The US4ABL Strategy: A Systematic Ultrasound-Guided Approach for Left Atrial and Ventricular Ablation Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Population
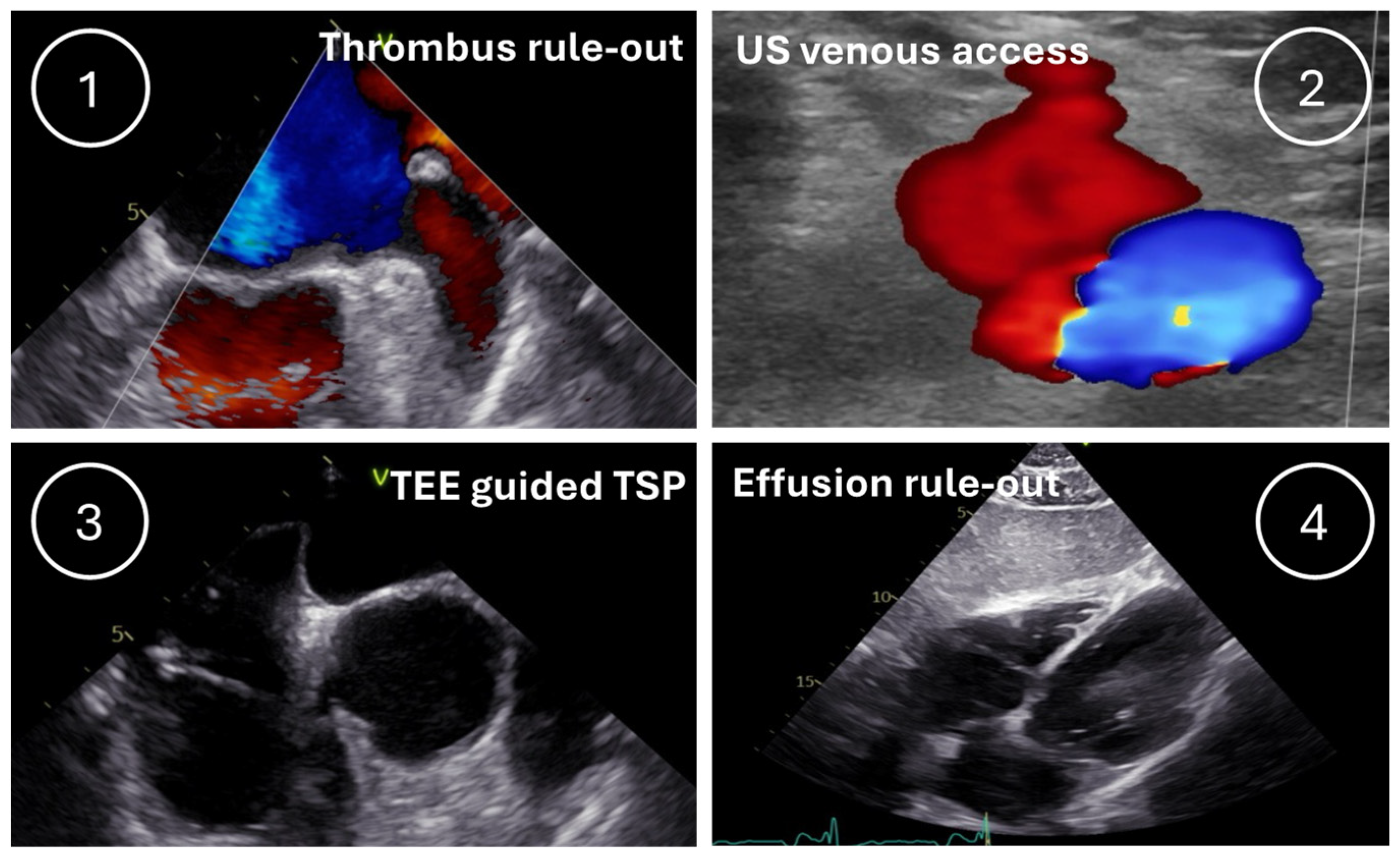
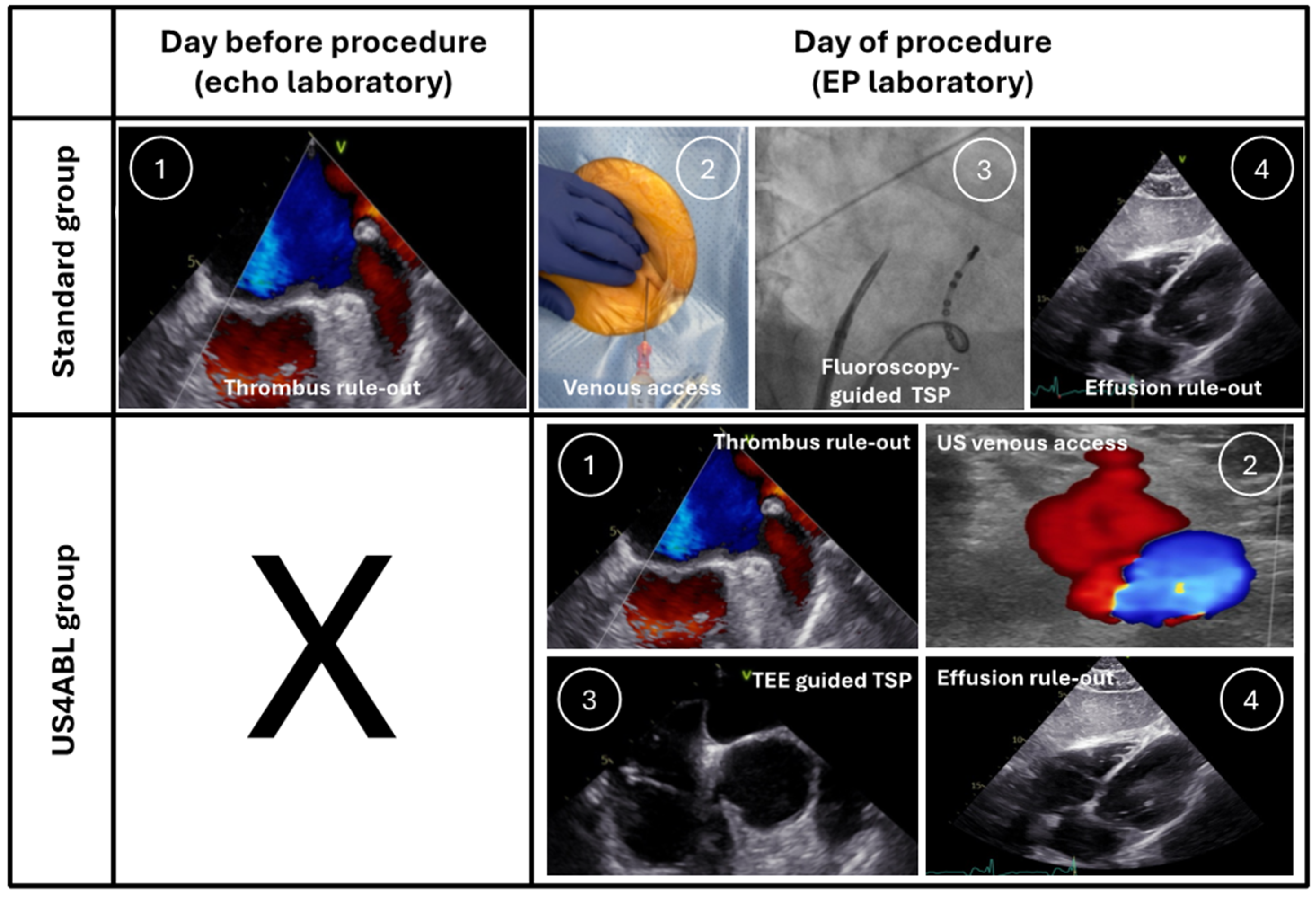
2.3. Procedural Workflows
- In pulmonary vein isolations (PVIs), a wide-area circumferential ablation was performed (ablation index guided), the procedural objective was the disappearance of the PV signal recorded on a circular mapping catheter or high-density mapping catheter (HDMC). The ablation was performed using the CLOSE protocol [15]. Briefly, we used a minimum contact force of 5 g, aiming to reach 10–20 g, 50 W irrespective of the anterior–posterior segment, with an ablation index target of 400 posterior and 550 anterior.
- In left atrial tachycardias, mapping with a HDMC was performed, and the final lesion sets were at the operator’s discretion, with an objective of non-inducibility.
- In left ventricular cases, mapping and ablation were performed with the ablation catheter, with the objective of the complete elimination of the PVC.
2.3.1. Standard Group
2.3.2. US4ABL Group
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benali, K.; Khairy, P.; Hammache, N.; Petzl, A.; Da Costa, A.; Verma, A.; Andrade, J.G.; Macle, L. Procedure-Related Complications of Catheter Ablation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2023, 81, 2089–2099. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Developed by the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC), with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; D’Amato, S.A.; Al-Kazaz, M.; Markowitz, S.M.; Liu, C.F.; Thomas, G.; Ip, J.E.; Sharma, S.K.; Yang, H.; Singh, P.; et al. Prevalence of Left Atrial Thrombus Detection by Transesophageal Echocardiography: A Comparison of Continuous Non-Vitamin K Antagonist Oral Anticoagulant Versus Warfarin Therapy in Patients Undergoing Catheter Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2016, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Wichterle, D.; Roubíček, T.; Jarkovský, P.; Sato, Y.; Kogure, T.; Peichl, P.; Konečný, P.; Jansová, H.; Kučera, P.; et al. Ultrasound-Guided versus Conventional Femoral Venipuncture for Catheter Ablation of Atrial Fibrillation: A Multicentre Randomized Efficacy and Safety Trial (ULTRA-FAST Trial). EP Eur. 2018, 20, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Wynn, G.J.; Haq, I.; Hung, J.; Bonnett, L.J.; Lewis, G.; Webber, M.; Waktare, J.E.P.; Modi, S.; Snowdon, R.L.; Hall, M.C.S.; et al. Improving Safety in Catheter Ablation for Atrial Fibrillation: A Prospective Study of the Use of Ultrasound to Guide Vascular Access. J. Cardiovasc. Electrophysiol. 2014, 25, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kupo, P.; Riesz, T.J.; Saghy, L.; Vamos, M.; Bencsik, G.; Makai, A.; Kohari, M.; Benak, A.; Miklos, M.; Pap, R. Ultrasound Guidance for Femoral Venous Access in Patients Undergoing Pulmonary Vein Isolation: A Quasi-Randomized Study. J. Cardiovasc. Electrophysiol. 2023, 34, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, R.; Herling, A.; Schmidt, M.T.; Bachmann, M.; Winnik, S.; Duru, F.; Eriksson, U. Transesophageal Echocardiography-Guided Transseptal Left Atrial Access to Improve Safety in Patients Undergoing Pulmonary Vein Isolation. J. Clin. Med. 2022, 11, 2546. [Google Scholar] [CrossRef] [PubMed]
- Chokesuwattanaskul, R.; Ananwattanasuk, T.; Hughey, A.B.; Stuart, E.A.; Shah, M.M.; Atreya, A.R.; Chugh, A.; Bogun, F.; Crawford, T.; Pelosi, F.; et al. Three-Dimensional-Guided and ICE-Guided Transseptal Puncture for Cardiac Ablations: A Propensity Score Match Study. J. Cardiovasc. Electrophysiol. 2023, 34, 382–388. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. EP Eur. 2024, 26, euae043. [Google Scholar] [CrossRef]
- Metzner, A.; Reubold, S.D.; Schönhofer, S.; Reißmann, B.; Ouyang, F.; Rottner, L.; Schleberger, R.; Dinshaw, L.; Moser, J.; Moser, F.; et al. Management of Pericardial Tamponade in the Electrophysiology Laboratory: Results from a National Survey. Clin. Res. Cardiol. 2023, 112, 1727–1737. [Google Scholar] [CrossRef]
- Erden, İ.; Erden, E.Ç.; Golcuk, E.; Aksu, T.; Yalin, K.; Güler, T.E.; Özcan, K.S.; Turan, B. Impact of Transesophageal Echocardiography during Transseptal Puncture on Atrial Fibrillation Ablation. J. Arrhythmia 2016, 32, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Developed by the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Bejinariu, A.G.; Makimoto, H.; Wakili, R.; Mathew, S.; Kosiuk, J.; Linz, D.; Steinfurt, J.; Dechering, D.G.; Meyer, C.; Veltmann, C.; et al. One-Year Course of Periprocedural Anticoagulation in Atrial Fibrillation Ablation: Results of a German Nationwide Survey. Cardiology 2020, 145, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Phlips, T.; Taghji, P.; El Haddad, M.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Duytschaever, M. Improving Procedural and One-Year Outcome after Contact Force-Guided Pulmonary Vein Isolation: The Role of Interlesion Distance, Ablation Index, and Contact Force Variability in the ‘CLOSE’-Protocol. EP Eur. 2018, 20, f419–f427. [Google Scholar] [CrossRef]
- Maclean, E.; Mahtani, K.; Roelas, M.; Vyas, R.; Butcher, C.; Ahluwalia, N.; Honarbakhsh, S.; Creta, A.; Finlay, M.; Chow, A.; et al. Transseptal Puncture for Left Atrial Ablation: Risk Factors for Cardiac Tamponade and a Proposed Causative Classification System. J. Cardiovasc. Electrophysiol. 2022, 33, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Kupó, P.; Pap, R.; Sághy, L.; Tényi, D.; Bálint, A.; Debreceni, D.; Basu-Ray, I.; Komócsi, A. Ultrasound Guidance for Femoral Venous Access in Electrophysiology Procedures—Systematic Review and Meta-Analysis. J. Interv. Card. Electrophysiol. 2020, 59, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Bejinariu, A.G.; Spieker, M.; Makimoto, H.; Augustin, N.; Kelm, M.; Rana, O.R. A Zero-Exchange Approach for Left Atrial Access in Pulmonary Vein Isolation with Pulsed Field Ablation. J. Cardiovasc. Electrophysiol. 2024, 35, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, D.G.; Magnocavallo, M.; Gianni, C.; Mohanty, S.; Al-Ahmad, A.; Bassiouny, M.; Denora, M.; La Fazia, V.M.; Lavalle, C.; Gallinghouse, G.J.; et al. Three-Dimensional Intracardiac Echocardiography for Left Atrial Appendage Sizing and Percutaneous Occlusion Guidance. EP Eur. 2024, 26, euae010. [Google Scholar] [CrossRef]
- Bulava, A.; Hanis, J.; Eisenberger, M. Catheter Ablation of Atrial Fibrillation Using Zero-Fluoroscopy Technique: A Randomized Trial. Pacing Clin. Electrophysiol. 2015, 38, 797–806. [Google Scholar] [CrossRef] [PubMed]
| Standard Group n = 299 | US4ABL Group n = 212 | p Value | |
|---|---|---|---|
| Age, years | 69 (59–76) | 68 (58–75) | 0.96 |
| Male gender—n (%) | 180 (60) | 116 (55) | 0.21 |
| BMI—kg/m2 (IQR) | 26 (24–30) | 27 (24–31) | 0.95 |
| Indication for TSP—n (%) | 0.01 | ||
| Paroxysmal AF | 115 (38.5) | 109 (51.4) | |
| Persistent AF | 100 (33.4) | 81 (38.2) | |
| Left atrial tachycardia | 40 (13.4) | 15 (7.1) | |
| Left ventricular PVC | 44 (14.7) | 7 (3.3) | |
| Hypertension—n (%) | 182 (61) | 139 (66) | 0.28 |
| History of stroke—n (%) | 33 (11) | 30 (14) | 0.29 |
| Coronary artery disease—n (%) | 90 (30) | 71 (33.5) | 0.41 |
| Peripheral artery disease—n (%) | 15 (5) | 15 (7) | 0.36 |
| Heart failure—n (%) | 61 (20) | 39 (18) | 0.21 |
| Obstructive sleep apnea—n (%) | 19 (6) | 22 (10) | 0.10 |
| COPD—n (%) | 16 (5) | 19 (9) | 0.11 |
| CHA2DS2-VASc score (IQR) | 3 (1–4) | 3 (2–4) | 0.51 |
| Standard Group n = 299 | US4ABL Group n = 212 | p Value | |
|---|---|---|---|
| Major vascular access complication—n (%) | 7 (2.3) | 0 (0) | 0.025 |
| Cardiac tamponade—n (%) | 4 (1.3) | 0 (0) | 0.091 |
| Stroke—n (%) | 0 (0) | 0 (0) | |
| TEE-related complication—n (%) | 0 (0) | 0 (0) | |
| Esophageal injury—n (%) | 0 (0) | 0 (0) | |
| Total—n (%) | 11 (3.7) | 0 (0) | 0.005 |
| Standard Group n = 299 | US4ABL Group n = 212 | p Value | |
|---|---|---|---|
| Procedural duration—min (IQR) | 134 (100–180) | 103 (75–135) | <0.01 |
| Paroxysmal AF | 121 (92–159) | 95 (70–136) | <0.01 |
| Persistent AF | 142 (102–191) | 105 (75–130) | <0.01 |
| Left atrial tachycardia | 135 (97–176) | 105 (78–150) | 0.15 |
| Left ventricular PVC | 165 (119–236) | 165 (88–195) | 0.36 |
| Fluoroscopy time—min (IQR) | 17 (12–25) | 15 (11–22) | 0.06 |
| Dose–area product—cGy ⋅cm2 (IQR) | 905 (564–1511) | 853 (500–1721) | 0.55 |
| Standard Group n = 215 | US4ABL Group n = 190 | p Value | |
|---|---|---|---|
| Procedural duration—min (IQR) | 174 (139–217) | 145 (106–179) | <0.01 |
| Fluoroscopy time—min (IQR) | 19 (12–27) | 15 (11–22) | <0.01 |
| Dose–area product—cGy⋅cm2 (IQR) | 889 (572–1660) | 828 (485–1698) | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejinariu, A.G.; Augustin, N.; Spieker, M.; auf der Heiden, C.; Angendohr, S.; Höckmann, M.; Clasen, L.; Hartl, S.; Makimoto, H.; Busch, L.; et al. The US4ABL Strategy: A Systematic Ultrasound-Guided Approach for Left Atrial and Ventricular Ablation Procedures. J. Clin. Med. 2025, 14, 103. https://doi.org/10.3390/jcm14010103
Bejinariu AG, Augustin N, Spieker M, auf der Heiden C, Angendohr S, Höckmann M, Clasen L, Hartl S, Makimoto H, Busch L, et al. The US4ABL Strategy: A Systematic Ultrasound-Guided Approach for Left Atrial and Ventricular Ablation Procedures. Journal of Clinical Medicine. 2025; 14(1):103. https://doi.org/10.3390/jcm14010103
Chicago/Turabian StyleBejinariu, Alexandru Gabriel, Nora Augustin, Maximilian Spieker, Carsten auf der Heiden, Stephan Angendohr, Moritz Höckmann, Lukas Clasen, Stefan Hartl, Hisaki Makimoto, Lucas Busch, and et al. 2025. "The US4ABL Strategy: A Systematic Ultrasound-Guided Approach for Left Atrial and Ventricular Ablation Procedures" Journal of Clinical Medicine 14, no. 1: 103. https://doi.org/10.3390/jcm14010103
APA StyleBejinariu, A. G., Augustin, N., Spieker, M., auf der Heiden, C., Angendohr, S., Höckmann, M., Clasen, L., Hartl, S., Makimoto, H., Busch, L., Kelm, M., & Rana, O. (2025). The US4ABL Strategy: A Systematic Ultrasound-Guided Approach for Left Atrial and Ventricular Ablation Procedures. Journal of Clinical Medicine, 14(1), 103. https://doi.org/10.3390/jcm14010103






