Efficacy and Safety of Three Cycles of TIP and Sequential High Dose Chemotherapy in Patients with Testicular Non-Seminomatous Germ Cell Tumors
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillessen, S.; Sauvé, N.; Collette, L.; Daugaard, G.; de Wit, R.; Albany, C.; Tryakin, A.; Fizazi, K.; Stahl, O.; Gietema, J.A.; et al. Predicting Outcomes in Men With Metastatic Nonseminomatous Germ Cell Tumors (NSGCT): Results From the IGCCCG Update Consortium. J. Clin. Oncol. 2021, 39, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J. Paclitaxel (Taxol) combination therapy for resistant germ cell tumors. Semin. Oncol. 2000, 27 (Suppl. 1), 33–35. [Google Scholar]
- Chovanec, M.; Adra, N.; Abu Zaid, M.; Abonour, R.; Einhorn, L. High-dose chemotherapy for relapsed testicular germ cell tumours. Nat. Rev. Urol. 2023, 20, 217–225. [Google Scholar] [CrossRef]
- Connolly, E.A.; Weickhardt, A.; Grimison, P.; Asher, R.; Heller, G.Z.; Lewin, J.; Liow, E.; Toner, G.; Tung, I.L.Y.; Tran, B.; et al. High-dose chemotherapy for relapsed germ cell tumours: Outcomes in low-volume specialized centres. BJU Int. 2022, 130 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Yildiran Keskin, G.S.; Erturk, I.; Aykan, M.B.; Acar, R.; Dumludag, A.; Topal, A.; Koseoglu, C.; Kuzu, O.F.; Ornek, E.; Karadurmus, N. High-Dose Chemotherapy and Autologous Stem Cell Transplantation for Salvage Therapy of Relapsed/Refractory Germ Cell Tumors: A Single-Center Experience. Oncol. Res. Treat. 2024, 47, 262–272. [Google Scholar] [CrossRef]
- Albany, C. Systemic immune-inflammation index in germ-cell tumours: Search for a biological prognostic biomarker. Br. J. Cancer 2018, 118, 761–762. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.J.; Feldman, D.R. Conventional-Dose versus High-Dose Chemotherapy for Relapsed Germ Cell Tumors. Adv. Urol. 2018, 2018, 7272541. [Google Scholar] [CrossRef]
- Erturk, I.; Karadurmus, N.; Kızıloz, H.; Acar, R.; Yildiz, B.; Aykan, M.B.; Esen, R.; Buyukturan, G.; Urun, Y.; Erdem, G.; et al. Treating relapsed and refractory metastatic germ cell tumours with high-dose chemotherapy with carboplatin and etoposide and autologous haematopoietic stem cell transplantation. J. Oncol. Pharm. Pract. 2021, 27, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Adra, N.; Abonour, R.; Althouse, S.K.; Albany, C.; Hanna, N.H.; Einhorn, L.H. High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience. J. Clin. Oncol. 2017, 35, 1096–1102. [Google Scholar] [CrossRef]
- Pico, J.L.; Rosti, G.; Kramar, A.; Wandt, H.; Koza, V.; Salvioni, R.; Theodore, C.; Lelli, G.; Siegert, W.; Horwich, A.; et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann. Oncol. 2005, 16, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.H.; Williams, S.D.; Chamness, A.; Brames, M.J.; Perkins, S.M.; Abonour, R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N. Engl. J. Med. 2007, 357, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Sheinfeld, J.; Bajorin, D.F.; Fischer, P.; Turkula, S.; Ishill, N.; Patil, S.; Bains, M.; Reich, L.M.; Bosl, G.J.; et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: Results and prognostic factor analysis. J. Clin. Oncol. 2010, 28, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Knezevic, A.; Bromberg, M.; Patil, S.; Sheinfeld, J.; Carver, B.S.; Bains, M.; Jones, D.R.; Bajorin, D.F.; Bosl, G.J.; et al. Paclitaxel, Ifosfamide, and Cisplatin as Initial Salvage Chemotherapy for Germ Cell Tumors: Long-Term Follow-Up and Outcomes for Favorable- and Unfavorable-Risk Disease. J. Clin. Oncol. 2024, 42, 3130–3139. [Google Scholar] [CrossRef]
- Feldman, D.R.; Huddart, R.; Hall, E.; Beyer, J.; Powles, T. Is high dose therapy superior to conventional dose therapy as initial treatment for relapsed germ cell tumors? The TIGER Trial. J. Cancer 2011, 2, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Tellini, R.; Grosso, A.A.; Zaccaro, C.; Viola, L.; Di Maida, F.; Mari, A.; Carini, M.; Minervini, A.; Masieri, L. Can we predict the development of symptomatic lymphocele following robot-assisted radical prostatectomy and lymph node dissection? Results from a tertiary referral Centre. Scand. J. Urol. 2020, 54, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- van Soest, R.J.; Templeton, A.J.; Vera-Badillo, F.E.; Mercier, F.; Sonpavde, G.; Amir, E.; Tombal, B.; Rosenthal, M.; Eisenberger, M.A.; Tannock, I.F.; et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for men with metastatic castration-resistant prostate cancer receiving first-line chemotherapy: Data from two randomized phase III trials. Ann. Oncol. 2015, 26, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Sander, S.; Roth, L.; Gross, O.; Eberli, D.; Sulser, T.; Seifert, B.; Beyer, J.; Hermanns, T. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br. J. Cancer 2018, 118, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Cursano, M.C.; Kopf, B.; Scarpi, E.; Menna, C.; Casadei, C.; Schepisi, G.; Lolli, C.; Altavilla, A.; Gallà, V.; Santini, D.; et al. Prognostic Role of Systemic Inflammatory Indexes in Germ Cell Tumors Treated With High-Dose Chemotherapy. Front. Oncol. 2020, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Aykan, M.B.; Yildiran, G.S.; Akcan, E.; Acar, R.; Erturk, I.; Karadurmus, N. Efficacy of Gemcitabine, Paclitaxel, and Oxaliplatin Protocol in the Treatment of Relapsed or Refractory Germ Cell Tumours. J. Coll. Physicians Surg. Pak. 2022, 32, 880–884. [Google Scholar]
- Ayash, L.J.; Clarke, M.; Silver, S.M.; Braun, T.; Uberti, J.; Ratanatharathorn, V.; Reynolds, C.; Ferrara, J.; Broun, E.R.; Adams, P.T. Double dose-intensive chemotherapy with autologous stem cell support for relapsed and refractory testicular cancer: The University of Michigan experience and literature review. Bone Marrow Transplant. 2001, 27, 939–947. [Google Scholar] [CrossRef][Green Version]
- Beyer, J.; Kingreen, D.; Krause, M.; Schleicher, J.; Schwaner, I.; Schwella, N.; Huhn, D.; Siegert, W. Long-term survival of patients with recurrent or refractory germ cell tumors after high dose chemotherapy. Cancer 1997, 79, 161–168. [Google Scholar] [CrossRef]
- Zujuan, S.; Xin, D.; Yang, H.; Guifu, Z. Potential Next Generation Markers of Testicular Germ Cell Tumors: miRNA-371a-3p. Int. Urol. Nephrol. 2024, 1–10. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 141) |
|---|---|
| Gender, male, n (%) | 141 (%100) |
| Primary, gonadal, n (%) | 141 (100) |
| Age, years, median (min–max) | 27 (18–51) |
| Age, <30 years, n (%) | 86 (61) |
| Histology, Non-seminoma, n (%) | 141 (100) |
| Non-seminoma subtype, n (%) | |
| 108 (76.6) |
| 4 (2.8) |
| 16 (11.3) |
| 5 (3.5) |
| 8 (5.7) |
| Clinical stage at first diagnosis, (AJCC, 8th), n (%) | |
| 50 (35.5) |
| 91 (64.5) |
| IGCCCG, n (%) | |
| 35 (24.8) |
| 23 (16.3) |
| 83 (58.9) |
| History of first line chemotherapy, n (%) | |
| 138 (97.9) |
| 3 (2.1) |
| Metastatic areas before HDC, n (%) | |
| 98 (69.5) |
| 130 (92.2) |
| 27 (19.1) |
| 15 (10.6) |
| 18 (12.8) |
| Liver, Bone, Brain metastasis before HDC, n (%) | |
| 49 (34.8) |
| Platinum Refractory Disease, n (%) | |
| 6 (4.3) |
| Late Relapse, n (%) | |
| 20 (14.2) |
| AFP before HDC, n (%) | |
| 29 (20.6) |
| Beta HCG before HDC, n (%) | |
| 36 (25.5) |
| AFP and/or Beta HCG elevation before HDC | |
| 58 (41.1) |
| IPFSG, n (%) | |
| 26 (18.4) |
| 74 (52.5) |
| 25 (17.7) |
| 16 (11.3) |
| HDC protocol, n (%) | |
| 135 (95.7) |
| 6 (4.3) |
| Follow up (months), median (SE) | 35.2 (2.9) |
| Response after HDC, n (%) | |
| 84 (59.6) |
| 24 (17) |
| 33 (23.4) |
| 108 (76.6) |
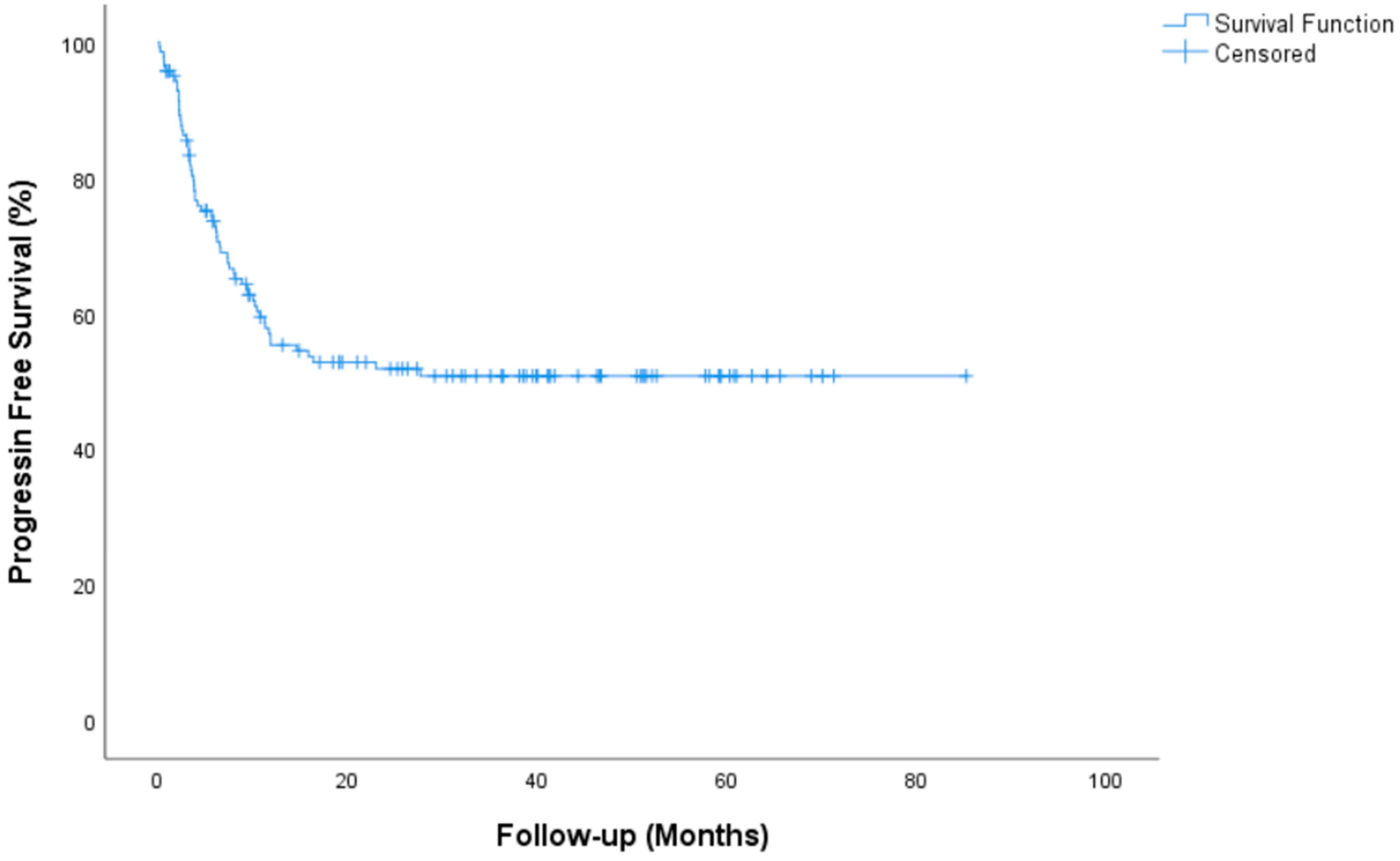
| Progression Free Survival, median (IQR) | NR (NR-NR) |
| 2 year PFS rate, % (SE) | 51.8 (4.4) |
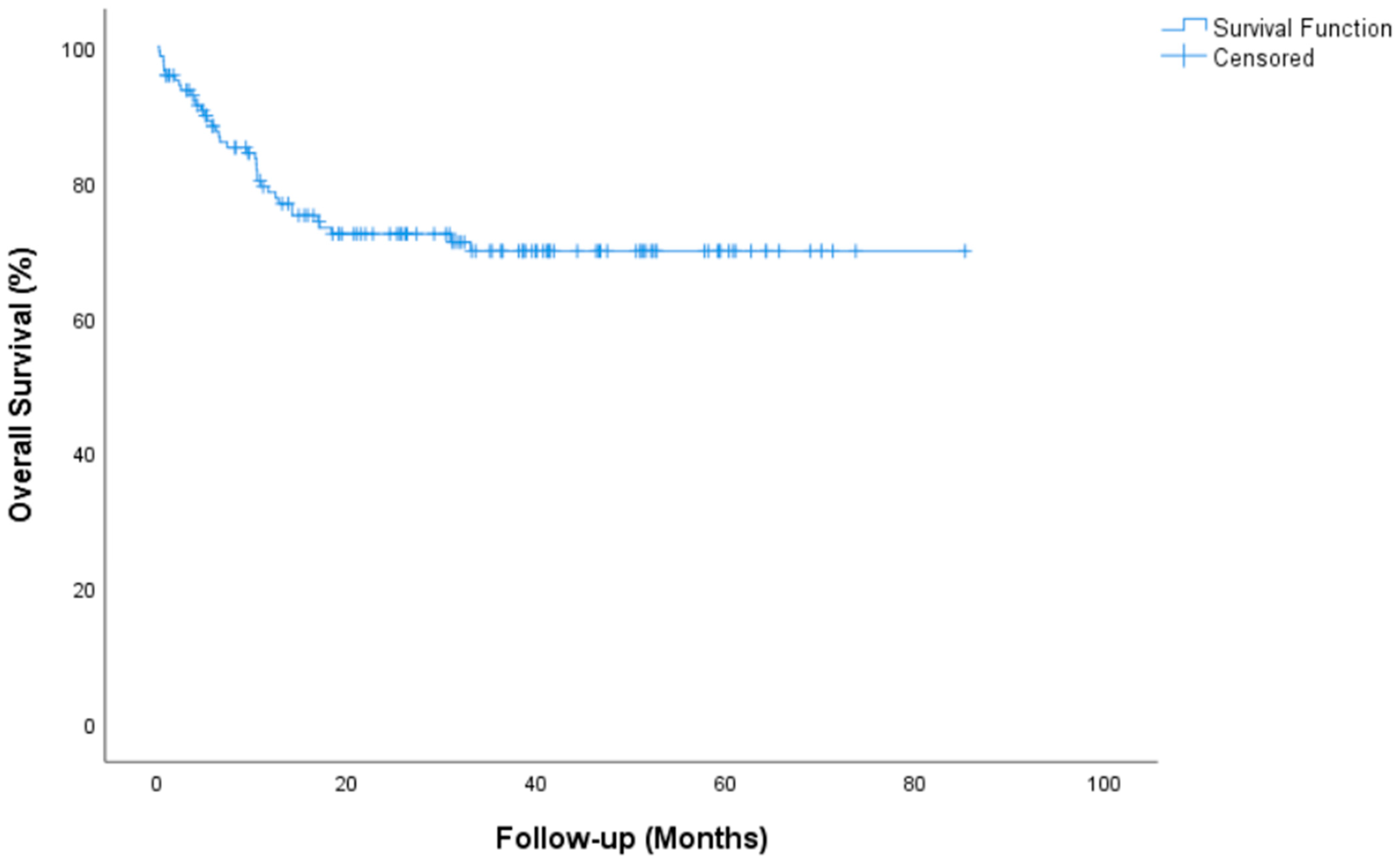
| Overall Survival, median (IQR) | NR (NR-NR) |
| 2 year OS rate, % (SE) | 72.3 (4) |
| Resection after HDC, n (%) | |
| 20 (14.2) |
| SII * | |
| 73 (51.8) |
| 68 (48.2) |
| Characteristics | Total (n = 141) |
|---|---|
| Engraftment Day, (median) IQR | 12 (3) |
| Neutropenia, n (%) | |
| 141 (100) |
| Anemia, n (%) | |
| 1 (0.7) |
| 36 (25.4) |
| 94 (66.7) |
| 9 (6.4) |
| 1 (0.7) |
| Thrombocytopenia, n (%) | |
| 2 (1.4) |
| 0 (0) |
| 3 (2.1) |
| 136 (96.5) |
| Number of ES transfusions after HDC, median (IQR) | 2 (2) |
| Number of TS transfusions after HDC, median (IQR) | 4 (4) |
| Kidney Failure, n (%) | |
| 3 (2.1) |
| Liver enzyme elevation, n (%) | |
| 91 (64.5) |
| 26 (18.4) |
| 15 (10.6) |
| 7 (5) |
| 2 (1.4) |
| Transplantation-related mortality, n (%) | |
| 10 (7.1) |
| Variables | n (%) | Median PFS, month (95% CI) | Univariable p-Value | Multivariable p-Value | HR (95% CI) |
|---|---|---|---|---|---|
| Age | |||||
| <30 | 86 (61) | NR | 0.077 | ||
| ≥30 | 55 (39) | 10.7 (5.5–15.9) | |||
| Histology, Non-seminoma | |||||
| Mixt Germ cell tumors | 108 (76.6) | NR | 0.032 | 0.093 | NE |
| Coryocarcinoma | 4 (2.8) | 8 (2.3–13.8) | 0.657 | 0.2–2.74 | |
| Embriyonal carcinoma | 16 (11.3) | NR | 0.568 | 0.52–3.2 | |
| Yolk sac tumor | 5 (3.5) | 3.5 (1.4–5.5) | 0.011 | 1.34–9.9 | |
| Immature teratoma | 8 (5.7) | NR | 0.327 | 0.15–1.86 | |
| Clinical stage at first diagnosis, (AJCC, 8th) | |||||
| Stage I–II | 50 (35.5) | NR | 0.142 | ||
| Stage III | 91 (64.5) | 15.8 (-) | |||
| IGCCCG | |||||
| Good | 35 (24.8) | NR | 0.046 | 0.79 | NE |
| Intermediate | 23 (16.3) | NR | 0.71 | 0.31–2.21 | |
| Poor | 83 (58.9) | 11.8 (3.6–20) | 0.72 | 0.57–2.2 | |
| Pulmonary metastasis | |||||
| Yes | 43 (30.5) | NR | 0.021 | 0.15 | 0.84–2.96 |
| No | 98 (69.5) | 11.8 (0–27.6) | |||
| Liver, Bone, Brain metastasis | |||||
| Yes | 49 (34.8) | NR | 0.020 | 0.092 | 0.92–3 |
| No | 92 (65.2) | 7.5 (3.2–11.7) | |||
| Platinum Refractory Disease | |||||
| Yes | 6 (4.3) | NR | 0.001 | 0.021 | 1.22–11.2 |
| No | 135 (95.7) | 3 (2.4–3.5) | |||
| Late Relapse | |||||
| Absent | 121 (85.8) | NR | 0.133 | ||
| Present | 20 (14.2) | 11.7 (5.8–17.5) | |||
| IPFSG | |||||
| Low risk | 26 (18.4) | NR | 0.001 | 0.976 | |
| Intermediate risk | 74 (52.5) | NR | |||
| High risk | 25 (17.7) | 11.8 (0–30.9) | |||
| Very high risk | 16 (11.3) | 3.9 (1.35–6.4) | |||
| AFP and/or Beta HCG elevation before HDC | |||||
| Absent | 83 (58.9) | NR | 0.001 | 0.001 | 2.9–9.6 |
| Present | 58 (41.1) | 6.23 (3.3–9.0) | |||
| HDC protocol, n (%) | |||||
| CE | 135 (95.7) | NR | 0.444 | ||
| ICE | 6 (4.3) | 10.4 (0.1–20.7) | |||
| Response after HDC | |||||
| CR or marker negative PR | 84 (59.6) | NR | 0.001 | ||
| Marker positive PR or SD | 24 (17) | 7.5 (4.4–10.5) | |||
| PD | 33 (23.4) | 2.6 (1.7–3.6) | |||
| Resection after HDC | |||||
| Absent | 121 (85.8) | NR | |||
| Present | 20 (14.2) | 27.66 (0.17–55.1) | 0.754 | ||
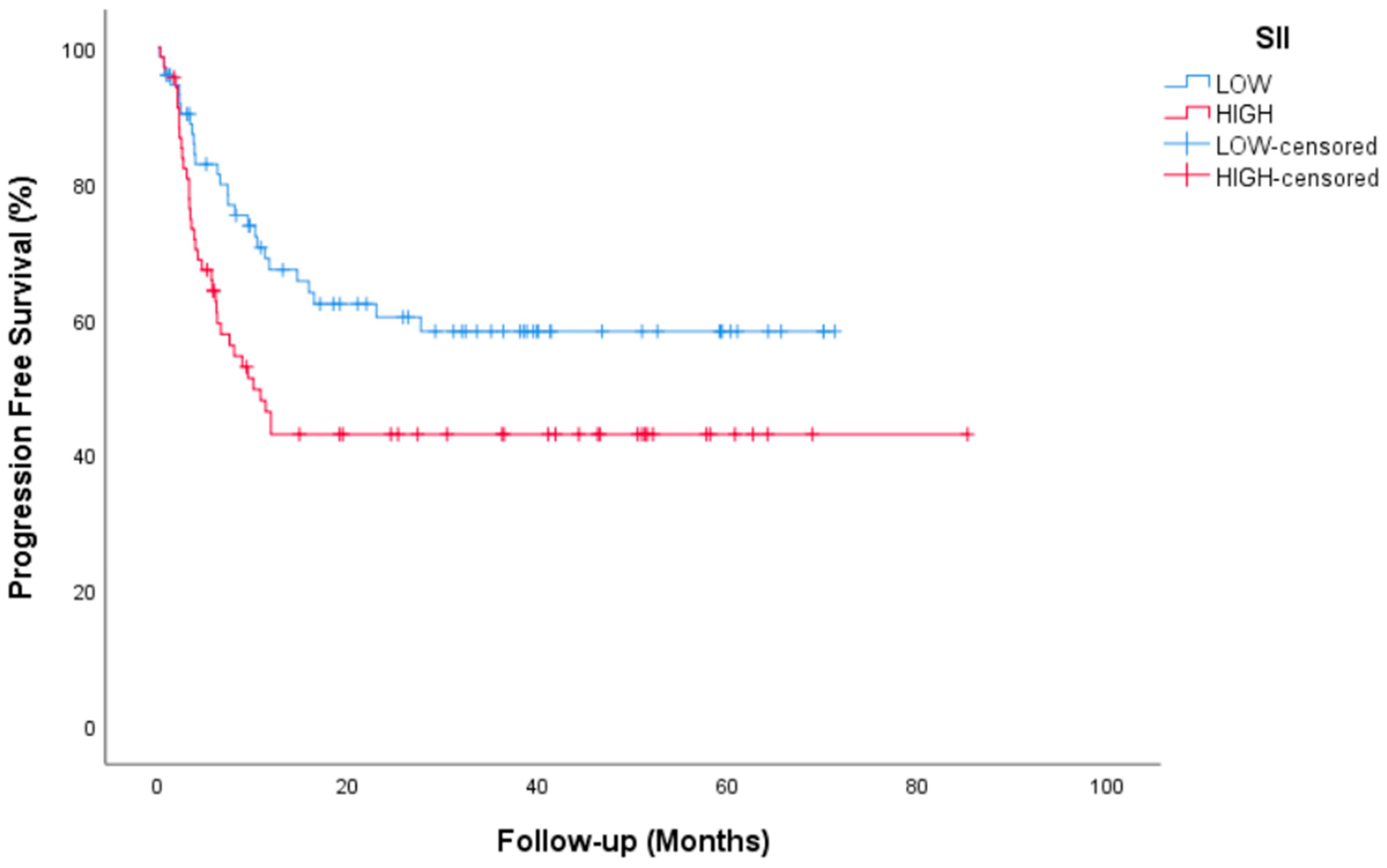
| SII | |||||
| Low | 73 (51.8) | NR | 0.026 | 0.027 | 1–3.2 |
| High | 68 (48.2) | 10 (5.9–14) | |||
| Variables | n (%) | Median OS, month (95% CI) | Univariable p-Value | Multivariable p-Value | HR (95% CI) |
|---|---|---|---|---|---|
| Age | |||||
| <30 | 86 (61) | NR | 0.295 | ||
| ≥30 | 55 (39) | NR | |||
| Histology, Non-seminoma | |||||
| Mixt Germ cell tumors | 108 (76.6) | NE | 0.046 | 0.555 | NE |
| Coryocarcinoma | 4 (2.8) | NE | 0.663 | 0.33–5.6 | |
| Embriyonal carcinoma | 16 (11.3) | NE | 0.466 | 0.44–5.7 | |
| Yolk sac tumor | 5 (3.5) | NE | 0.094 | 0.82–12.3 | |
| Immature teratoma | 8 (5.7) | NE | 0.979 | NE | |
| Clinical stage at first diagnosis, (AJCC, 8th) | |||||
| Stage I–II | 50 (35.5) | NR | 0.143 | ||
| Stage III | 91 (64.5) | NR | |||
| IGCCCG | |||||
| Good | 35 (24.8) | NR | 0.013 | 0.223 | NE |
| Intermediate | 23 (16.3) | NR | 0.867 | 0.24–5.4 | |
| Poor | 83 (58.9) | NR | 0.131 | 0.78–6.5 | |
| Pulmonary metastasis | |||||
| Yes | 43 (30.5) | NR | 0.042 | 0.298 | 0.66–3.8 |
| No | 98 (69.5) | NR | |||
| Liver, Bone, Brain metastasis | |||||
| Yes | 49 (34.8) | NR | 0.014 | 0.852 | 0.5–2.27 |
| No | 92 (65.2) | NR | |||
| Platinum Refractory Disease | |||||
| Yes | 6 (4.3) | 6.56 (3.7–9.4) | 0.001 | 0.030 | 1.14–13.21 |
| No | 135 (95.7) | NR | |||
| Late Relapse | |||||
| Absent | 121 (85.8) | NR | 0.750 | ||
| Present | 20 (14.2) | NR | |||
| IPFSG | |||||
| Low risk | 26 (18.4) | NR | 0.001 | 0.002 | NE |
| Intermediate risk | 74 (52.5) | NR | 0.140 | 0.6–37.03 | |
| High risk | 25 (17.7) | NR | 0.278 | 0.37–30.54 | |
| Very high risk | 16 (11.3) | 7.33 (1.06–13.6) | 0.010 | 1.96–150.53 | |
| AFP and/or Beta HCG elevation before HDC | |||||
| Absent | 83 (58.9) | NR | 0.001 | 0.001 | 2.36–11.84 |
| Present | 58 (41.1) | 17.06 (0–38.1) | |||
| HDC protocol, n (%) | |||||
| CE | 135 (95.7) | NR | 0.844 | ||
| ICE | 6 (4.3) | NR | |||
| Response after HDC | |||||
| CR or marker negative PR | 84 (59.6) | NR | 0.001 | ||
| Marker positive PR or SD | 24 (17) | NR | |||
| PD | 33 (23.4) | 6.16 (4.04–8.2) | |||
| Resection after HDC | |||||
| Absent | 121 (85.8) | NR | 0.122 | ||
| Present | 20 (14.2) | NR | |||
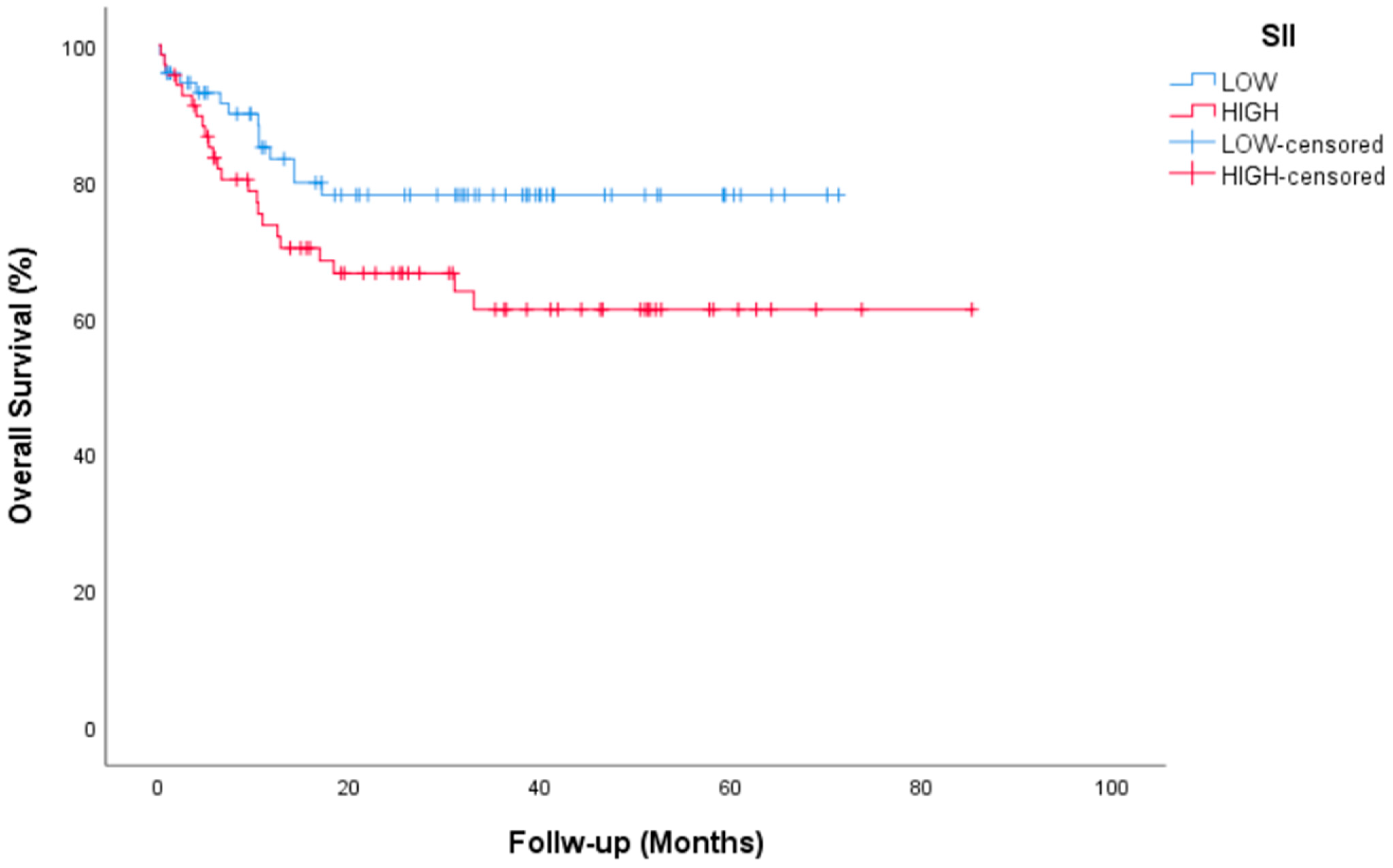
| SII | |||||
| Low | 73 (51.8) | NR | 0.063 | ||
| High | 68 (48.2) | NR | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aykan, M.B.; Yildiran Keskin, G.; Erturk, İ.; Acar, R.; Kose, A.F.; Karadurmus, N. Efficacy and Safety of Three Cycles of TIP and Sequential High Dose Chemotherapy in Patients with Testicular Non-Seminomatous Germ Cell Tumors. J. Clin. Med. 2025, 14, 131. https://doi.org/10.3390/jcm14010131
Aykan MB, Yildiran Keskin G, Erturk İ, Acar R, Kose AF, Karadurmus N. Efficacy and Safety of Three Cycles of TIP and Sequential High Dose Chemotherapy in Patients with Testicular Non-Seminomatous Germ Cell Tumors. Journal of Clinical Medicine. 2025; 14(1):131. https://doi.org/10.3390/jcm14010131
Chicago/Turabian StyleAykan, Musa Baris, Gulsema Yildiran Keskin, İsmail Erturk, Ramazan Acar, Ahmet Fatih Kose, and Nuri Karadurmus. 2025. "Efficacy and Safety of Three Cycles of TIP and Sequential High Dose Chemotherapy in Patients with Testicular Non-Seminomatous Germ Cell Tumors" Journal of Clinical Medicine 14, no. 1: 131. https://doi.org/10.3390/jcm14010131
APA StyleAykan, M. B., Yildiran Keskin, G., Erturk, İ., Acar, R., Kose, A. F., & Karadurmus, N. (2025). Efficacy and Safety of Three Cycles of TIP and Sequential High Dose Chemotherapy in Patients with Testicular Non-Seminomatous Germ Cell Tumors. Journal of Clinical Medicine, 14(1), 131. https://doi.org/10.3390/jcm14010131






