Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- Patients’ characteristics (sex, body weight, and age at intervention);
- -
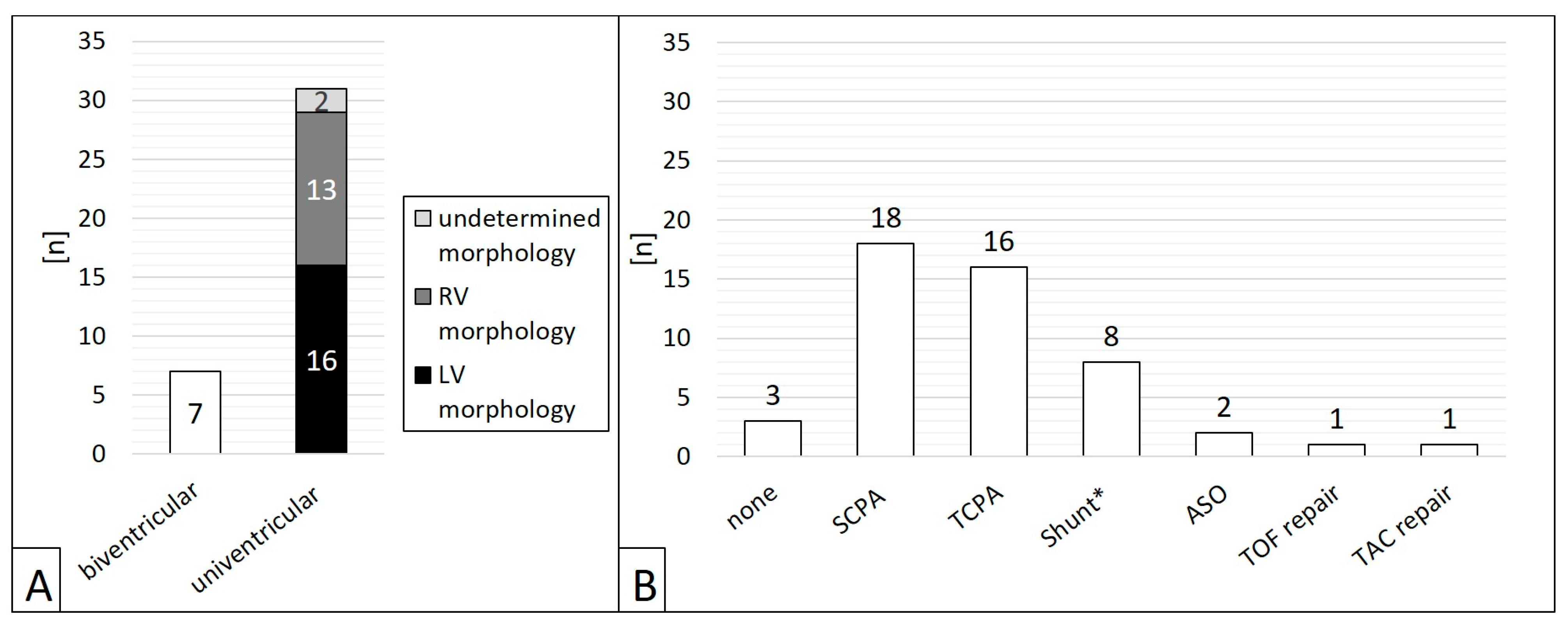
- Ventricular morphology (biventricular or univentricular anatomy);
- -
- Preceding surgery;
- -
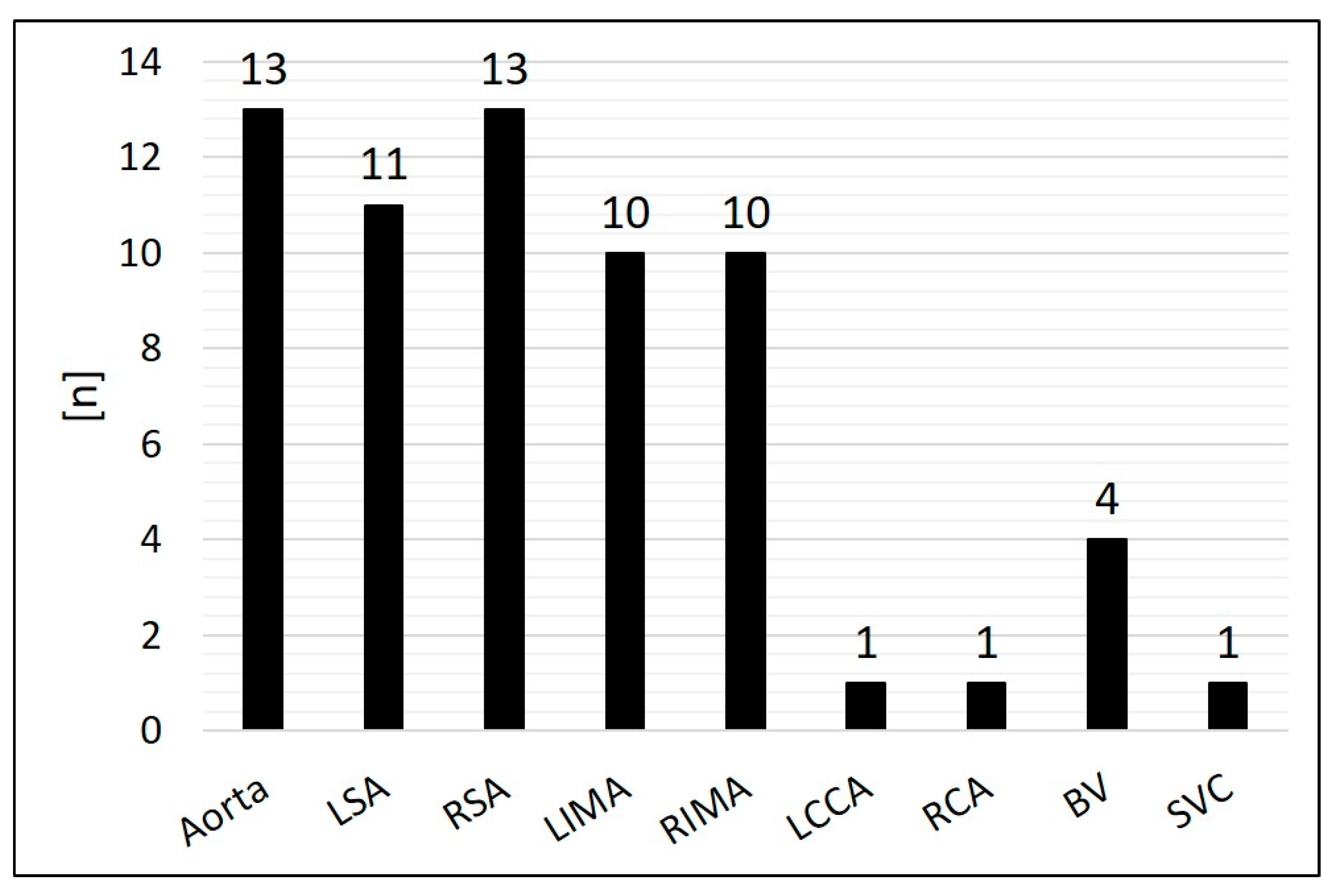
- Origin and diameter of the embolized SPCs;
- -
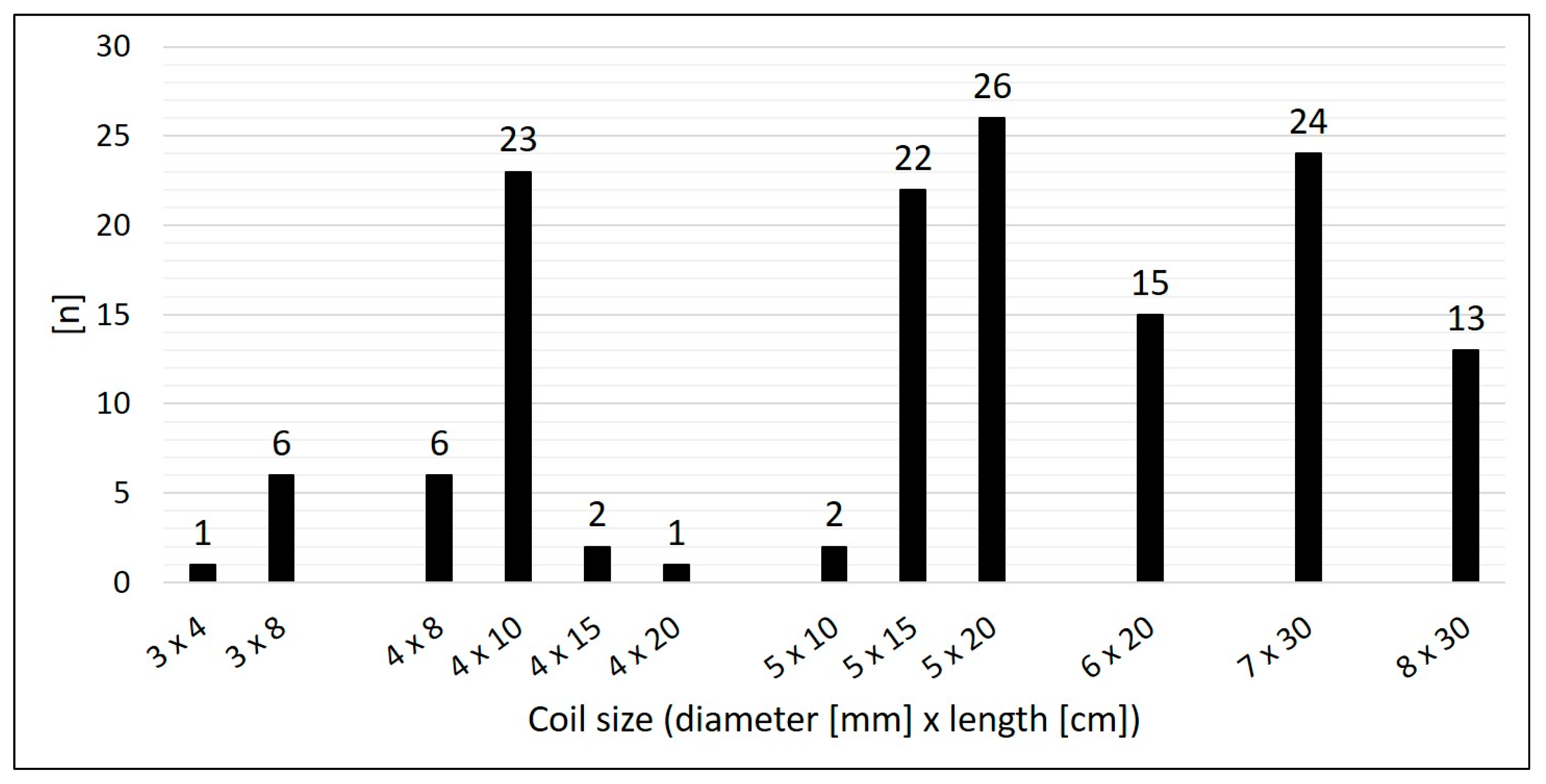
- Number and size of the implanted CHMs;
- -
- Oversizing of the CHMs;
- -
- Primary success of the embolization;
- -
- Procedure time (defined as time from the insertion of the sheath to the removal of the sheath) and fluoroscopy time;
- -
- Contrast dose and radiation exposure (dose area product);
- -
- Follow-up;
- -
- Complications (vascular injuries, haemorrhage, coil migration, non-target embolization, thrombosis, embolism, and mortality);
- -
- Use of plugs or coils other than CHMs for SPCs.
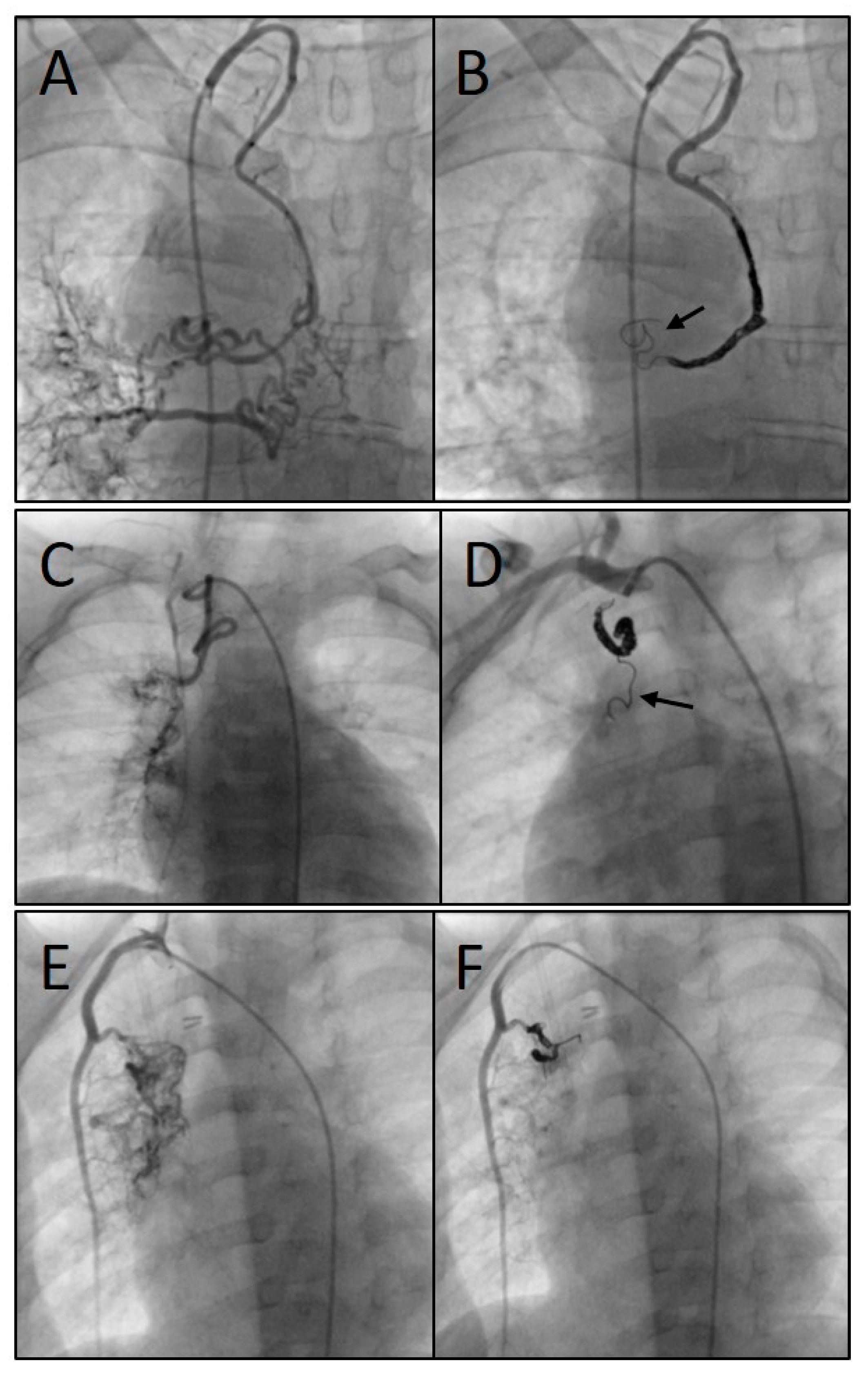
2.2. Catheterization and Transcatheter Embolization
2.3. Statistics
3. Results
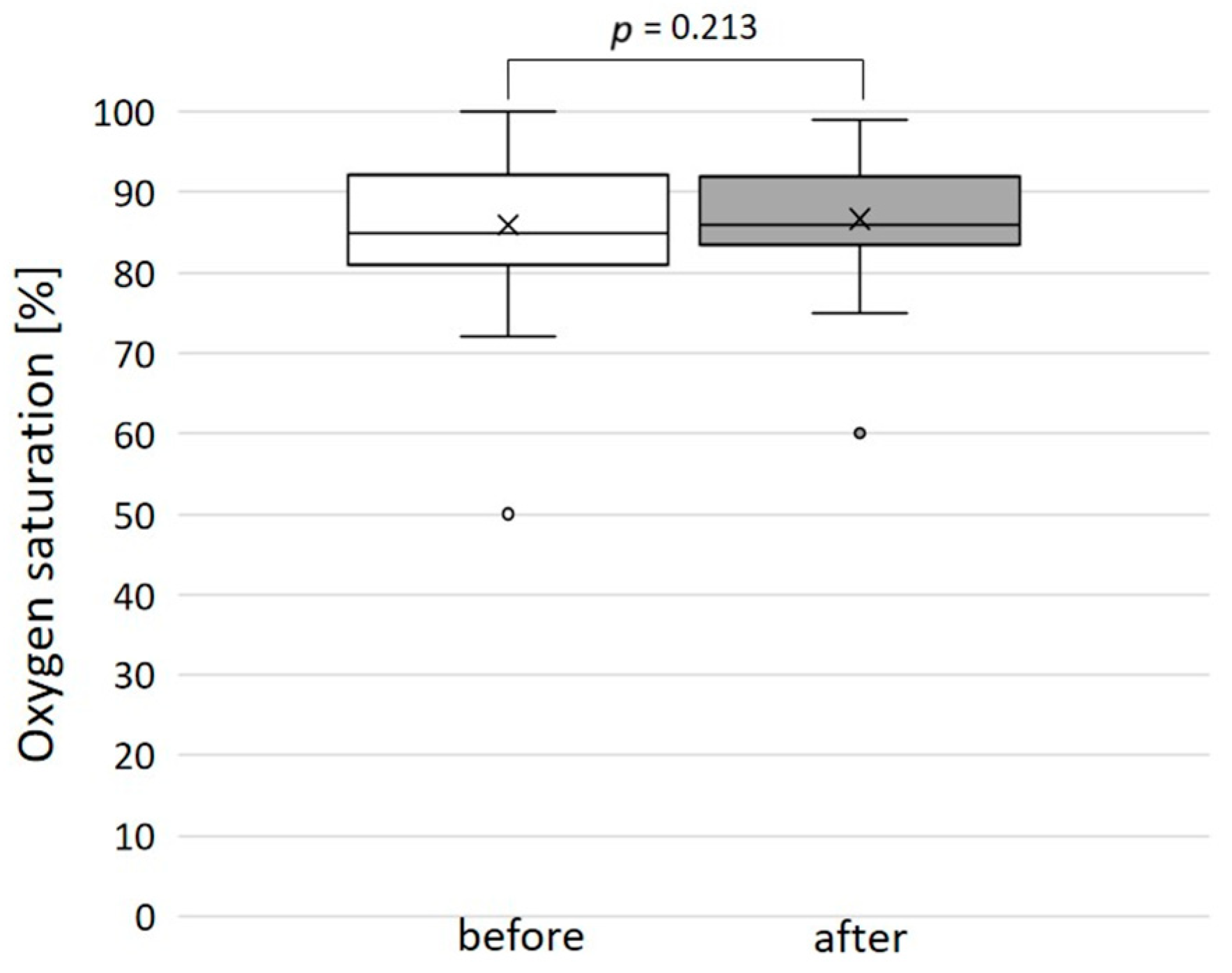
3.1. Patients’ Characteristics, Contrast Doses, and Radiation Exposure
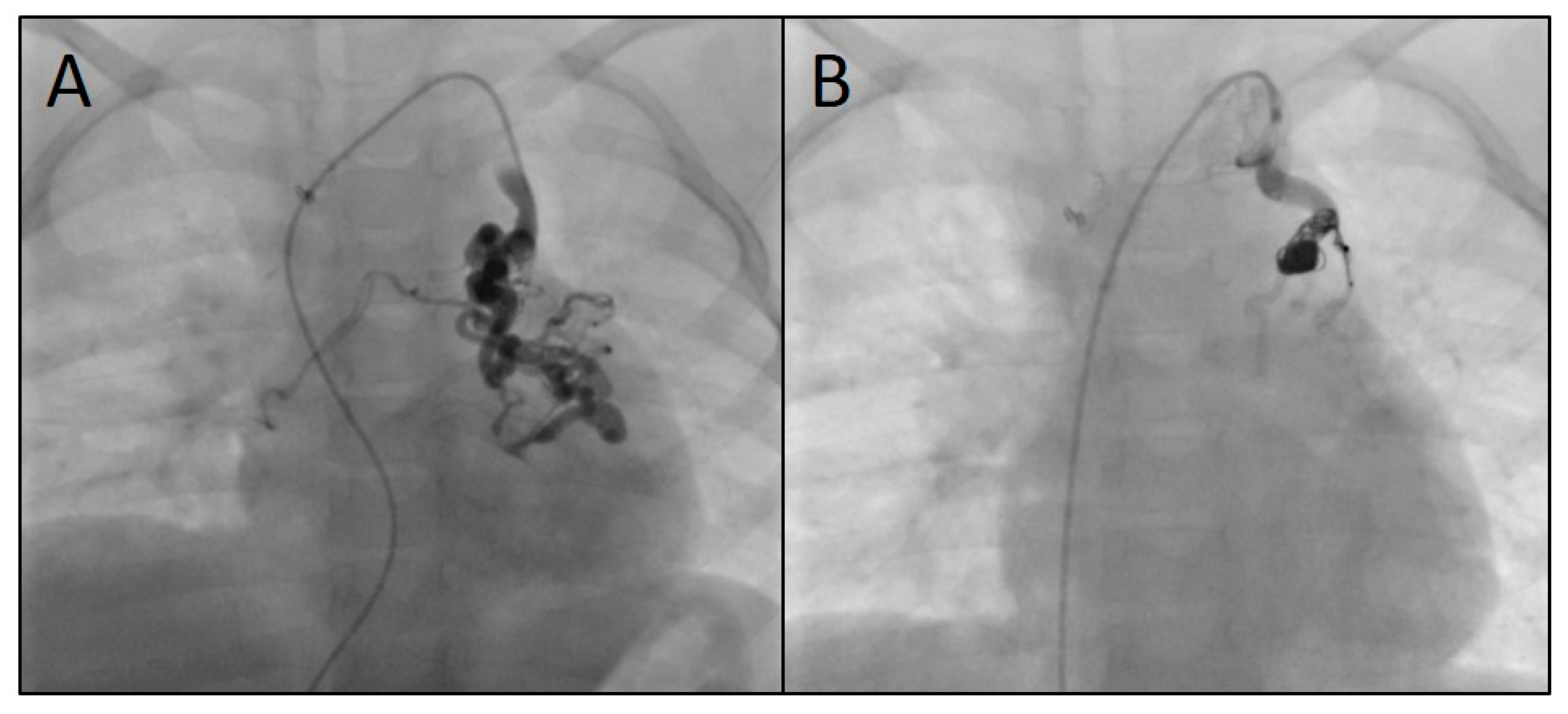
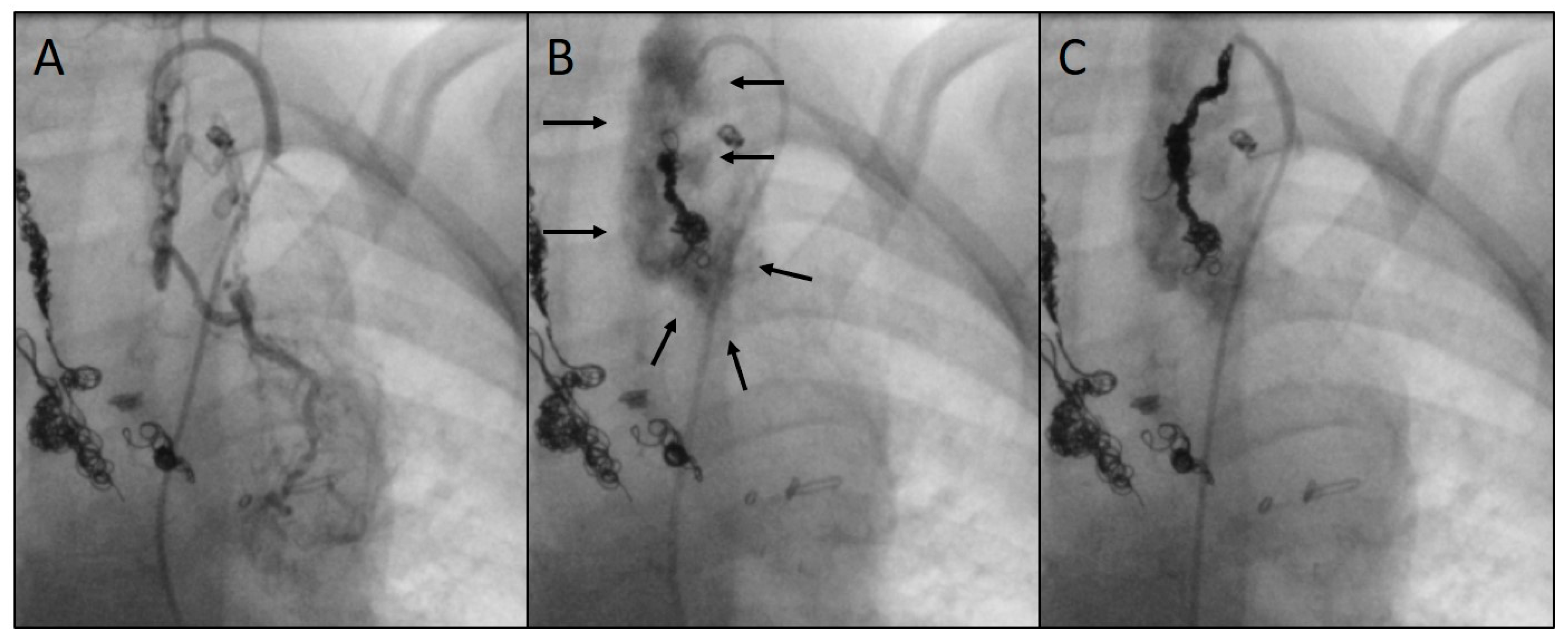
3.2. Characteristics of the Target Vessels, Characteristics of the Used Coils, and Coil Oversizing
3.3. Use of Devices Other than CHMs
3.4. Outcome, Complications, and Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHD | congenital heart disease |
| CHM | Concerto™ Helix microcoil (nylon-fibered) |
| MAPCA | major aorto–pulmonary collateral arteries |
| SCPA | superior cavo–pulmonary anastomosis |
| SPC | systemic-to-pulmonary collateral(s) |
| TCPA | total cavo–pulmonary anastomosis |
References
- Alex, A.; Ayyappan, A.; Valakkada, J.; Kramadhari, H.; Sasikumar, D.; Menon, S. Major Aortopulmonary Collateral Arteries. Radiol. Cardiothorac. Imaging 2022, 4, e210157. [Google Scholar] [CrossRef]
- Mohammad Nijres, B.; Aregullin, E.O.; Al-Khatib, Y.; Samuel, B.P.; Abdulla, R.I.; Hijazi, Z.M.; Vettukattil, J.J. Aortopulmonary Collaterals in Single Ventricle Physiology: Variation in Understanding Occlusion Practice Among Interventional Cardiologists. Pediatr. Cardiol. 2020, 41, 1608–1616. [Google Scholar] [CrossRef]
- Schmiel, M.; Ono, M.; Staehler, H.; Georgiev, S.; Burri, M.; Heinisch, P.P.; Strbad, M.; Ewert, P.; Hager, A.; Hörer, J. Impact of Anatomical Sub-types and Shunt Types on Aortopulmonary Collaterals in Hypoplastic Left Heart Syndrome. Semin. Thorac. Cardiovasc. Surg. 2023, 35, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, Q.; Meng, H.; Zhang, G.J.; Yang, J.X. Aortopulmonary Collateral Arteries in Noncyanotic Congenital Heart Disease. Ann. Thorac. Surg. 2022, 113, e125–e127. [Google Scholar] [CrossRef]
- Adamson, G.T.; McElhinney, D.B.; Zhang, Y.; Feinstein, J.A.; Peng, L.F.; Ma, M.; Algaze, C.A.; Hanley, F.L.; Perry, S.B. Angiographic Anatomy of Major Aortopulmonary Collateral Arteries and Association with Early Surgical Outcomes in Tetralogy of Fallot. J. Am. Heart Assoc. 2020, 9, e017981. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.T.; Peng, L.F.; Perry, S.B.; Hanley, F.L.; McElhinney, D.B. Comprehensive diagnostic catheterization in children with major aortopulmonary collateral arteries: A review of catheterization technique and anatomic nomenclature. Catheter. Cardiovasc. Interv. 2022, 99, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Averin, K.; Byrnes, J.W.; Benscoter, D.T.; Whiteside, W.; DeSena, H.; Hirsch, R.; Goldstein, B.H. Life-threatening airway bleeding after palliation of single ventricle congenital heart disease. Heart 2018, 104, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Van De Bruaene, A.; Budts, W. Collaterals in congenital heart disease: When and how to treat? Cardiovasc. Diagn. Ther. 2023, 13, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Batlivala, S.P.; Briscoe, W.E.; Ebeid, M.R. Particle embolization of systemic-to-pulmonary collateral artery networks in congenital heart disease: Technique and special considerations. Ann. Pediatr. Cardiol. 2018, 11, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, J.; Gheibeh, A.; Fries, P.; Poryo, M.; Rentzsch, A.; Abdul-Khaliq, H. Transcatheter Embolization in Congenital Cardiovascular Malformations—Variable Use of Vascular Plugs. Cardiovascular Therapeutics 2024, 2024, 4778469. [Google Scholar] [CrossRef]
- Schmiel, M.; Kido, T.; Georgiev, S.; Burri, M.; Heinisch, P.P.; Vodiskar, J.; Strbad, M.; Ewert, P.; Hager, A.; Hörer, J.; et al. Aortopulmonary collaterals in single ventricle: Incidence, associated factors and clinical significance. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac190. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.C.; Boshoff, D.E.; Eyskens, B.; Mertens, L.; Gewillig, M. Use of a microcatheter in a telescopic system to reach difficult targets in complex congenital heart disease. Catheter. Cardiovasc. Interv. 2009, 73, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.A.; Levi, D.S.; Moore, J.W. Embolization and transcatheter retrieval of coils and devices. Pediatr. Cardiol. 2005, 26, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kudumula, V.; Stumper, O.; Noonan, P.; Mehta, C.; De Giovanni, J.; Stickley, J.; Dhillon, R.; Bhole, V. Transcatheter Retrieval of Cardiovascular Foreign Bodies in Children: A 15-Year Single Centre Experience. Pediatr. Cardiol. 2017, 38, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, G.; Read, M.; Sohn, J.; Shah, C.; Shrivastava, S. In-vitro thrombogenicity assessment of polymer filament modified and native platinum embolic coils. J. Neurol. Sci. 2014, 339, 97–101. [Google Scholar] [CrossRef]
- Trerotola, S.O.; Pressler, G.A.; Premanandan, C. Nylon Fibered Versus Non-Fibered Embolization Coils: Comparison in a Swine Model. J. Vasc. Interv. Radiol. 2019, 30, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.J.; Lydon, E.; Gauvreau, K.; Jenkins, K.J.; Slater, D.; Bergersen, L. Exploring procedure duration and risk for serious adverse events during congenital cardiac catheterization. BMJ Surg. Interv. Health Technol. 2023, 5, e000142. [Google Scholar] [CrossRef] [PubMed]
- Haas, N.A.; Happel, C.M.; Mauti, M.; Sahyoun, C.; Tebart, L.Z.; Kececioglu, D.; Laser, K.T. Substantial radiation reduction in pediatric and adult congenital heart disease interventions with a novel X-ray imaging technology. Int. J. Cardiol. Heart Vasc. 2015, 6, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, N.; Ullah, M.; Younis, U.; Akhtar, K.; Mehmood, A. Perioperative major aortopulmonary collateral arteries (MAPCAs) coiling in tetralogy of fallot patients. J. Cardiol. Curr. Res. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Senthilnathan, S.; Gauvreau, K.; Marshall, A.C.; Lock, J.E.; Bergersen, L. Contrast administration in pediatric cardiac catheterization: Dose and adverse events. Catheter. Cardiovasc. Interv. 2009, 73, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Ohno, T.; Kakizaki, S.; Takegawa, M.; Yuuki, H.; Nakada, M.; Takahashi, S.; Zuguchi, M. Radiation dose to the pediatric cardiac catheterization and intervention patient. AJR Am. J. Roentgenol. 2010, 195, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, P.C.; Armstrong, A.K.; Glatz, A.C.; Goldstein, B.H.; Gudausky, T.M.; Leahy, R.A.; Petit, C.J.; Shahanavaz, S.; Trucco, S.M.; Bergersen, L.J. Radiation dose benchmarks in pediatric cardiac catheterization: A prospective multi-center C3PO-QI study. Catheter. Cardiovasc. Interv. 2017, 90, 269–280. [Google Scholar] [CrossRef]
- Goldstein, B.H.; Holzer, R.J.; Trucco, S.M.; Porras, D.; Murphy, J.; Foerster, S.R.; El-Said, H.G.; Beekman, R.H., 3rd; Bergersen, L. Practice Variation in Single-Ventricle Patients Undergoing Elective Cardiac Catheterization: A Report from the Congenital Cardiac Catheterization Project on Outcomes (C3PO). Congenit. Heart Dis. 2016, 11, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.M. Management of aortopulmonary collateral arteries in Fontan patients: Routine occlusion is not warranted. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2002, 5, 55–67. [Google Scholar] [CrossRef]
- Bradley, S.M.; McCall, M.M.; Sistino, J.J.; Radtke, W.A. Aortopulmonary collateral flow in the Fontan patient: Does it matter? Ann. Thorac. Surg. 2001, 72, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Powell, A.J. Aortopulmonary collaterals in single-ventricle congenital heart disease: How much do they count? Circ. Cardiovasc. Imaging 2009, 2, 171–173. [Google Scholar] [CrossRef]
- Prakash, A. Significance of systemic to pulmonary artery collaterals in single ventricle physiology: New insights from CMR imaging. Heart 2012, 98, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Ridderbos, F.S.; Chan, F.P.; van Melle, J.P.; Ebels, T.; Feinstein, J.A.; Berger, R.M.F.; Willems, T.P. Quantification of systemic-to-pulmonary collateral flow in univentricular physiology with 4D flow MRI. Cardiol. Young 2023, 33, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Glatz, A.C.; Hanna, B.D.; Gillespie, M.J.; Harris, M.A.; Keller, M.S.; Fogel, M.A.; Rome, J.J.; Whitehead, K.K. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: A pilot study. Circ. Cardiovasc. Interv. 2013, 6, 101–106. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Drolet, C.; Dragulescu, A.; Kotani, Y.; Chaturvedi, R.; Lee, K.J.; Mertens, L.; Taylor, K.; La Rotta, G.; van Arsdell, G.; et al. Aortopulmonary collateral flow volume affects early postoperative outcome after Fontan completion: A multimodality study. J. Thorac. Cardiovasc. Surg. 2012, 144, 1329–1336. [Google Scholar] [CrossRef]
- Ascuitto, R.J.; Ross-Ascuitto, N.T. Systematic-to-pulmonary collaterals: A source of flow energy loss in Fontan physiology. Pediatr. Cardiol. 2004, 25, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Glatz, A.C.; Rome, J.J.; Small, A.J.; Gillespie, M.J.; Dori, Y.; Harris, M.A.; Keller, M.S.; Fogel, M.A.; Whitehead, K.K. Systemic-to-pulmonary collateral flow, as measured by cardiac magnetic resonance imaging, is associated with acute post-Fontan clinical outcomes. Circ. Cardiovasc. Imaging 2012, 5, 218–225. [Google Scholar] [CrossRef]
- Osawa, T.; Schaeffer, T.; Borgmann, K.; Schmiel, M.; Staehler, H.; Di Padua, C.; Heinisch, P.P.; Piber, N.; Mutsuga, M.; Hager, A.; et al. Impact of aortopulmonary collaterals on adverse events after total cavopulmonary connection. Eur. J. Cardiothorac. Surg. 2023, 64, ezad408. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, D.; Tanase, D.; Ewert, P.; Georgiev, S.; Cleuziou, J.; Renner, D.; Borgmann, K.; Eicken, A. Catheter interventional closure of veno-venous collaterals in cyanotic patients after partial cavopulmonary shunts in pediatric patients: Clinical practice review. Cardiovasc. Diagn. Ther. 2023, 13, 599–608. [Google Scholar] [CrossRef]
- Nguyen Cong, M.B.H.; Schaeffer, T.; Osawa, T.; Palm, J.; Georgiev, S.; Di Padua, C.; Niedermaier, C.; Heinisch, P.P.; Piber, N.; Hager, A.; et al. Impact of veno-venous collaterals on outcome after the total cavopulmonary connection. Int. J. Cardiol. 2024, 410, 132229. [Google Scholar] [CrossRef] [PubMed]
- Doulamis, I.P.; Marathe, S.P.; Oh, N.A.; Saeed, M.Y.; Muter, A.; Del Nido, P.J.; Nathan, M. Major Aortopulmonary Collateral Arteries Requiring Percutaneous Intervention Following the Arterial Switch Operation: A Case Series and Systematic Review. World J. Pediatr. Congenit. Heart Surg. 2022, 13, 146–154. [Google Scholar] [CrossRef]
- Wipf, A.; Christmann, M.; Navarini-Meury, S.; Dave, H.; Quandt, D.; Knirsch, W.; Kretschmar, O. Aortopulmonary collaterals in neonates with d-transposition of the great arteries—Clinical significance early after arterial switch operation. Int. J. Cardiol. 2018, 258, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Mainwaring, R.D. Midline unifocalization for pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J. Thorac. Dis. 2020, 12, 1263–1273. [Google Scholar] [CrossRef]
- Patrick, W.L.; Mainwaring, R.D.; Reinhartz, O.; Punn, R.; Tacy, T.; Hanley, F.L. Major Aortopulmonary Collateral Arteries with Anatomy Other Than Pulmonary Atresia/Ventricular Septal Defect. Ann. Thorac. Surg. 2017, 104, 907–916. [Google Scholar] [CrossRef] [PubMed]
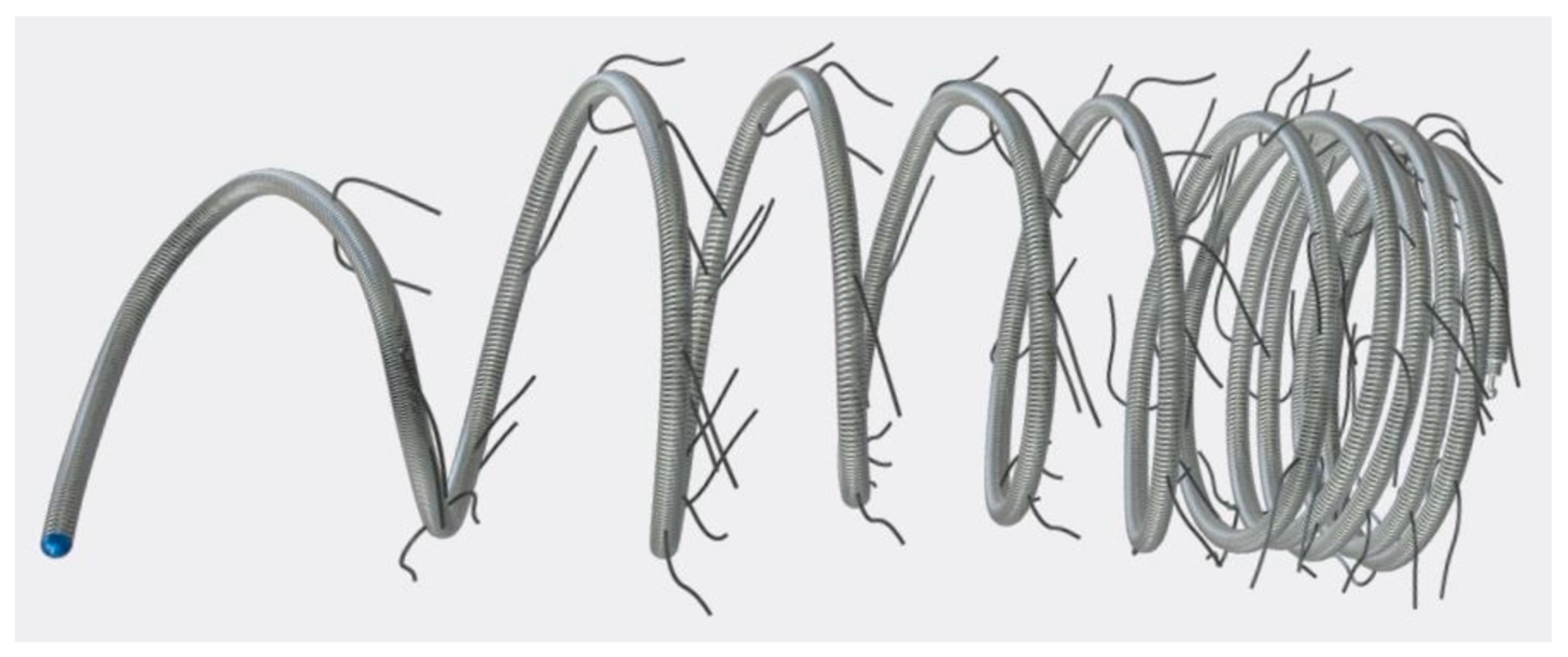
| Procedure No. | Sex | Age (Months) | Weight (kg) | Diagnosis | Univentricular Heart | Most Recent Surgery | Embolized SPC (n) | Origin of SPC | Diameter of SPC (mm) | Implanted Coils (n) | Size of Coil (Diameter (mm) × Length (cm)) | Coil/SPC Ratio | Procedure Time (min) | Fluoroscopy Time (min) | Contrast Volume (mL) | Dose Area Product (cGy·cm2) | Complications | Follow-Up Time (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 11 | 9.0 | TOF | No | TOF repair | 1 | Ao | 1.4 | 2 | 5 × 20 7 × 30 | 3.6 5.0 | 105 | 32.5 | 160 | 344.5 | No | 0.5 |
| 2 | F | 135 | 39.4 | DORV, D-TGA | Yes | TCPA | 1 | LSA | 2.2 | 2 | 5 × 20 7 × 30 | 2.3 3.2 | 128 | 41.1 | 125 | 1764.4 | No | 15 |
| 3 | F | 48 | 16.0 | LVH, D-TGA | Yes | SCPA | 1 | LSA | 1.7 | 1 | 5 × 20 | 2.9 | 92 | 22.8 | 80 | 368.0 | No | 96 |
| 4 | F | 20 | 9.3 | DORV, D-TGA | Yes | SCPA | 1 | LIMA | 1.5 | 2 | 4 × 10 8 × 30 | 2.7 5.3 | 60 | 10.0 | 135 | 225.0 | No | 71 |
| 5 | F | 41 | 12.0 | TA | Yes | SCPA | 1 | RSA | 1.5 | 1 | 5 × 15 | 3.3 | 102 | 29.0 | 50 | 259.1 | No | 38 |
| 6 | M | 57 | 17.0 | HLHS | Yes | TCPA | 2 | RIMA | 1.0 | 2 | 3 × 8 4 × 8 | 3.0 4.0 | 92 | 18.4 | 79 | 1959.3 | No | 90 |
| LSA | 2.1 | 1 | 7 × 30 | 3.3 | ||||||||||||||
| 7 | M | 162 | 53.3 | DORV, D-TGA | Yes | Shunt * | 2 | RSA | 2.7 | 4 | 5 × 20 (n = 2) 6 × 20 7 × 30 | 1.9 2.2 2.6 | 101 | 35.0 | 70 | 7947.3 | No | 88 |
| LSA | 3.0 | 1 | 5 × 20 | 1.7 | ||||||||||||||
| 8 | M | 16 | 11.3 | L-TGA, VSD | No | Shunt * | 1 | Ao | 2.3 | 4 | 6 × 20 (n = 3) 7 × 30 | 2.6 3.0 | 106 | 31.5 | 70 | 699.9 | No | 77 |
| 9 | M | 23 | 12.0 | L-TGA, VSD | No | Shunt * | 1 | RSA | 1.7 | 3 | 4 × 20 5 × 20 (n = 2) | 2.4 2.9 | 105 | 36.5 | 64 | 909.4 | No | 70 |
| 10 | M | 77 | 19.2 | L-TGA, VSD | No | Shunt * | 3 | LSA | 1.6 | 3 | 5 × 20 6 × 20 7 × 30 | 3.1 3.8 4.4 | 102 | 23.5 | 55 | 897.1 | Yes ‡ | 16 |
| LIMA | 2.7 | 3 | 7 × 30 8 × 30 (n = 2) | 2.6 3.0 | ||||||||||||||
| LIMA | 2.3 | 2 | 5 × 20 6 × 20 | 2.2 2.6 | ||||||||||||||
| 11 | F | 4 | 5.7 | RVH, PS, VSD | Yes | None | 1 | Ao | 1.8 | 2 | 4 × 10 5 × 20 | 2.2 2.8 | 68 | 15.7 | 27 | 92.5 | No | 42 |
| 12 | F | 26 | 12.2 | TA | Yes | SCPA | 1 | RSA | 1.9 | 4 | 4 × 8 4 × 10 5 × 15 5 × 20 | 2.1 2.1 2.6 2.6 | 123 | 26.7 | 20 | 262.2 | No | 84 |
| 13 | F | 35 | 14.1 | TA | Yes | SCPA | 1 | LIMA | 1.9 | 2 | 4 × 15 5 × 15 | 2.1 2.6 | 122 | 13.2 | 55 | 238.9 | No | 69 |
| 14 | M | 118 | 24.7 | TA | Yes | TCPA | 1 | Ao | 1.7 | 4 | 4 × 10 (n = 2) 5 × 10 (n = 2) | 2.4 2.9 | 73 | 20.5 | 70 | 1142.5 | No | 71 |
| 15 | M | 12 | 8.8 | D-TGA | No | ASO | 1 | RSA | 1.7 | 2 | 4 × 10 5 × 15 | 2.4 2.9 | 84 | 22.3 | 40 | 275.4 | No | 47 |
| 16 | M | 52 | 12.5 | LVH, AoH | Yes | SCPA | 2 | Ao | 1.8 | 2 | 4 × 10 5 × 15 | 2.2 2.8 | 163 | 23.8 | 42 | 364.1 | No | 38 |
| RSA | 2.0 | 2 | 4 × 8 5 × 15 | 2.0 2.5 | ||||||||||||||
| 17 | F | 39 | 12.0 | DORV, LVH | Yes | SCPA | 2 | LSA | 1.5 | 3 | 3 × 8 5 × 15 5 × 20 | 2.0 3.3 3.3 | 132 | 35.0 | 54 | 295.3 | No | 26 |
| RIMA | 2.4 | 3 | 7 × 30 8 × 30 (n = 2) | 2.9 3.3 | ||||||||||||||
| 18 | F | 46 | 13.0 | DORV, LVH | Yes | SCPA | 1 | Ao | 1.8 | 1 | 4 × 10 | 2.2 | 85 | 16.2 | 60 | 139.4 | No | 19 |
| 19 | M | 117 | 32.7 | TA | Yes | TCPA | 2 | LSA | 2.3 | 4 | 4 × 8 4 × 10 6 × 20 (n = 2) | 1.7 1.7 2.6 | 104 | 22.3 | 155 | 3106.4 | No | 78 |
| LIMA | 2.1 | 2 | 5 × 15 6 × 20 | 2.4 2.9 | ||||||||||||||
| 20 | M | 7 | 8.1 | PDA | No | none | 2 | Ao | 1.6 | 1 | 4 × 10 | 2.5 | 66 | 11.8 | 30 | 102.4 | No | 64 |
| Ao | 2.0 | 2 | 4 × 8 5 × 15 | 2.0 2.5 | ||||||||||||||
| 21 | F | 10 | 7.0 | DILV, D-TGA | Yes | Shunt * | 2 | LIMA | 2.0 | 3 | 5 × 15 6 × 20 (n = 2) | 2.5 3.0 | 75 | 25.5 | 17 | 108.3 | No | 95 |
| LSA | 2.5 | 1 | 4 × 15 | 1.6 | ||||||||||||||
| 22 | F | 42 | 14.9 | DILV, D-TGA | Yes | SCPA | 2 | RSA | 1.6 | 3 | 4 × 10 5 × 15 5 × 20 | 2.5 3.1 3.1 | 90 | 25.5 | 12 | 455.0 | No | 63 |
| RIMA | 2.4 | 1 | 7 × 30 | 2.9 | ||||||||||||||
| 23 | M | 53 | 13.0 | DILV, PA | Yes | TCPA | 2 | RIMA | 2.5 | 2 | 5 × 20 (n = 2) | 2.0 | 111 | 28.1 | 132 | 588.0 | No | 77 |
| LBV | 3.1 | 3 | 4 × 10 7 × 30 8 × 30 | 1.3 2.3 2.6 | ||||||||||||||
| 24 | M | 81 | 19.6 | TA | Yes | TCPA | 1 | RIMA | 1.5 | 1 | 5 × 20 | 3.3 | 71 | 9.7 | 64 | 268.6 | No | 43 |
| 25 | M | 168 | 45.0 | AVSD, LVH | Yes | TCPA | 1 | RBV | 2.6 | 4 | 7 × 30 (n = 2) 8 × 30 (n = 2) | 2.7 3.1 | 76 | 16.1 | 65 | 283.2 | No | 60 |
| 26 | M | 14 | 10.9 | D-TGA | No | ASO | 1 | RSA | 2.0 | 3 | 4 × 10 (n = 2) 5 × 15 | 2.0 2.5 | 49 | 17.3 | 75 | 657.5 | No | 71 |
| 27 | F | 49 | 14.8 | PA, VSD | No | Shunt * | 1 | Ao | 3.7 | 3 | 7 × 30 (n = 2) 8 × 30 | 1.9 2.2 | 133 | 46.0 | 110 | 773.4 | No | 93 |
| 28 | M | 40 | 11.0 | DORV, LVH | Yes | SCPA | 1 | RSA | 2.1 | 2 | 5 × 15 5 × 20 | 2.4 2.4 | 122 | 35.5 | 34 | 435.4 | No | 38 |
| 29 | M | 42 | 13.2 | DORV, LVH | Yes | TCPA | 1 | RCA | 2.4 | 2 | 4 × 10 5 × 15 | 1.7 2.1 | 80 | 16.8 | 65 | 413.6 | No | 36 |
| 30 | M | 490 | 64.0 | PA, VSD | Yes | none | 2 | RSA | 2.4 | 3 | 7 × 30 8 × 30 (n = 2) | 2.9 3.3 2.4 | 79 | 20.7 | 123 | 6955.2 | No | 36 |
| LSA | 2.5 | 2 | 6 × 20 7 × 30 | 2.8 | ||||||||||||||
| 31 | M | 206 | 59.0 | TA | Yes | TCPA | 1 | LIMA | 1.6 | 3 | 5 × 15 (n = 2) 5 × 20 | 3.1 3.1 | 82 | 20.0 | 115 | 3094.8 | No | 63 |
| 32 | M | 71 | 18.5 | HLHS | Yes | TCPA | 1 | Ao | 1.4 | 1 | 3 × 8 | 2.1 | 84 | 19.9 | 85 | 706.2 | No | 23 |
| 33 | F | 31 | 10.0 | TAC | No | TAC repair | 1 | RIMA | 2.1 | 3 | 6 × 20 (n = 2) 7 × 30 | 2.9 3.3 | 51 | 18.4 | 90 | 713.0 | No | 19 |
| 34 | M | 155 | 39.2 | DORV, D-TGA, LVH | Yes | TCPA | 1 | LIMA | 1.3 | 1 | 4 × 10 | 3.1 | 82 | 19.7 | 100 | 1438.3 | No | 34 |
| 35 | M | 9 | 7.8 | DILV, TGA, PS | Yes | SPCA | 1 | RSA | 1.8 | 2 | 4 × 8 4 × 10 | 2.2 2.2 | 52 | 19.2 | 18 | 59.0 | No | 34 |
| 36 | M | 20 | 12.3 | DORV, TGA, LVH | Yes | SPCA | 2 | Ao | 1.6 | 1 | 4 × 10 | 2.5 | 101 | 25.9 | 39 | 442.6 | No | 30 |
| RIMA | 1.5 | 2 | 5 × 15 5 × 20 | 3.3 3.3 | ||||||||||||||
| 37 | F | 8 | 7.0 | TA | Yes | Shunt * | 2 | Ao | 1.5 | 3 | 3 × 4 3 × 8 (n = 2) | 2.0 2.0 | 82 | 20.0 | 15 | 61.2 | No | 91 |
| RSA | 1.8 | 1 | 4 × 10 | 2.2 | ||||||||||||||
| 38 | F | 24 | 9.6 | TA | Yes | SPCA | 1 | LSA | 1.5 | 1 | 5 × 15 | 3.3 | 66 | 11.0 | 126 | 204.8 | No | 75 |
| 39 | M | 232 | 50.0 | TA | Yes | TCPA | 1 | LBV | 2.9 | 3 | 4 × 10 6 × 20 7 × 30 | 1.4 2.1 2.4 | 52 | 11.7 | 95 | 3785.0 | No | 95 |
| 40 | F | 183 | 44.0 | DILV, D-TGA | Yes | TCPA | 1 | LCCA | 2.0 | 1 | 5 × 20 | 2.5 | 131 | 34.2 | 60 | 5752.8 | No | 75 |
| 41 | F | 219 | 50.3 | DILV, D-TGA | Yes | TCPA | 1 | RSA | 1.8 | 2 | 3 × 8 5 × 15 | 1.6 2.8 | 110 | 29.5 | 65 | 5157.2 | No | 39 |
| 42 | M | 2 | 4.2 | D-TGA, VSD, PS | Yes | Shunt * | 1 | Ao | 2.3 | 2 | 5 × 20 (n = 2) | 2.2 | 49 | 9.5 | 38 | 61.7 | No | 60 |
| 43 | M | 148 | 37.9 | TA | Yes | TCPA | 1 | SVC | 3.0 | 3 | 7 × 30 (n = 2) 8 × 30 | 2.3 2.7 | 75 | 16.5 | 121 | 860.7 | No | 64 |
| 44 | M | 28 | 10.6 | DORV, LVH | Yes | SPCA | 1 | LSA | 2.8 | 2 | 5 × 15 5 × 20 | 1.8 1.8 | 117 | 32.5 | 20 | 231.6 | No | 66 |
| 45 | M | 75 | 16.2 | DORV, LVH | Yes | TCPA | 1 | LBV | 2.9 | 3 | 5 × 20 7 × 30 (n = 2) | 1.7 2.4 | 60 | 12.9 | 12 | 200.0 | No | 19 |
| 46 | M | 8 | 6.4 | DORV, LVH | Yes | SCPA | 2 | LIMA | 1.7 | 1 | 5 × 20 | 2.9 | 101 | 27.1 | 40 | 102.6 | No | 86 |
| RIMA | 2.7 | 3 | 7 × 30 (n = 2) 8 × 30 | 2.6 3.0 | ||||||||||||||
| 47 | F | 25 | 13.3 | DORV, PS, LVH | Yes | SCPA | 1 | RIMA | 1.7 | 1 | 4 × 10 | 2.4 | 88 | 17.4 | 57 | 292.6 | No | 48 |
| 48 | F | 36 | 14.7 | DORV, PS, LVH | Yes | SCPA | 1 | RIMA | 1.8 | 1 | 4 × 10 | 2.2 | 59 | 15.8 | 45 | 233.6 | No | 37 |
| 49 | M | 37 | 18.9 | TA | Yes | SCPA | 1 | LIMA | 1.9 | 3 | 4 × 10 5 × 15 (n = 2) | 2.1 2.6 | 73 | 17.4 | 100 | 701.0 | No | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, J.; Poryo, M.; Gheibeh, A.; Rentzsch, A.; Abdul-Khaliq, H. Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils. J. Clin. Med. 2025, 14, 113. https://doi.org/10.3390/jcm14010113
Pfeifer J, Poryo M, Gheibeh A, Rentzsch A, Abdul-Khaliq H. Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils. Journal of Clinical Medicine. 2025; 14(1):113. https://doi.org/10.3390/jcm14010113
Chicago/Turabian StylePfeifer, Jochen, Martin Poryo, Anas Gheibeh, Axel Rentzsch, and Hashim Abdul-Khaliq. 2025. "Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils" Journal of Clinical Medicine 14, no. 1: 113. https://doi.org/10.3390/jcm14010113
APA StylePfeifer, J., Poryo, M., Gheibeh, A., Rentzsch, A., & Abdul-Khaliq, H. (2025). Transcatheter Embolization of Systemic-to-Pulmonary Collaterals: A New Approach Using Concerto™ Helix Nylon-Fibered Microcoils. Journal of Clinical Medicine, 14(1), 113. https://doi.org/10.3390/jcm14010113







