8 |
| Non-CV CMs | |
| Dyslipidaemia (%) | 75 |
| Iron deficiency (%) | 74 |
| Kidney dysfunction (%) | 57 |
| Obesity (%) | 35 |
| DM (%) | 35 |
| Hyperuricaemia (%) | 34 |
| Anaemia (%) | 22 |
| Hypo-/hyperthyroidism (%) | 17 |
| Asthma/COPD (%) | 17 |
| Sleep-disordered breathing (%) | 2 |
| Number of CMs, median [IQR] | 5 [4–7] |
| ≥1 CMs (%) | 99 |
| ≥2 CMs (%) | 96 |
| ≥3 CMs (%) | 89 |
| ≥4 CMs (%) | 77 |
| ≥5 CMs (%) | 62 |
| ≥6 CMs (%) | 44 |
| ≥7 CMs (%) | 30 |
| ≥8 CMs (%) | 16 |
| ≥9 CMs (%) | 9 |
| Number of CV CMs, median [IQR] | 2 [1–3] |
| ≥1 CV CMs (%) | 90 |
| ≥2 CV CMs (%) | 60 |
| ≥3 CV CMs (%) | 28 |
| ≥4 CV CMs (%) | 9 |
| Number of non-CV CMs, median [IQR] | 3 [2–4] |
| ≥1 non-CV CMs (%) | 98 |
| ≥2 non-CV CMs (%) | 88 |
| ≥3 non-CV CMs (%) | 67 |
| ≥4 non-CV CMs (%) | 46 |
| ≥5 non-CV CMs (%) | 24 |
| ≥6 non-CV CMs (%) | 12 |
| Medical and Device Therapy | At Admission n = 388 | At Discharge n = 388 | p-Value |
|---|---|---|---|
| RASi (%) | 61 | 91 | <0.001 |
| ACEi/ARB (%) | 46 | 68 | <0.001 |
| ARNI (%) | 15 | 23 | <0.001 |
| βB (%) | 61 | 85 | <0.001 |
| MRA (%) | 46 | 95 | <0.001 |
| TT (%) | 14 | 82 | <0.001 |
| SGLT2i (%) | 34 | 59 | <0.001 |
| QT (%) | 9 | 54 | <0.001 |
| TD RASi (%) | 16 | 18 | 0.644 |
| TD ACEi/ARB (%) | 11 | 15 | 0.229 |
| TD ARNI (%) | 5 | 3 | 0.031 |
| TD βB (%) | 14 | 7 | <0.001 |
| TD MRA (%) | 18 | 65 | <0.001 |
| TD TT (%) | 3 | 2 | 0.727 |
| TD QT (%) | 2 | 2 | 1.000 |
| CRT-P/CRT-D (%) | 12 | 16 | <0.001 |
| ICD (except for CRT-D) (%) | 10 | 15 | <0.001 |
| 1-Year All-Cause Mortality—Univariate Cox-Regression | ||||
| HR | 95% CI | p-Value | ||
| Age (/1 year) | 1.031 | 1.011 | 1.052 | 0.003 |
| Female gender | 0.816 | 0.434 | 1.537 | 0.530 |
| Heart rate (/1 min−1) | 0.992 | 0.980 | 1.003 | 0.158 |
| Systolic blood pressure (/1 mmHg) | 0.987 | 0.975 | 0.998 | 0.024 |
| Potassium at discharge > 4.5 mmol/L | 1.249 | 0.713 | 2.191 | 0.437 |
| LVEF (/1%) | 0.958 | 0.925 | 0.993 | 0.018 |
| De novo HF | 0.341 | 0.177 | 0.657 | 0.001 |
| TT/QT at discharge | 0.290 | 0.173 | 0.489 | <0.001 |
| CRT at discharge | 2.119 | 1.208 | 3.715 | 0.009 |
| ≥5 CMs | 3.005 | 1.562 | 5.780 | 0.001 |
| CAD | 1.079 | 0.650 | 1.793 | 0.768 |
| Hypertension | 0.939 | 0.555 | 1.587 | 0.813 |
| Atrial fibrillation/flutter | 2.088 | 1.249 | 3.490 | 0.005 |
| VHD | 2.804 | 1.666 | 4.721 | <0.001 |
| Stroke | 1.736 | 0.854 | 3.527 | 0.127 |
| PAD | 1.976 | 0.939 | 4.161 | 0.073 |
| Obesity | 0.468 | 0.241 | 0.908 | 0.025 |
| DM | 2.077 | 1.251 | 3.446 | 0.005 |
| Kidney dysfunction | 2.217 | 1.236 | 3.977 | 0.008 |
| Hyperuricaemia | 0.939 | 0.555 | 1.587 | 0.813 |
| Hypo-/hyperthyroidism | 2.088 | 1.249 | 3.490 | 0.005 |
| Sleep-disordered breathing | 2.217 | 1.236 | 3.977 | 0.008 |
| Asthma/COPD | 1.201 | 0.713 | 2.021 | 0.491 |
| Anaemia | 1.246 | 0.662 | 2.346 | 0.496 |
| Iron deficiency | 1.776 | 0.787 | 4.005 | 0.167 |
| Dyslipidaemia | 1.209 | 0.665 | 2.200 | 0.534 |
| 1-Year All-Cause Mortality—Multivariate Cox-Regression | ||||
| Adjusted HR | 95% CI | p-Value | ||
| Age (/1 year) | 1.021 | 0.999 | 1.044 | 0.066 |
| Systolic blood pressure (/1 mmHg) | 0.991 | 0.980 | 1.002 | 0.116 |
| LVEF (/1%) | 0.962 | 0.927 | 0.999 | 0.043 |
| De novo HF | 0.499 | 0.241 | 1.031 | 0.060 |
| TT/QT at discharge | 0.391 | 0.229 | 0.669 | 0.001 |
| CRT at discharge | 1.217 | 0.664 | 2.229 | 0.525 |
| ≥5 CMs | 2.373 | 1.133 | 4.971 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bánfi-Bacsárdi, F.; Kazay, Á.; Gergely, T.G.; Forrai, Z.; Füzesi, T.P.; Hanuska, L.F.; Schäffer, P.P.; Pilecky, D.; Vámos, M.; Vértes, V.; et al. Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act. J. Clin. Med. 2025, 14, 139. https://doi.org/10.3390/jcm14010139
Bánfi-Bacsárdi F, Kazay Á, Gergely TG, Forrai Z, Füzesi TP, Hanuska LF, Schäffer PP, Pilecky D, Vámos M, Vértes V, et al. Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act. Journal of Clinical Medicine. 2025; 14(1):139. https://doi.org/10.3390/jcm14010139
Chicago/Turabian StyleBánfi-Bacsárdi, Fanni, Ádám Kazay, Tamás G. Gergely, Zsolt Forrai, Tamás Péter Füzesi, Laura Fanni Hanuska, Pál Péter Schäffer, Dávid Pilecky, Máté Vámos, Vivien Vértes, and et al. 2025. "Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act" Journal of Clinical Medicine 14, no. 1: 139. https://doi.org/10.3390/jcm14010139
APA StyleBánfi-Bacsárdi, F., Kazay, Á., Gergely, T. G., Forrai, Z., Füzesi, T. P., Hanuska, L. F., Schäffer, P. P., Pilecky, D., Vámos, M., Vértes, V., Dékány, M., Andréka, P., Piróth, Z., Nyolczas, N., & Muk, B. (2025). Therapeutic Consequences and Prognostic Impact of Multimorbidity in Heart Failure: Time to Act. Journal of Clinical Medicine, 14(1), 139. https://doi.org/10.3390/jcm14010139






