The Prevalence of Cancer in Dutch Female Patients with Idiopathic Scoliosis Compared with the General Population
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
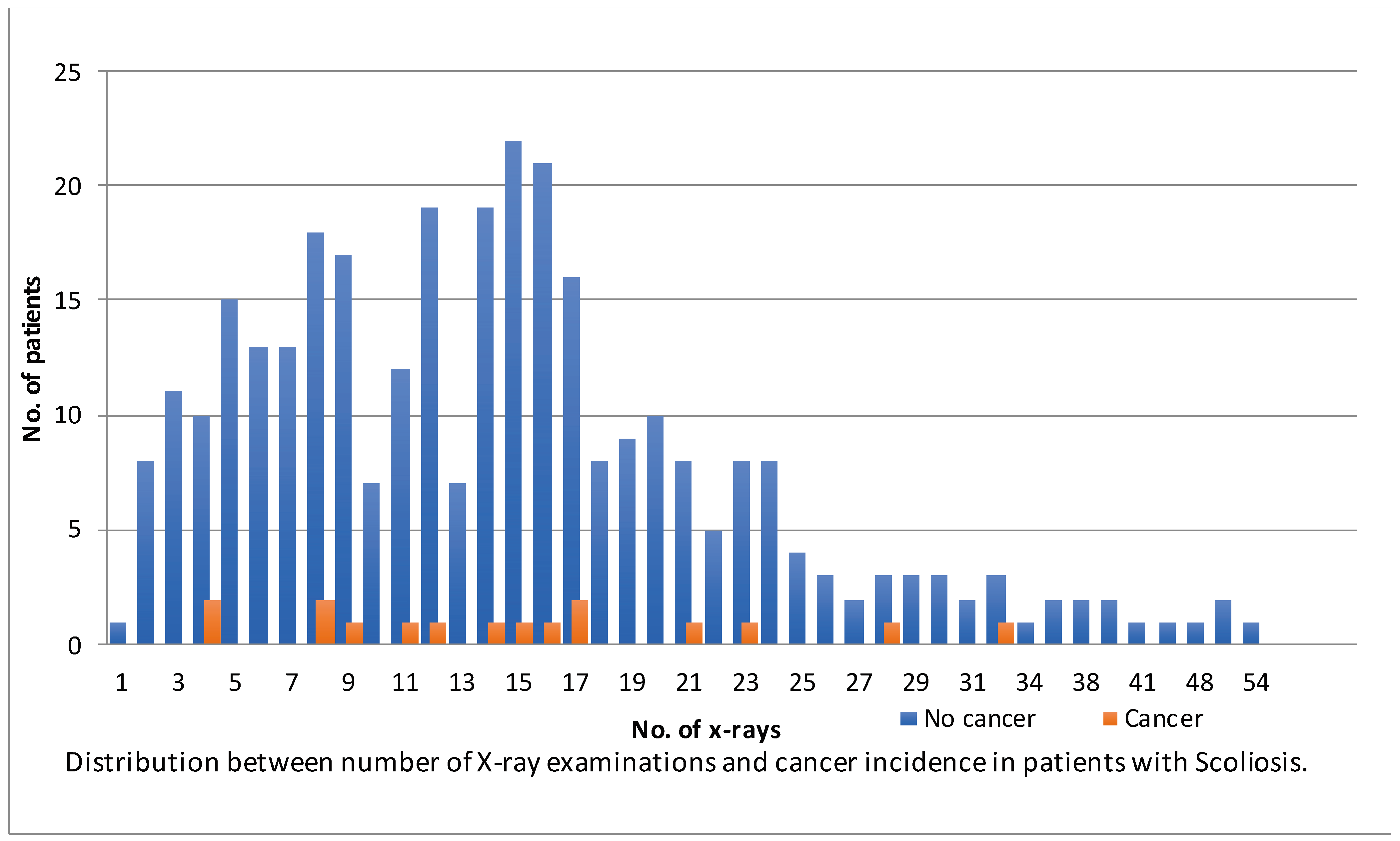
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howe, G.R.; McLaughlin, J. Breast cancer mortality between 1950 and 1987 after exposure to fractionated moderate-dose-rate ionizing radiation in the Canadian fluoroscopy cohort study and a comparison with breast cancer mortality in the atomic bomb survivors study. Radiat Res. 1996, 145, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Sugiyama, H.; Nishi, N.; Sakata, R.; Shimizu, Y.; Grant, E.J.; Soda, M.; Hsu, W.-L.; Suyama, A.; Kodama, K.; et al. Ionizing Radiation and Leukemia Mortality among Japanese Atomic Bomb Survivors, 1950–2000. Radiat. Res. 2009, 172, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P. Cancer and non-cancer effects in Japanese atomic bomb survivors. J. Radiol. Prot. 2009, 29, A43–A59. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Low-dose extrapolation of radiation-related cancer risk. Ann. ICRP 2005, 35, 1–140. [Google Scholar] [PubMed]
- Oakley, P.A.; Navid Ehsani, N.; Harrison, D.E. 5 Reasons Why Scoliosis X-rays Are Not Harmful. Dose Response 2020, 18, 1559325820957797. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Board on Radiation Effects Research. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII, Phase I, Letter Report (1998); National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Kane, W.J. Scoliosis prevalence: A call for a statement of terms. Clin. Orthop. Relat. Res. 1977, 126, 43–46. [Google Scholar] [CrossRef]
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Stirling, A.J.; Howel, D.; Millner, P.A.; Sadiq, S.; Sharples, D.; Dickson, R.A. Late-onset idiopathic scoliosis in children six to fourteen years old. A cross-sectional prevalence study. J. Bone Jt. Surg. Am. 1996, 78, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Doody, M.M.; Lonstein, J.E.; Stovall, M.; Hacker, D.G.; Luckyanov, N.; Land, C.E. Breast cancer mortality after diagnostic radiography: Findings from the U.S. Scoliosis Cohort Study. Spine 2000, 25, 2052–2063. [Google Scholar] [CrossRef]
- Levy, A.R.; Goldberg, M.S.; Mayo, N.E.; Hanley, J.A.; Poitras, B. Reducing the lifetime risk of cancer from spinal radiographs among people with adolescent idiopathic scoliosis. Spine 1996, 21, 1540–1547; discussion 1548. [Google Scholar] [CrossRef]
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of cancer in adolescent idiopathic scoliosis patients treated 25 years previously. Eur. Spine J. 2016, 25, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- Knott, P.; Pappo, E.; Cameron, M.; Demauroy, J.; Rivard, C.; Kotwicki, T.; Zaina, F.; Wynne, J.; Stikeleather, L.; Bettany-Saltikov, J.; et al. SOSORT 2012 consensus paper: Reducing X-ray exposure in pediatric patients with scoliosis. Scoliosis 2014, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Bone, C.M.; Hsieh, G.H. The risk of carcinogenesis from radiographs to pediatric orthopaedic patients. J. Pediatr. Orthop. 2000, 20, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.A.; Lonstein, J.E.; Morin, M.M.; Visscher, W.; Harris, B.S., 3rd; Boice, J.D., Jr. Breast cancer in women with scoliosis exposed to multiple diagnostic X rays. J. Natl. Cancer Inst. 1989, 81, 1307–1312. [Google Scholar] [CrossRef]
- Nash, C.L., Jr.; Gregg, E.C.; Brown, R.H.; Pillai, K. Risks of exposure to X-rays in patients undergoing long-term treatment for scoliosis. J Bone Jt. Surg. Am. 1979, 61, 371–374. [Google Scholar] [CrossRef]
- Ronckers, C.M.; Doody, M.M.; Lonstein, J.E.; Stovall, M.; Land, C.E. Multiple diagnostic X-rays for spine deformities and risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.R.; Goldberg, M.S.; Hanley, J.A.; Mayo, N.E.; Poitras, B. Projecting the lifetime risk of cancer from exposure to diagnostic ionizing radiation for adolescent idiopathic scoliosis. Health Phys. 1994, 66, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Buls, N.; Laumen, A.; Van Gompel, G.; Verhelle, F.; de Mey, J. Lowered dose full-spine radiography in pediatric patients with idiopathic scoliosis. Eur. Spine J. 2018, 27, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.R.; Muchow, R.D.; Peppler, W.W.; Anderson, P.A. Theoretical breast cancer induction risk from thoracic spine CT in female pediatric trauma patients. Pediatrics 2012, 130, e1614–e1620. [Google Scholar] [CrossRef]
- Jansen, L.; Dubois, B.F.H.; Hollmann, M.W. The Effect of Propofol versus Inhalation Anesthetics on Survival after Oncological Surgery. J. Clin. Med. 2022, 11, 6741. [Google Scholar] [CrossRef]
- Springvloet, L.; Bommelé, J.; Willemsen, M.; van Laar, M. Health Survey/Lifestyle Monitor, Statistics Netherlands (CBS), in Collaboration with National Institute for Public Health and the Environment (RIVM) and Trimbos Institute, 2017. English Report. Available online: https://www.trimbos.nl/wp-content/uploads/sites/31/2021/09/af1636-smoking-in-the-netherlands-key-statistics-2017.pdf (accessed on 24 February 2024).
- Thompson, D.E.; Mabuchi, K.; Ron, E.; Soda, M.; Tokunaga, M.; Ochikubo, S.; Sugimoto, S.; Ikeda, T.; Terasaki, M.; Izumi, S.; et al. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat. Res. 1994, 137 (Suppl. S2), S17–S67. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, S.; Hauptmann, M.; Sigurdson, A.J.; Doody, M.M.; Freedman, D.M.; Alexander, B.H.; Linet, M.S.; Ron, E.; Mabuchi, K. Nonmelanoma skin cancer in relation to ionizing radiation exposure among U.S. radiologic technologists. Int. J. Cancer 2005, 115, 828–834. [Google Scholar] [CrossRef] [PubMed]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332.
- Stokes, O.M.; O’Donovan, E.J.; Samartzis, D.; Bow, C.H.; Luk, K.D.; Cheung, K.M. Reducing radiation exposure in early-onset scoliosis surgery patients: Novel use of ultrasonography to measure lengthening in magnetically-controlled growing rods. Spine J. Off. J. N. Am. Spine Soc. 2014, 14, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Minehiro, K.; Demura, S.; Ichikawa, K.; Sasagawa, T.; Takahashi, N.; Minami, S.; Murakami, H.; Tsuchiya, H. Dose Reduction Protocol for Full Spine X-ray Examination Using Copper Filters in Patients with Adolescent Idiopathic Scoliosis. Spine 2019, 44, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Amzallag-Bellenger, E.; Uyttenhove, F.; Nectoux, E.; Moraux, A.; Bigot, J.; Herbaux, B.; Boutry, N. Idiopathic scoliosis in children and adolescents: Assessment with a biplanar X-ray device. Insights Imaging 2014, 5, 571–583. [Google Scholar] [CrossRef]
- Ilharreborde, B.; Ferrero, E.; Alison, M.; Mazda, K. EOS microdose protocol for the radiological follow-up of adolescent idiopathic scoliosis. Eur. Spine J. 2016, 25, 526–531. [Google Scholar] [CrossRef]
| Total | Observation | Brace | Surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 337) | (n = 87) | (n = 175) | (n = 75) | ||||||
| Sex | |||||||||
| Female | 298 (88%) | 70 (80%) | 160 (91%) | 68 (91%) | |||||
| Male | 39 (12%) | 17 (20%) | 15 (8.6%) | 7 (9.3%) | |||||
| Age at follow-up, years | |||||||||
| Mean, SD | 44 ± 6.6 | 44 ± 5.7 | 44 ± 6.2 | 43 ± 8.0 | |||||
| Year of birth | |||||||||
| <1960 | 8 (2.4%) | 1 (1.1%) | 2 (1.1%) | 5 (6.7%) | |||||
| 1960–1969 | 93 (28%) | 19 (22%) | 53 (30%) | 21 (28%) | |||||
| 1970–1979 | 207 (61%) | 61 (70%) | 110 (63%) | 36 (48%) | |||||
| ≥1980 | 29 (8.6%) | 6 (6.9%) | 10 (5.7%) | 13 (17%) | |||||
| Age at first radiograph | |||||||||
| Median, IQR | 13 (11–15) | 14 (12–16) | 13 (11–14) | 13 (11–15) | |||||
| <10 | 48 (14%) | 10 (11%) | 26 (15%) | 12 (16%) | |||||
| 10–13 | 161 (48%) | 33 (38%) | 98 (56%) | 30 (40%) | |||||
| 14–18 | 128 (38%) | 44 (51%) | 51 (29%) | 33 (44%) | |||||
| Diagnosis | |||||||||
| Juvenile | 81 (24%) | 12 (14%) | 44 (25%) | 25 (33%) | |||||
| Adolescent | 256 (76%) | 75 (86%) | 131 (75%) | 50 (67%) | |||||
| Cobbs angle, degrees † | |||||||||
| <20 | 41 (12%) | 33 (38%) | 7 (4.0%) | 1 (1.3%) | |||||
| 20–29 | 70 (21%) | 24 (28%) | 44 (25%) | 2 (2.7%) | |||||
| 30–39 | 101 (30%) | 18 (21%) | 73 (42%) | 10 (13%) | |||||
| 40–49 | 65 (19%) | 5 (5.7%) | 36 (21%) | 24 (32%) | |||||
| ≥50 | 60 (18%) | 7 (8.0%) | 15 (8.6%) | 38 (51%) | |||||
| Total no. of X-rays | |||||||||
| Median, IQR | 14 (8–19) | 6 (4–9) | 15 (12–20) | 17 (12–24) | |||||
| 1–9 | 111 (33%) | 68 (78%) | 27 (15%) | 16 (21%) | |||||
| 10–19 | 147 (44%) | 19 (22%) | 99 (57%) | 29 (39%) | |||||
| 20–29 | 57 (17%) | 0 (0%) | 39 (22%) | 18 (24%) | |||||
| ≥30 | 22 (6.5%) | 0 (0%) | 10 (5.7%) | 12 (16%) | |||||
| No. of X-rays <18 years old | |||||||||
| Median, IQR | 12 (6–17) | 5 (3–8) | 14 (11–18) | 15 (9–22) | |||||
| 1–9 | 129 (38%) | 73 (84%) | 34 (19%) | 22 (29%) | |||||
| 10–19 | 147 (44%) | 14 (16%) | 106 (61%) | 27 (36%) | |||||
| 20–29 | 46 (14%) | 0 (0%) | 28 (16%) | 18 (24%) | |||||
| ≥30 | 15 (4.4%) | 0 (0%) | 7 (4.0%) | 8 (11%) | |||||
| Time between X-rays in years | |||||||||
| Median, IQR | 5.8 (3.1–10) | 2.6 (1.2–6.8) | 7.1 (4.4–10) | 7 (3.8–13) | |||||
| Age of menarche | |||||||||
| <12 | 26 (7.7%) | 7 (8.1%) | 14 (8.0%) | 5 (6.7%) | |||||
| 12–14 | 204 (60%) | 48 (55%) | 116 (66%) | 40 (53%) | |||||
| ≥15 | 21 (6.2%) | 2 (2.3%) | 12 (6.9%) | 7 (9.3%) | |||||
| Unknown | 86 (26%) | 30 (34%) | 33 (19%) | 23 (31%) | |||||
| BMI (mean, SD) | |||||||||
| At first radiograph | 18 ± 2.7 | 19 ± 2.5 | 18 ± 2.6 | 19 ± 3.1 | |||||
| At last radiograph | 20 ± 2.9 | 20 ± 2.6 | 20 ± 2.8 | 21 ± 3.2 | |||||
| Smoking * | |||||||||
| Never | 246 (74%) | 62 (71%) | 126 (73%) | 58 (78%) | |||||
| Past | 41 (12%) | 14 (16%) | 19 (11%) | 8 (11%) | |||||
| Occasional | 19 (5.7%) | 4 (4.6%) | 11 (6.4%) | 4 (5.4%) | |||||
| Current | 27 (8.1%) | 7 (8.0%) | 16 (9.3%) | 4 (5.4%) | |||||
| Deceased | |||||||||
| <40 | 4 (1.2%) | 0 (0%) | 3 (1.7%) | 1 (1.3%) | |||||
| 40–49 | 7 (2.1%) | 1 (1.1%) | 3 (1.7%) | 3 (4.0%) | |||||
| ≥50 | 3 (0.9%) | 0 (0%) | 0 (0%) | 3 (4.0%) | |||||
| Total No. of Patients | Female Sex | Age at Diagnosis in Years * | |
|---|---|---|---|
| Breast cancer † | 4 (1.2%) | 4 | 49 (45–53) |
| Melanoma | 3 (0.9%) | 3 | 39 (29–44) |
| Ovarian cancer | 2 (0.6%) | 2 | 35 |
| Cervical cancer | 3 (0.3%) | 3 | 49 (47–50) |
| Hodgkin’s lymphoma | 1 (0.3%) | 1 | 37 |
| Lung cancer | 1 (0.3%) | 1 | 26 |
| Brain cancer (Astrocytoma) | 1 (0.3%) | 1 | 26 |
| Renal cell carcinoma | 1 (0.3%) | 1 | 33 (46) |
| Anal cancer | 1 (0.3%) | 0 | 47 |
| Total | 17 (5.6%) | 16 | 39 (31–46) |
| Age | National Population Cancer Rates (%) | Number of Women in Our Cohort | Observed Cancer * | Expected Cancer Rates (Rounded) |
|---|---|---|---|---|
| 0 | - | - | - | - |
| 1–5 | - | - | - | - |
| 6–10 | - | - | - | - |
| 11–15 | 0.06 | 0 | 0 | 0.00 |
| 16–20 | 0.17 | 2 | 0 | 0.00 |
| 21–25 | 0.35 | 1 | 0 | 0.00 |
| 26–30 | 0.69 | 7 | 3 | 0.05 |
| 31–35 | 1.31 | 11 | 2 | 0.14 |
| 36–40 | 2.29 | 64 | 5 | 1.47 |
| 41–45 | 3.77 | 83 | 1 | 3.13 |
| 46–50 | 6.06 | 96 | 4 | 5.82 |
| 51–55 | 9.29 | 24 | 0 | 2.23 |
| 56–60 | 13.38 | 8 | 0 | 1.07 |
| 61–65 | 18.72 | 2 | 0 | 0.37 |
| Total | 298 | 15 (5.0%) | 14.3 (4.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heijboer, R.R.O.; Heemskerk, J.L.; Vorrink, S.N.W.; Kempen, D.H.R. The Prevalence of Cancer in Dutch Female Patients with Idiopathic Scoliosis Compared with the General Population. J. Clin. Med. 2024, 13, 2616. https://doi.org/10.3390/jcm13092616
Heijboer RRO, Heemskerk JL, Vorrink SNW, Kempen DHR. The Prevalence of Cancer in Dutch Female Patients with Idiopathic Scoliosis Compared with the General Population. Journal of Clinical Medicine. 2024; 13(9):2616. https://doi.org/10.3390/jcm13092616
Chicago/Turabian StyleHeijboer, Reinout R. O., Johan L. Heemskerk, Sigrid N. W. Vorrink, and Diederik H. R. Kempen. 2024. "The Prevalence of Cancer in Dutch Female Patients with Idiopathic Scoliosis Compared with the General Population" Journal of Clinical Medicine 13, no. 9: 2616. https://doi.org/10.3390/jcm13092616
APA StyleHeijboer, R. R. O., Heemskerk, J. L., Vorrink, S. N. W., & Kempen, D. H. R. (2024). The Prevalence of Cancer in Dutch Female Patients with Idiopathic Scoliosis Compared with the General Population. Journal of Clinical Medicine, 13(9), 2616. https://doi.org/10.3390/jcm13092616





