Endobronchial Suture of Tracheoesophageal Fistula Through Rigid Bronchoscopy Without Tracheostomy: A Preliminary, Observational Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Intervention and Follow-Up
2.2.1. Pre-Operative Assessment
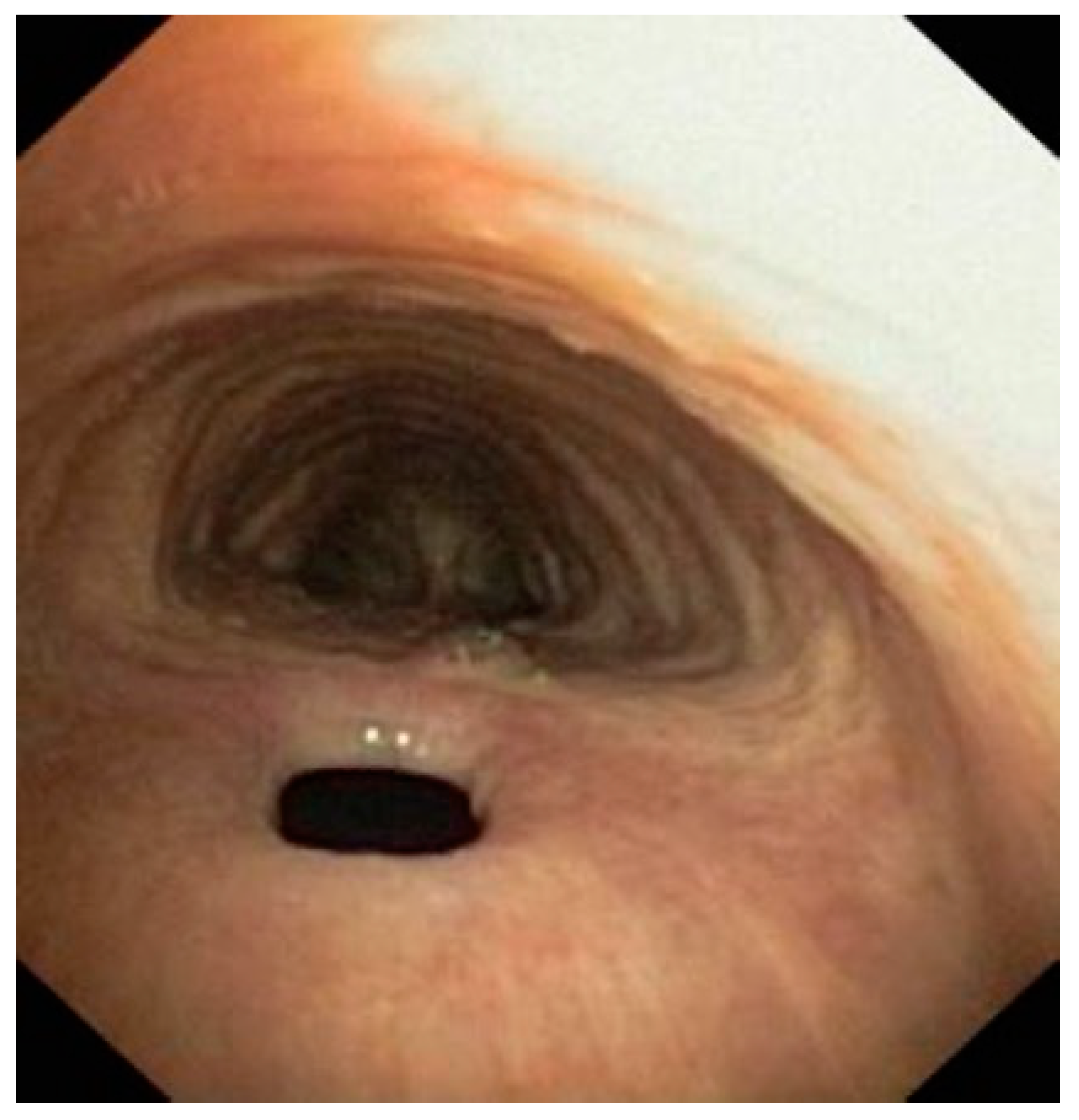
2.2.2. Procedure Description
2.2.3. Post-Procedure Management
2.3. Outcomes
2.4. Statistical Methods
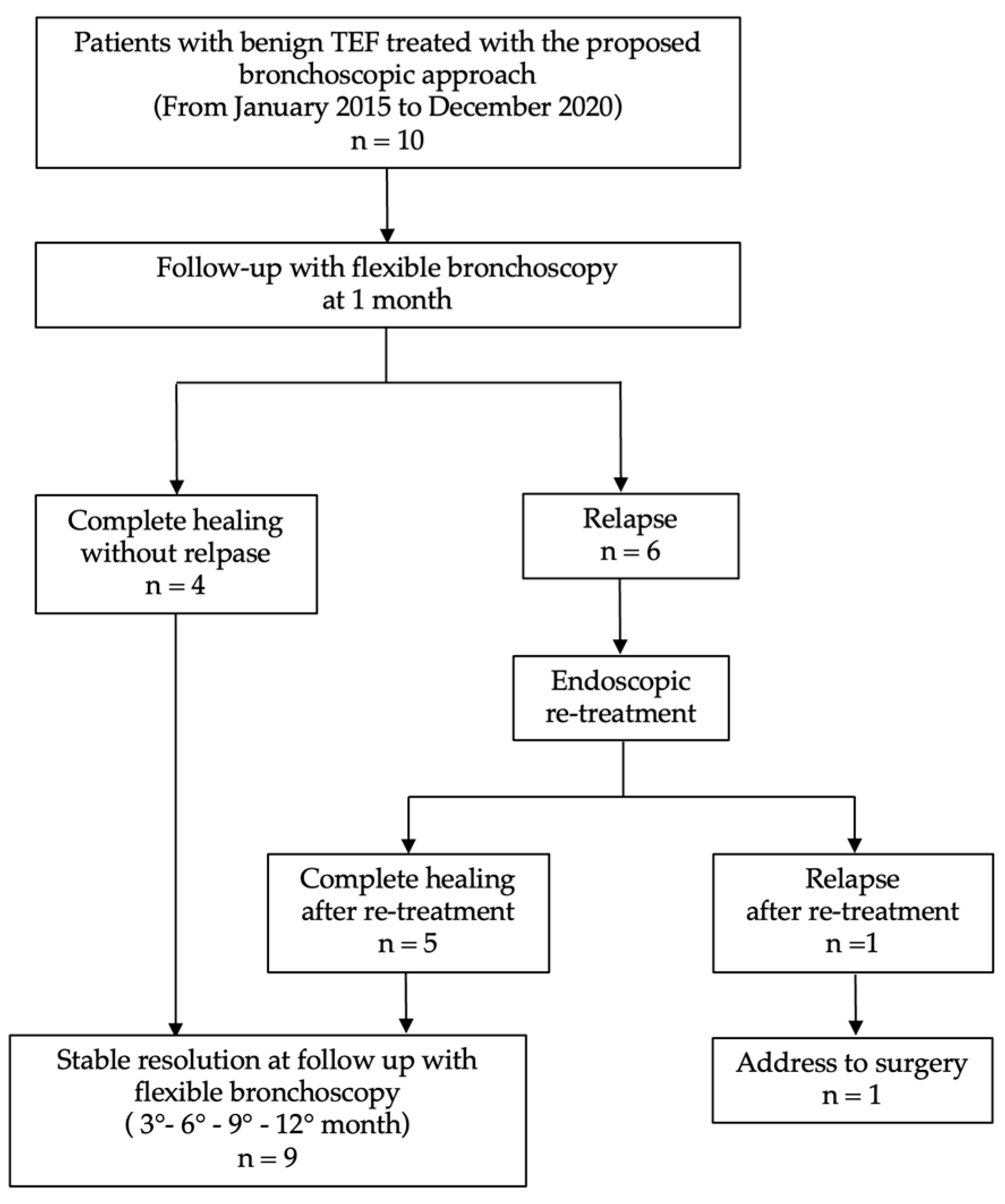
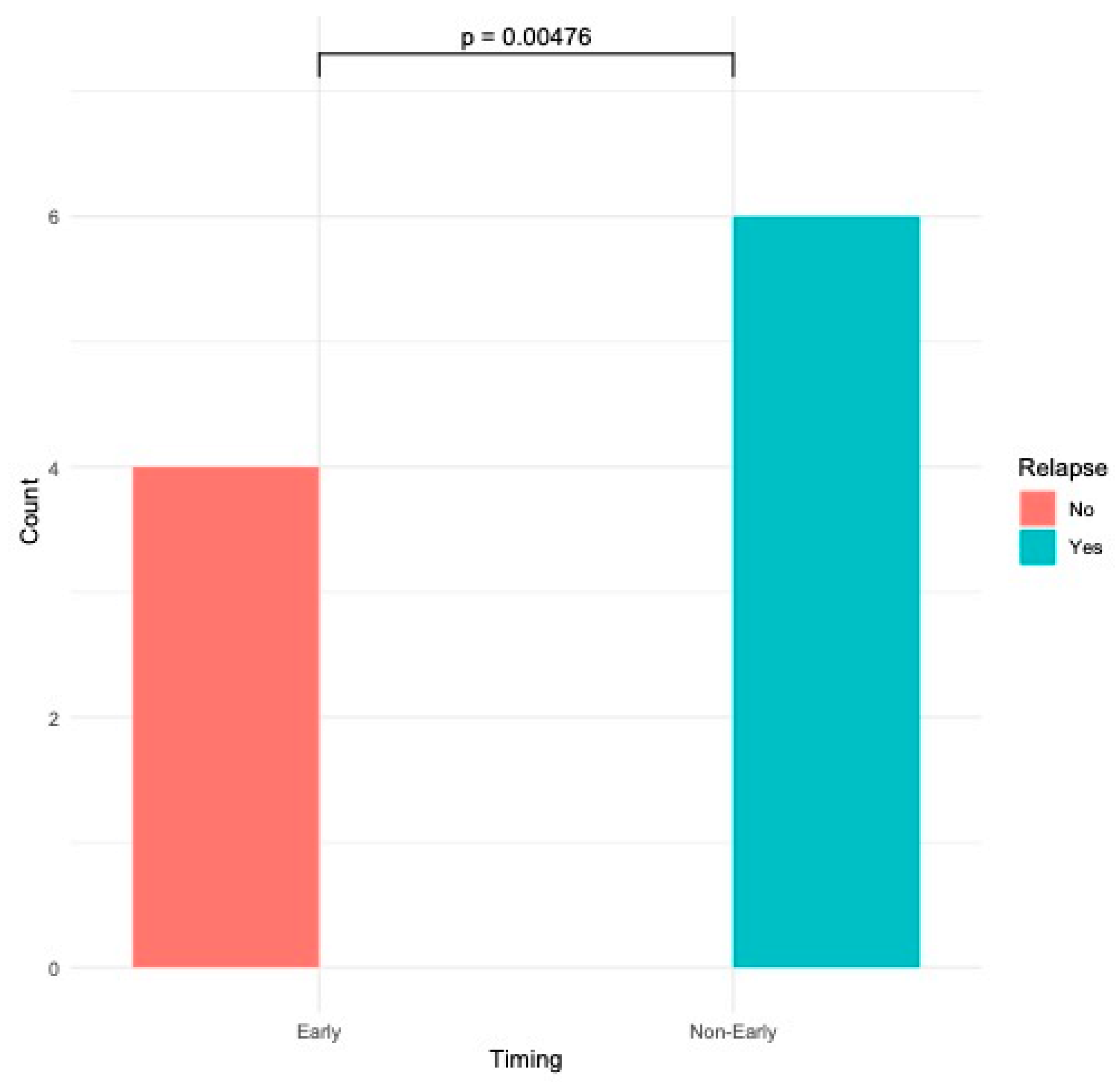
3. Results
Outcomes: Success Rate and Procedure-Related Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.S.; Khemasuwan, D.; Diaz-Mendoza, J.; Mehta, A.C. Management of Tracheo-Oesophageal Fistula in Adults. Eur. Respir. Rev. 2020, 29, 200094. [Google Scholar] [CrossRef]
- Ramai, D.; Bivona, A.; Latson, W.; Ofosu, A.; Ofori, E.; Reddy, M.; Adler, D.G. Endoscopic Management of Tracheoesophageal Fistulas. Ann. Gastroenterol. 2019, 32, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Marulli, G.; Loizzi, M.; Cardillo, G.; Battistella, L.; De Palma, A.; Ialongo, P.; Zampieri, D.; Rea, F. Early and Late Outcome after Surgical Treatment of Acquired Non-Malignant Tracheo-Oesophageal Fistulae. Eur. J. Cardio-Thoracic Surg. 2013, 43, e155–e161. [Google Scholar] [CrossRef]
- Ke, M.; Wu, X.; Zeng, J. The Treatment Strategy for Tracheoesophageal Fistula. J. Thorac. Dis. 2015, 7, S389–S397. [Google Scholar] [CrossRef] [PubMed]
- Ciecierega, T.; Sharaiha, R.Z. Endoscopic Treatment of Tracheoesophageal Fistula: Use of Stents and Endoclips. Curr. Challenges Thorac. Surg. 2021, 4. [Google Scholar] [CrossRef]
- Sharaiha, R.Z.; Kumta, N.A.; DeFilippis, E.M.; Dimaio, C.J.; Gonzalez, S.; Gonda, T.; Rogart, J.; Siddiqui, A.; Berg, P.S.; Samuels, P.; et al. A Large Multicenter Experience With Endoscopic Suturing for Management of Gastrointestinal Defects and Stent Anchorage in 122 Patients: A Retrospective Review. J. Clin. Gastroenterol. 2016, 50, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Silon, B.; Siddiqui, A.A.; Taylor, L.J.; Arastu, S.; Soomro, A.; Adler, D.G. Endoscopic Management of Esophagorespiratory Fistulas: A Multicenter Retrospective Study of Techniques and Outcomes. Dig. Dis. Sci. 2017, 62, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Ambrogi, M.C.; Mussi, A.; Ribechini, A.; Angeletti, C.A. Posterior Wall Laceration of the Thoracic Trachea: The Transcervical-Transtracheal Approach. Eur. J. Cardio-Thoracic Surg. 2001, 19, 932–934. [Google Scholar] [CrossRef]
- Galluccio, G. Endoscopic Treatment of Tracheo-Oesophageal Fistulae: An Innovative Procedure. Multimed. Man Cardiothorac. Surg. 2016, 2017, mmw015. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Sohrabi, C.; Kerwan, A.; Franchi, T.; Mathew, G.; Nicola, M.; Agha, R.A. The STROCSS 2024 Guideline: Strengthening the Reporting of Cohort, Cross-Sectional, and Case-Control Studies in Surgery. Int. J. Surg. 2024, 110, 3151–3165. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.M.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.W. Towards Complete and Accurate Reporting of Studies of Diagnostic Accuracy: The STARD Initiative. BMJ 2003, 326, 41–44. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Li, S.; Le, Y.; Deng, Y.; Chen, J.; Peng, Q.; Xu, R.; Li, J. Efficacy of Mesenchymal Stem Cells in Treating Tracheoesophageal Fistula via the TLR4/NF-Κb Pathway in Beagle Macrophages. Heliyon 2024, 10, e32903. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yue, L.; Jia, W.; Li, J.; Liu, Y.; Zhu, X. Local Injection of Platelet-Rich Plasma Offers a New Therapeutic Option for the Treatment of Esophagotracheal Fistula: A Case Report. J. Int. Med. Res. 2024, 52, 3000605231220874. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, C.; Voigtländer, T.; Drews, J.; Engelke, C.; Marquardt, J.U.; Heidrich, B.; Klein, F.; Wedemeyer, H.; Kirstein, M.M.; von Hahn, T. Safety and Efficacy of Conventional Compared to Segmented Esophageal Fully Covered Self-Expanding Metal Stents: A Retrospective Multicenter Case–Control Study. Surg. Endosc. 2024, 38, 7158–7164. [Google Scholar] [CrossRef]
- Upadhyaya, V.D.; Gopal, S.C.; Gangopadhyaya, A.N.; Gupta, D.K.; Sharma, S.; Upadyaya, A.; Kumar, V.; Pandey, A. Role of Fibrin Glue as a Sealant to Esophageal Anastomosis in Cases of Congenital Esophageal Atresia with Tracheoesophageal Fistula. World J. Surg. 2007, 31, 2412–2415. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.T.; Ryckman, F.; Brown, R.L.; Rutter, M.J. Endoscopic Management of Recurrent Tracheoesophageal Fistula. J. Pediatr. Surg. 2008, 43, 238–245. [Google Scholar] [CrossRef]
- Gregory, S.; Chun, R.H.; Parakininkas, D.; Amos, L.; Fons, R.; Lerner, D.G.; Lal, D.R.; Sulman, C. Endoscopic Esophageal and Tracheal Cauterization for Closure of Recurrent Tracheoesophageal Fistula: A Case Report and Review of the Literature. Int. J. Pediatr. Otorhinolaryngol. 2017, 98, 158–161. [Google Scholar] [CrossRef]
- van Bodegraven, A.A.; Kuipers, E.J.; Bonenkamp, H.J.; Meuwissen, S.G.M. Esophagopleural Fistula Treated Endoscopically with Argon Beam Electrocoagulation and Clips. Gastrointest. Endosc. 1999, 50, 407–410. [Google Scholar] [CrossRef]
- Zakaluzny, S.A.; Lane, J.D.; Mair, E.A. Complications of Tracheobronchial Airway Stents. Otolaryngol. Neck Surg. 2003, 128, 478–488. [Google Scholar] [CrossRef]
- Jeong, B.-H.; Ng, J.; Jeong, S.H.; Kim, H. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina 2020, 56, 367. [Google Scholar] [CrossRef] [PubMed]

| Total | n = 10 |
|---|---|
| Age mean (SD) | 45.1 (±9.8) |
| Gender | n (%) |
| Male | 7 (70%) |
| Female | 3 (30%) |
| Smoking | n (%) |
| Smokers | 6 (60%) |
| Non-smokers | 4 (40%) |
| Cause of TEF | |
| Post-intubation | 8 (80%) |
| Trauma | 1 (10%) |
| Post–esophagectomy (esophageal cancer) | 1 (10%) |
| Symptoms | n (%) |
| Cough | 9 (90%) |
| Dyspnea | 7 (70%) |
| Hemoptysis | 6 (60%) |
| Dysphagia | 6 (60%) |
| Sialorrhea | 5 (50%) |
| Comorbidities | n (%) |
| COPD | 5 (50%) |
| Diabetes mellitus | 3 (30%) |
| Systemic arterial hypertension | 6 (60%) |
| Ischemic heart disease | 2 (20%) |
| Patients (n) | Total (n = 10) |
|---|---|
| Complete healing (without relapse) | 4 (40%) |
| Relapse | 6 (60%) |
| ○ Endoscopic re–treatment | 5 (83%) |
| ○ Complete reopening | 1 (17%) |
| (referral for surgery) | |
| Complications | |
| ○ Massive bleeding | n = 0 |
| ○ Mortality during the procedure | n = 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluccio, G.; D’Agnano, V.; Menichini, I.; Napolitano, A.G.; Masi, U.; Bianco, A. Endobronchial Suture of Tracheoesophageal Fistula Through Rigid Bronchoscopy Without Tracheostomy: A Preliminary, Observational Retrospective Study. J. Clin. Med. 2025, 14, 110. https://doi.org/10.3390/jcm14010110
Galluccio G, D’Agnano V, Menichini I, Napolitano AG, Masi U, Bianco A. Endobronchial Suture of Tracheoesophageal Fistula Through Rigid Bronchoscopy Without Tracheostomy: A Preliminary, Observational Retrospective Study. Journal of Clinical Medicine. 2025; 14(1):110. https://doi.org/10.3390/jcm14010110
Chicago/Turabian StyleGalluccio, Giovanni, Vito D’Agnano, Ilaria Menichini, Antonio Giulio Napolitano, Umberto Masi, and Andrea Bianco. 2025. "Endobronchial Suture of Tracheoesophageal Fistula Through Rigid Bronchoscopy Without Tracheostomy: A Preliminary, Observational Retrospective Study" Journal of Clinical Medicine 14, no. 1: 110. https://doi.org/10.3390/jcm14010110
APA StyleGalluccio, G., D’Agnano, V., Menichini, I., Napolitano, A. G., Masi, U., & Bianco, A. (2025). Endobronchial Suture of Tracheoesophageal Fistula Through Rigid Bronchoscopy Without Tracheostomy: A Preliminary, Observational Retrospective Study. Journal of Clinical Medicine, 14(1), 110. https://doi.org/10.3390/jcm14010110








