Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts
Abstract
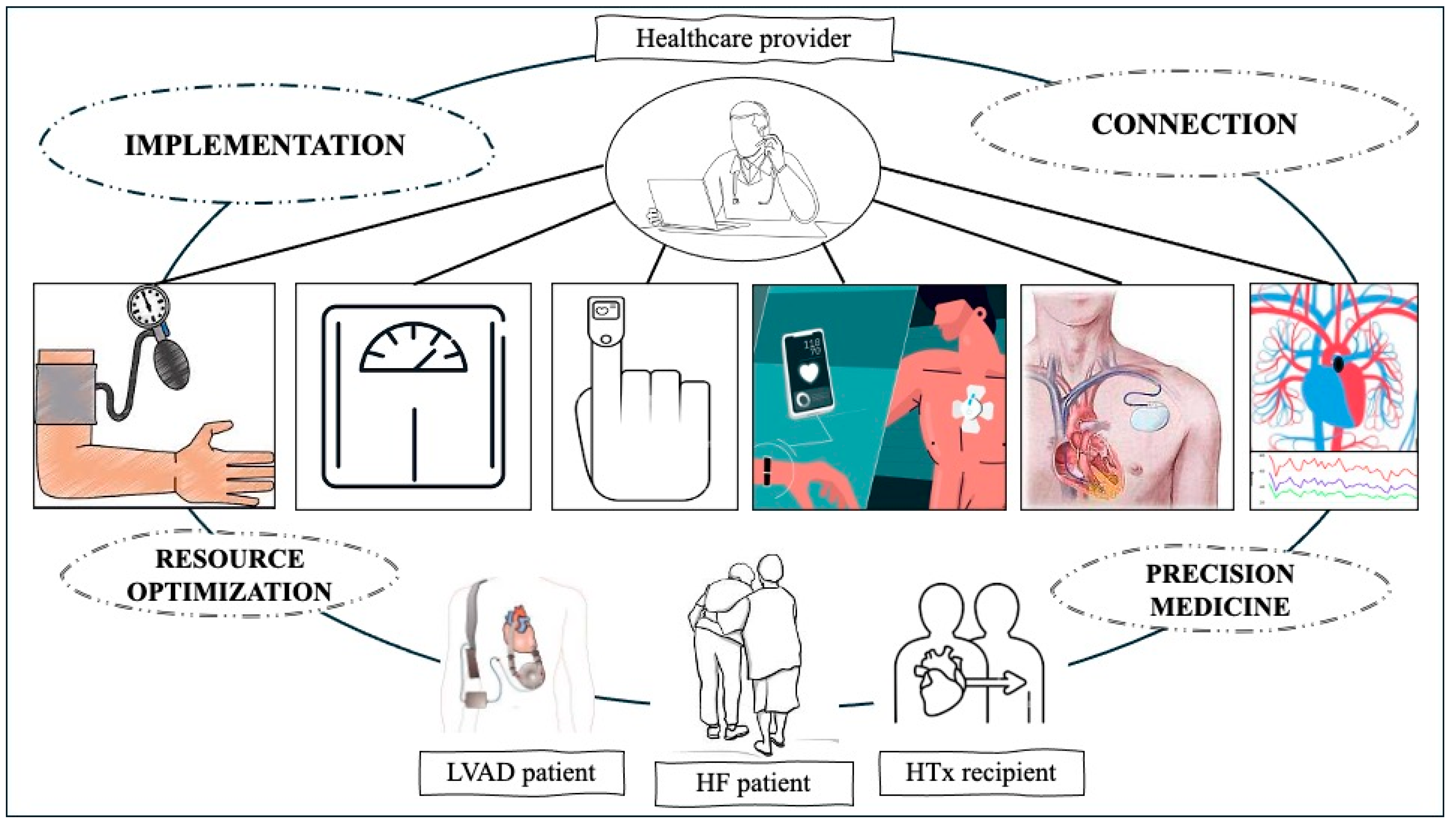
1. Introduction
2. A Brief Chronicle of Telemedicine in Heart Failure: In the Drama of the Pandemic, the Turn towards the Future
3. Telehealth Consultation
4. Wearable Technology
5. Cardiac Implantable Electronic Devices
6. Telemedicine as a Tool for Implementing Medical Therapy: Towards a Paradigm Shift
7. Remote Invasive Hemodynamic Monitoring
7.1. Who Is the Ideal Candidate for Remote Invasive Pressure Monitoring?
7.2. What Is the Role of Remote Invasive Pressure Monitoring in Advanced Heart Failure?
| Clinical Trial | Trial Summary | Results | Main Inclusion Criteria | Main Exclusion Criteria |
|---|---|---|---|---|
| COMPASS [75] |
|
|
|
|
| CHAMPION [76] |
|
|
|
|
| GUIDE-HF [77] |
|
|
|
|
| MEMS-HF [82] |
|
|
|
|
| LAPTOP [90] |
|
|
|
|
| VECTOR-HF [91] |
|
|
|
|
8. Telehealth in Left Ventricular Assist Device and Heart-Transplanted Patients
9. The Role of Artificial Intelligence in Telemedicine: The Future Is Now
10. Cost-Effectiveness of Telehealth Interventions in Heart Failure
11. Conclusions and Future Directions
- A.
- Optimize patient selection for telemonitoring programs by identifying the most suitable devices, considering their clinical history, comorbidities, technological capability, and cognitive capacity.
- B.
- Implement an efficient yet user-friendly device and include a short training phase to make device usage accessible even for vulnerable subjects.
- C.
- Expand the use of TC to facilitate medication up-titration and enhance patient adherence to GDMT.
- D.
- romote the adoption of invasive hemodynamic monitoring devices, which have been proven to be safe and effective in reducing hospitalizations and mortality.
- E.
- Enhance the role of telehealth for rehabilitative purposes, as successfully demonstrated in heart transplant recipients.
- F.
- Improve artificial intelligence algorithms to enable multiparametric integration of data collected through available systems, enhancing the accuracy with which HF relapses can be predicted.
Author Contributions
Funding
Conflicts of Interest
References
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for Healthcare: Capabilities, Features, Barriers, and Applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef] [PubMed]
- Ghilencea, L.-N.; Chiru, M.-R.; Stolcova, M.; Spiridon, G.; Manea, L.-M.; Stănescu, A.-M.A.; Bokhari, A.; Kilic, I.D.; Secco, G.G.; Foin, N.; et al. Telemedicine: Benefits for Cardiovascular Patients in the COVID-19 Era. Front. Cardiovasc. Med. 2022, 9, 868635. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.A.; Schwamm, L.H.; Adeoye, O.M.; Alabi, O.; Jahangir, E.; Misra, S.; Still, C.H. Overview of Telehealth in the Management of Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e558–e568. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Golbus, J.R.; Spaulding, E.M.; Hwang, P.H.; Ciminelli, A.L.A.; Lacar, K.; Hernandez, M.F.; Gilotra, N.A.; Din, N.; Brant, L.C.C.; et al. Challenge of Optimizing Medical Therapy in Heart Failure: Unlocking the Potential of Digital Health and Patient Engagement. J. Am. Heart Assoc. 2024, 13, e030952. [Google Scholar] [CrossRef] [PubMed]
- Fedson, S.; Bozkurt, B. Telehealth in Heart Failure. Heart Fail. Clin. 2022, 18, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.; Martino, A.; Bollettino, M.; Scialla, L.; Cicogna, F.; Tota, C.; Ponziani, B.; Oliviero, G.; Panuccio, M.; Fagagnini, A.; et al. Heart Failure and Telemedicine: Where Are We and Where Are We Going? Opportunities and Critical Issues. Eur. Heart J. Suppl. 2023, 25, C326–C330. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Magnocavallo, M.; Maraone, A.; Notari, C.; Papisca, I.; Mancone, M.; Fedele, F. Clinical Support through Telemedicine in Heart Failure Outpatients during the COVID-19 Pandemic Period: Results of a 12-Months Follow Up. J. Clin. Med. 2022, 11, 2790. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Bennett, T.D.; El Hajj, S.; Kueffer, F.J.; Baicu, C.F.; Abraham, W.T.; Bourge, R.C.; Warner Stevenson, L. Intracardiac Pressures Measured Using an Implantable Hemodynamic Monitor: Relationship to Mortality in Patients With Chronic Heart Failure. Circ. Heart Fail. 2017, 10, e003594. [Google Scholar] [CrossRef]
- Lindenfeld, J.; Costanzo, M.R.; Zile, M.R.; Ducharme, A.; Troughton, R.; Maisel, A.; Mehra, M.R.; Paul, S.; Sears, S.F.; Smart, F.; et al. Implantable Hemodynamic Monitors Improve Survival in Patients with Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2024, 83, 682–694. [Google Scholar] [CrossRef]
- Veenis, J.F.; Birim, O.; Brugts, J.J. Pulmonary Artery Pressure Telemonitoring by CardioMEMS in a Patient Pre- and Post-Left Ventricular Assist Device Implantation. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2019, 56, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Bart, N.K.; Emmanuel, S.; Friits-Lamora, R.; Larkins, E.; Kotlyar, E.; Muthiah, K.; Jabbour, A.; Hayward, C.; Jansz, P.C.; Keogh, A.M.; et al. Rapid Triage and Transition to Telehealth for Heart Transplant Patients in the COVID-19 Pandemic Setting. J. Telemed. Telecare 2023, 1–6. [Google Scholar] [CrossRef]
- Beddows, K.; Bansal, N.; Abraham, L.; Hsu, D.T.; Lamour, J.M. Impact of Telemedicine on Pediatric Heart Transplant Patients during the COVID-19 Pandemic. J. Heart Lung Transplant. 2021, 40, S26. [Google Scholar] [CrossRef]
- Mariani, M.V.; Pierucci, N.; Forleo, G.B.; Schiavone, M.; Bernardini, A.; Gasperetti, A.; Mitacchione, G.; Mei, M.; Giunta, G.; Piro, A.; et al. The Feasibility, Effectiveness and Acceptance of Virtual Visits as Compared to In-Person Visits among Clinical Electrophysiology Patients during the COVID-19 Pandemic. J. Clin. Med. 2023, 12, 620. [Google Scholar] [CrossRef]
- Silva-Cardoso, J.; Juanatey, J.R.G.; Comin-Colet, J.; Sousa, J.M.; Cavalheiro, A.; Moreira, E. The Future of Telemedicine in the Management of Heart Failure Patients. Card. Fail. Rev. 2021, 7, e11. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Canonico, F.; Rodolico, D.; Borovac, J.A.; Vergallo, R.; Montone, R.A.; Galli, M.; Migliaro, S.; Restivo, A.; Massetti, M.; et al. Telemedicine, Artificial Intelligence and Humanisation of Clinical Pathways in Heart Failure Management: Back to the Future and Beyond. Card. Fail. Rev. 2020, 6, e16. [Google Scholar] [CrossRef]
- Sanchez-Ross, M.; Oghlakian, G.; Maher, J.; Patel, B.; Mazza, V.; Hom, D.; Dhruva, V.; Langley, D.; Palmaro, J.; Ahmed, S.; et al. The STAT-MI (ST-Segment Analysis Using Wireless Technology in Acute Myocardial Infarction) Trial Improves Outcomes. JACC Cardiovasc. Interv. 2011, 4, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Koehler, F.; Abraham, W.T. Telemedicine and Remote Management of Patients with Heart Failure. Lancet Lond. Engl. 2011, 378, 731–739. [Google Scholar] [CrossRef]
- Mahalwar, G.; Kumar, A.; Kalra, A. Virtual Cardiology: Past, Present, Future Directions, and Considerations. Curr. Cardiovasc. Risk Rep. 2023, 17, 117–122. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet Lond. Engl. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; D’Assante, R.; Stagnaro, F.M.; Valente, V.; Crisci, G.; Giardino, F.; Arcopinto, M.; Bossone, E.; Marra, A.M.; Cittadini, A. Heart Failure Management during the COVID-19 Outbreak in Italy: A Telemedicine Experience from a Heart Failure University Tertiary Referral Centre. Eur. J. Heart Fail. 2020, 22, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Reza, N.; Donald, E.; Givertz, M.M.; Lindenfeld, J.; Jessup, M. Considerations for Heart Failure Care during the COVID-19 Pandemic. JACC Heart Fail. 2020, 8, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Clark, R.A.; Pellicori, P.; Inglis, S.C. Caring for People with Heart Failure and Many Other Medical Problems through and beyond the COVID-19 Pandemic: The Advantages of Universal Access to Home Telemonitoring. Eur. J. Heart Fail. 2020, 22, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.L.; Fonarow, G.C. Home Monitoring for Heart Failure Management. J. Am. Coll. Cardiol. 2012, 59, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Almufleh, A.; Givertz, M.M. Virtual Health During a Pandemic: Redesigning Care to Protect Our Most Vulnerable Patients. Circ. Heart Fail. 2020, 13, e007317. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, E.Z.; Goyal, P.; Cox, Z.L.; Thibodeau, J.T.; Reay, R.E.; Rasmusson, K.; Rogers, J.G.; Starling, R.C. Virtual Visits for Care of Patients with Heart Failure in the Era of COVID-19: A Statement from the Heart Failure Society of America. J. Card. Fail. 2020, 26, 448–456. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Youngson, E.; Kaul, P.; Ezekowitz, J.A. Early Follow-Up After a Heart Failure Exacerbation: The Importance of Continuity. Circ. Heart Fail. 2016, 9, e003194. [Google Scholar] [CrossRef]
- Lee, K.K.; Thomas, R.C.; Tan, T.C.; Leong, T.K.; Steimle, A.; Go, A.S. The Heart Failure Readmission Intervention by Variable Early Follow-up (THRIVE) Study: A Pragmatic Randomized Trial. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006553. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Hilty, D.M.; Gentry, M.T.; McKean, A.J.; Cowan, K.E.; Lim, R.F.; Lu, F.G. Telehealth for Rural Diverse Populations: Telebehavioral and Cultural Competencies, Clinical Outcomes and Administrative Approaches. mHealth 2020, 6, 20. [Google Scholar] [CrossRef]
- Lee, K.C.-S.; Breznen, B.; Ukhova, A.; Martin, S.S.; Koehler, F. Virtual Healthcare Solutions in Heart Failure: A Literature Review. Front. Cardiovasc. Med. 2023, 10, 1231000. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, A.; Bruce, M.; Ning, H.; Freaney, P.M.; Glynn, P.; Ahmad, F.S.; Yancy, C.W.; Shah, S.J.; Allen, N.B.; Vupputuri, S.X.; et al. Predictive Accuracy of Heart Failure-Specific Risk Equations in an Electronic Health Record-Based Cohort. Circ. Heart Fail. 2020, 13, e007462. [Google Scholar] [CrossRef]
- Bekelman, D.B.; Plomondon, M.E.; Carey, E.P.; Sullivan, M.D.; Nelson, K.M.; Hattler, B.; McBryde, C.F.; Lehmann, K.G.; Gianola, K.; Heidenreich, P.A.; et al. Primary Results of the Patient-Centered Disease Management (PCDM) for Heart Failure Study: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Hovland-Tånneryd, A.; Melin, M.; Hägglund, E.; Hagerman, I.; Persson, H.E. From Randomised Controlled Trial to Real World Implementation of a Novel Home-Based Heart Failure Tool: Pooled and Comparative Analyses of Two Clinical Controlled Trials. Open Heart 2019, 6, e000954. [Google Scholar] [CrossRef]
- Hwang, D. Monitoring Progress and Adherence with Positive Airway Pressure Therapy for Obstructive Sleep Apnea: The Roles of Telemedicine and Mobile Health Applications. Sleep Med. Clin. 2016, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Brahmbhatt, D.H.; Ross, H.J.; O’Sullivan, M.; Artanian, V.; Mueller, B.; Runeckles, K.; Steve Fan, C.-P.; Rac, V.E.; Seto, E.; Medly Titrate Study Team. The Effect of Using a Remote Patient Management Platform in Optimizing Guideline-Directed Medical Therapy in Heart Failure Patients: A Randomized Controlled Trial. JACC Heart Fail. 2024, 12, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of Telemedical Interventional Management in Patients with Heart Failure (TIM-HF2): A Randomised, Controlled, Parallel-Group, Unmasked Trial. Lancet Lond. Engl. 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Marrero, S.; Yun, S.; Cainzos-Achirica, M.; Enjuanes, C.; Garay, A.; Farre, N.; Verdú, J.M.; Linas, A.; Ruiz, P.; Hidalgo, E.; et al. Impact of Telemedicine on the Clinical Outcomes and Healthcare Costs of Patients with Chronic Heart Failure and Mid-Range or Preserved Ejection Fraction Managed in a Multidisciplinary Chronic Heart Failure Programme: A Sub-Analysis of the iCOR Randomized Trial. J. Telemed. Telecare 2020, 26, 64–72. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the Medical Revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef]
- DeVore, A.D.; Wosik, J.; Hernandez, A.F. The Future of Wearables in Heart Failure Patients. JACC Heart Fail. 2019, 7, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous Wearable Monitoring Analytics Predict Heart Failure Hospitalization: The LINK-HF Multicenter Study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Rappaport, D.; Zafrir, B.; Abraham, W.T. A Novel Approach to Monitoring Pulmonary Congestion in Heart Failure: Initial Animal and Clinical Experiences Using Remote Dielectric Sensing Technology. Congest. Heart Fail. Greenwich Conn 2013, 19, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Amir, O.; Ben-Gal, T.; Weinstein, J.M.; Schliamser, J.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Evaluation of Remote Dielectric Sensing (ReDS) Technology-Guided Therapy for Decreasing Heart Failure Re-Hospitalizations. Int. J. Cardiol. 2017, 240, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Anker, S.; Burkhoff, D.; Cleland, J.; Gorodeski, E.; Jaarsma, T.; Small, R.; Lindenfeld, J.; Miller, A.; Ogenstad, S.; et al. Primary Results of the Sensible Medical Innovations Lung Fluid Status Monitor Allows Reducing Readmission Rate of Heart Failure Patients (Smile) Trial. J. Card. Fail. 2019, 25, 938. [Google Scholar] [CrossRef]
- Lala, A.; Barghash, M.H.; Giustino, G.; Alvarez-Garcia, J.; Konje, S.; Parikh, A.; Ullman, J.; Keith, B.; Donehey, J.; Mitter, S.S.; et al. Early Use of Remote Dielectric Sensing after Hospitalization to Reduce Heart Failure Readmissions. ESC Heart Fail. 2021, 8, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, N.M.; Virani, S.A.; Sperrin, M.; Buchan, I.E.; McMurray, J.J.V.; Krahn, A.D. Predicting Heart Failure Decompensation Using Cardiac Implantable Electronic Devices: A Review of Practices and Challenges. Eur. J. Heart Fail. 2016, 18, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Small, R.S.; Tang, W.H.W. Assessing Impedance in Heart Failure: From Device Diagnostics to Population Health Opportunities? Circ. Heart Fail. 2016, 9, e002761. [Google Scholar] [CrossRef]
- Zile, M.R.; Sharma, V.; Johnson, J.W.; Warman, E.N.; Baicu, C.F.; Bennett, T.D. Prediction of All-Cause Mortality Based on the Direct Measurement of Intrathoracic Impedance. Circ. Heart Fail. 2016, 9, e002543. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Warman, E.N.; Johnson, J.W.; Small, R.S.; Heywood, J.T. Threshold Crossing of Device-Based Intrathoracic Impedance Trends Identifies Relatively Increased Mortality Risk. Eur. Heart J. 2012, 33, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Drexler, H.; Oswald, H.; Rybak, K.; Bosch, R.; Butter, C.; Klein, G.; Gerritse, B.; Monteiro, J.; Israel, C.; et al. Fluid Status Telemedicine Alerts for Heart Failure: A Randomized Controlled Trial. Eur. Heart J. 2016, 37, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Wintrich, J.; Pavlicek, V.; Brachmann, J.; Bosch, R.; Butter, C.; Oswald, H.; Rybak, K.; Mahfoud, F.; Böhm, M.; Ukena, C. Remote Monitoring With Appropriate Reaction to Alerts Was Associated With Improved Outcomes in Chronic Heart Failure: Results From the OptiLink HF Study. Circ. Arrhythm. Electrophysiol. 2021, 14, e008693. [Google Scholar] [CrossRef] [PubMed]
- Palfy, J.A.; Benezet-Mazuecos, J.; Martinez Milla, J.; Iglesias, J.A.; de la Vieja, J.J.; Sanchez-Borque, P.; Miracle, A.; Rubio, J.M. CorVue Algorithm Efficacy to Predict Heart Failure in Real Life: Unnecessary and Potentially Misleading Information? Pacing Clin. Electrophysiol. PACE 2018, 41, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Whellan, D.J.; Ousdigian, K.T.; Al-Khatib, S.M.; Pu, W.; Sarkar, S.; Porter, C.B.; Pavri, B.B.; O’Connor, C.M.; PARTNERS Study Investigators. Combined Heart Failure Device Diagnostics Identify Patients at Higher Risk of Subsequent Heart Failure Hospitalizations: Results from PARTNERS HF (Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure) Study. J. Am. Coll. Cardiol. 2010, 55, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Taborsky, M.; Glikson, M.; Heinrich, U.; Schumacher, B.; Katz, A.; Brachmann, J.; Lewalter, T.; Goette, A.; Block, M.; et al. Implant-Based Multiparameter Telemonitoring of Patients with Heart Failure (IN-TIME): A Randomised Controlled Trial. Lancet Lond. Engl. 2014, 384, 583–590. [Google Scholar] [CrossRef]
- Egolum, U.O.; Parikh, K.; Lekavich, C.; Wosik, J.; Frazier-Mills, C.; Fudim, M. Applications of the Multisensor HeartLogic Heart Failure Monitoring Algorithm During the COVID-19 Global Pandemic. JACC Case Rep. 2020, 2, 2265–2269. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.S.; Singh, J.P.; Stancak, B.; Nair, D.G.; Cao, M.; Schulze, C.; Thakur, P.H.; An, Q.; Wehrenberg, S.; Hammill, E.F.; et al. HeartLogic Multisensor Algorithm Identifies Patients During Periods of Significantly Increased Risk of Heart Failure Events: Results From the MultiSENSE Study. Circ. Heart Fail. 2018, 11, e004669. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining Home Monitoring Temporal Trends from Implanted Defibrillators and Baseline Patient Risk Profile to Predict Heart Failure Hospitalizations: Results from the SELENE HF Study. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2022, 24, 234–244. [Google Scholar] [CrossRef]
- Varma, N.; Epstein, A.E.; Irimpen, A.; Schweikert, R.; Love, C.; TRUST Investigators. Efficacy and Safety of Automatic Remote Monitoring for Implantable Cardioverter-Defibrillator Follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) Trial. Circulation 2010, 122, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H.; CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial: The Value of Wireless Remote Monitoring with Automatic Clinician Alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote Monitoring Reduces Healthcare Use and Improves Quality of Care in Heart Failure Patients with Implantable Defibrillators: The Evolution of Management Strategies of Heart Failure Patients with Implantable Defibrillators (EVOLVO) Study. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Guédon-Moreau, L.; Lacroix, D.; Sadoul, N.; Clémenty, J.; Kouakam, C.; Hermida, J.-S.; Aliot, E.; Boursier, M.; Bizeau, O.; Kacet, S.; et al. A Randomized Study of Remote Follow-up of Implantable Cardioverter Defibrillators: Safety and Efficacy Report of the ECOST Trial. Eur. Heart J. 2013, 34, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.T.; Bersohn, M.M.; Waldo, A.L.; Wathen, M.S.; Choucair, W.K.; Lip, G.Y.H.; Ip, J.; Holcomb, R.; Akar, J.G.; Halperin, J.L.; et al. Randomized Trial of Atrial Arrhythmia Monitoring to Guide Anticoagulation in Patients with Implanted Defibrillator and Cardiac Resynchronization Devices. Eur. Heart J. 2015, 36, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.; Timmermans, I.; Widdershoven, J.; Kimman, G.-J.; Prevot, S.; Rauwolf, T.; Scholten, M.F.; Zitron, E.; Mabo, P.; Denollet, J.; et al. Effect of Remote Monitoring on Patient-Reported Outcomes in European Heart Failure Patients with an Implantable Cardioverter-Defibrillator: Primary Results of the REMOTE-CIED Randomized Trial. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2019, 21, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Fonarow, G.C.; DeVore, A.D.; Sharma, P.P.; Vaduganathan, M.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; McCague, K.; Patterson, J.H.; et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 2365–2383. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Venechuk, G.; McIlvennan, C.K.; Page, R.L.; Knoepke, C.E.; Helmkamp, L.J.; Khazanie, P.; Peterson, P.N.; Pierce, K.; Harger, G.; et al. An Electronically Delivered, Patient-Activation Tool for Intensification of Medications for Chronic Heart Failure with Reduced Ejection Fraction: The EPIC-HF Trial. Circulation 2021, 143, 427–437. [Google Scholar] [CrossRef]
- Hale, G.M.; Hassan, S.L.; Hummel, S.L.; Lewis, C.; Ratz, D.; Brenner, M. Impact of a Pharmacist-Managed Heart Failure Postdischarge (Bridge) Clinic for Veterans. Ann. Pharmacother. 2017, 51, 555–562. [Google Scholar] [CrossRef]
- Desai, A.S.; Maclean, T.; Blood, A.J.; Bosque-Hamilton, J.; Dunning, J.; Fischer, C.; Fera, L.; Smith, K.V.; Wagholikar, K.; Zelle, D.; et al. Remote Optimization of Guideline-Directed Medical Therapy in Patients With Heart Failure With Reduced Ejection Fraction. JAMA Cardiol. 2020, 5, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.E.; Park, J.-E.; Park, H.-Y.; Lee, H.-Y.; Park, D.-A. Comparative Effectiveness of Telemonitoring Versus Usual Care for Heart Failure: A Systematic Review and Meta-Analysis. J. Card. Fail. 2018, 24, 19–28. [Google Scholar] [CrossRef]
- Stevenson, L.W.; Ross, H.J.; Rathman, L.D.; Boehmer, J.P. Remote Monitoring for Heart Failure Management at Home. J. Am. Coll. Cardiol. 2023, 81, 2272–2291. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; St John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from Chronic Compensated to Acute Decompensated Heart Failure: Pathophysiological Insights Obtained from Continuous Monitoring of Intracardiac Pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Bourge, R.C.; Abraham, W.T.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M.; Magalski, A.; Zile, M.R.; Smith, A.L.; Smart, F.W.; O’Shaughnessy, M.A.; et al. Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients with Advanced Heart Failure: The COMPASS-HF Study. J. Am. Coll. Cardiol. 2008, 51, 1073–1079. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless Pulmonary Artery Haemodynamic Monitoring in Chronic Heart Failure: A Randomised Controlled Trial. Lancet Lond. Engl. 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Zile, M.R.; Mehra, M.R.; Ducharme, A.; Sears, S.F.; Desai, A.S.; Maisel, A.; Paul, S.; Smart, F.; Grafton, G.; Kumar, S.; et al. Hemodynamically-Guided Management of Heart Failure Across the Ejection Fraction Spectrum: The GUIDE-HF Trial. JACC Heart Fail. 2022, 10, 931–944. [Google Scholar] [CrossRef]
- Heywood, J.T.; Jermyn, R.; Shavelle, D.; Abraham, W.T.; Bhimaraj, A.; Bhatt, K.; Sheikh, F.; Eichorn, E.; Lamba, S.; Bharmi, R.; et al. Impact of Practice-Based Management of Pulmonary Artery Pressures in 2000 Patients Implanted With the CardioMEMS Sensor. Circulation 2017, 135, 1509–1517. [Google Scholar] [CrossRef]
- Ritzema, J.; Troughton, R.; Melton, I.; Crozier, I.; Doughty, R.; Krum, H.; Walton, A.; Adamson, P.; Kar, S.; Shah, P.K.; et al. Physician-Directed Patient Self-Management of Left Atrial Pressure in Advanced Chronic Heart Failure. Circulation 2010, 121, 1086–1095. [Google Scholar] [CrossRef]
- Perl, L.; Meerkin, D.; D’amario, D.; Avraham, B.B.; Gal, T.B.; Weitsman, T.; Hasin, T.; Ince, H.; Feickert, S.; D’ancona, G.; et al. The V-LAP System for Remote Left Atrial Pressure Monitoring of Patients With Heart Failure: Remote Left Atrial Pressure Monitoring. J. Card. Fail. 2022, 28, 963–972. [Google Scholar] [CrossRef]
- Guichard, J.L.; Cowger, J.A.; Chaparro, S.V.; Kiernan, M.S.; Mullens, W.; Mahr, C.; Mullin, C.; Forouzan, O.; Hiivala, N.J.; Sauerland, A.; et al. Rationale and Design of the Proactive-HF Trial for Managing Patients With NYHA Class III Heart Failure by Using the Combined Cordella Pulmonary Artery Sensor and the Cordella Heart Failure System. J. Card. Fail. 2023, 29, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Angermann, C.E.; Assmus, B.; Anker, S.D.; Brachmann, J.; Ertl, G.; Köhler, F.; Rosenkranz, S.; Tschöpe, C.; Adamson, P.B.; Böhm, M. Safety and Feasibility of Pulmonary Artery Pressure-Guided Heart Failure Therapy: Rationale and Design of the Prospective CardioMEMS Monitoring Study for Heart Failure (MEMS-HF). Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2018, 107, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef] [PubMed]
- Garascia, A.; Palazzini, M.; Tedeschi, A.; Sacco, A.; Oliva, F.; Gentile, P. Advanced Heart Failure: From Definitions to Therapeutic Options. Eur. Heart J. Suppl. J. Eur. Soc. Cardiol. 2023, 25, C283–C291. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Cantone, R.; Perna, E.; Ammirati, E.; Varrenti, M.; D’Angelo, L.; Verde, A.; Foti, G.; Masciocco, G.; Garascia, A.; et al. Haemodynamic Effects of Sacubitril/Valsartan in Advanced Heart Failure. ESC Heart Fail. 2022, 9, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, L.; Qin, X.; Guo, X. Effect of Sacubitril/Valsartan on the Right Ventricular Function and Pulmonary Hypertension in Patients With Heart Failure With Reduced Ejection Fraction: A Systematic Review and Meta-Analysis of Observational Studies. J. Am. Heart Assoc. 2022, 11, e024449. [Google Scholar] [CrossRef]
- Nassif, M.E.; Qintar, M.; Windsor, S.L.; Jermyn, R.; Shavelle, D.M.; Tang, F.; Lamba, S.; Bhatt, K.; Brush, J.; Civitello, A.; et al. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation 2021, 143, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Masciocco, G.; Palazzini, M.; Tedeschi, A.; Ruzzenenti, G.; Conti, N.; D’Angelo, L.; Foti, G.; Perna, E.; Verde, A.; et al. Intravenous Continuous Home Inotropic Therapy in Advanced Heart Failure: Insights from an Observational Retrospective Study. Eur. J. Intern. Med. 2023, 116, 65–71. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Costanzo, M.R.; Eigler, N.; Gold, M.; Klapholz, M.; Maurer, M.; Saxon, L.; Singh, J.; Troughton, R. Hemodynamic Monitoring in Advanced Heart Failure: Results from the LAPTOP-HF Trial. J. Card. Fail. 2016, 22, 940. [Google Scholar] [CrossRef]
- D’Amario, D.; Meerkin, D.; Restivo, A.; Ince, H.; Sievert, H.; Wiese, A.; Schaefer, U.; Trani, C.; Bayes-Genis, A.; Leyva, F.; et al. Safety, Usability, and Performance of a Wireless Left Atrial Pressure Monitoring System in Patients with Heart Failure: The VECTOR-HF Trial. Eur. J. Heart Fail. 2023, 25, 902–911. [Google Scholar] [CrossRef]
- Reiss, N.; Schmidt, T.; Boeckelmann, M.; Schulte-Eistrup, S.; Hoffmann, J.-D.; Feldmann, C.; Schmitto, J.D. Telemonitoring of Left-Ventricular Assist Device Patients-Current Status and Future Challenges. J. Thorac. Dis. 2018, 10, S1794–S1801. [Google Scholar] [CrossRef] [PubMed]
- Vidula, H.; Cheyne, C.; Martens, J.; Gosev, I.; Zareba, W.; Goldenberg, I. Telehealth for the Management of Left Ventricular Assist Device Patients: The University of Rochester TeleLVAD Study. J. Card. Fail. 2021, 27, 112–113. [Google Scholar] [CrossRef]
- Schmidt, T.; Mewes, P.; Hoffmann, J.-D.; Müller-von Aschwege, F.; Glitza, J.I.; Schmitto, J.D.; Schulte-Eistrup, S.; Sindermann, J.R.; Reiss, N. Improved Aftercare in LVAD Patients: Development and Feasibility of a Smartphone Application as a First Step for Telemonitoring. Artif. Organs 2020, 44, 248–256. [Google Scholar] [CrossRef]
- Kilic, A.; Katz, J.N.; Joseph, S.M.; Brisco-Bacik, M.A.; Uriel, N.; Lima, B.; Agarwal, R.; Bharmi, R.; Farrar, D.J.; Lee, S. Changes in Pulmonary Artery Pressure before and after Left Ventricular Assist Device Implantation in Patients Utilizing Remote Haemodynamic Monitoring. ESC Heart Fail. 2018, 6, 138–145. [Google Scholar] [CrossRef]
- Thohan, V.; Abraham, J.; Burdorf, A.; Sulemanjee, N.; Jaski, B.; Guglin, M.; Pagani, F.D.; Vidula, H.; Majure, D.T.; Napier, R.; et al. Use of a Pulmonary Artery Pressure Sensor to Manage Patients With Left Ventricular Assist Devices. Circ. Heart Fail. 2023, 16, e009960. [Google Scholar] [CrossRef]
- Ko, T.; Fujita, K.; Nomura, S.; Uemura, Y.; Yamada, S.; Tobita, T.; Katoh, M.; Satoh, M.; Ito, M.; Domoto, Y.; et al. Quantification of DNA Damage in Heart Tissue as a Novel Prediction Tool for Therapeutic Prognosis of Patients With Dilated Cardiomyopathy. JACC Basic Transl. Sci. 2019, 4, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Sherman, C.B.; Said, A.; Kriss, M.; Potluri, V.S.; Levitsky, J.; Reese, P.P.; Shea, J.A.; Serper, M. In-Person Outreach and Telemedicine in Liver and Intestinal Transplant: A Survey of National Practices, Impact of Coronavirus Disease 2019, and Areas of Opportunity. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2020, 26, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Abasi, S.; Yazdani, A.; Kiani, S.; Mahmoudzadeh-Sagheb, Z. Effectiveness of Mobile Health-Based Self-Management Application for Posttransplant Cares: A Systematic Review. Health Sci. Rep. 2021, 4, e434. [Google Scholar] [CrossRef]
- Hussain, T.; Nassetta, K.; Badawy, S.M. Adherence to Immunosuppression Medications among Heart Transplant Recipients: Challenges, Opportunities, and Potential Role of Digital Approaches in the COVID-19 Era. J. Cardiovasc. Dev. Dis. 2021, 8, 68. [Google Scholar] [CrossRef]
- Gomis-Pastor, M.; Mirabet Perez, S.; Roig Minguell, E.; Brossa Loidi, V.; Lopez Lopez, L.; Ros Abarca, S.; Galvez Tugas, E.; Mas-Malagarriga, N.; Mangues Bafalluy, M.A. Mobile Health to Improve Adherence and Patient Experience in Heart Transplantation Recipients: The mHeart Trial. Healthc. Basel Switz. 2021, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Schöde, A.; Homann, K.; Feueriegel, S.; Nöth, S.; Warnke, K.; Bounader, K.; Andreeva, A.; Li, T.; Dogan, G.; et al. Telemonitoring and Care Program for Left Ventricular Assist Device Patients During COVID-19 Outbreak: A European Experience. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2021, 67, 973–981. [Google Scholar] [CrossRef]
- Dykes, J.C.; Kipps, A.K.; Chen, A.; Nourse, S.; Rosenthal, D.N.; Selamet Tierney, E.S. Parental Acquisition of Echocardiographic Images in Pediatric Heart Transplant Patients Using a Handheld Device: A Pilot Telehealth Study. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019, 32, 404–411. [Google Scholar] [CrossRef]
- Chen, A.C.; Selamet Tierney, E.S. Telehealth in Pediatric Heart Transplant Patients: Exercise, Nutrition, and Parental Imaging. Pediatr. Clin. North Am. 2020, 67, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, F. The Perceptron: A Probabilistic Model for Information Storage and Organization in the Brain. Psychol. Rev. 1958, 65, 386–408. [Google Scholar] [CrossRef]
- Mohammad, M.A. Advancing Heart Failure Research Using Machine Learning. Lancet Digit. Health 2023, 5, e331–e332. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Shah, S.M.I.; Naeem, A.; Shujauddin, S.M.; Jabeen, A.; Kazmi, S.; Siddiqui, S.A.; Kumar, P.; Salman, S.; Hassan, S.A.; et al. Artificial Intelligence in the Diagnosis and Detection of Heart Failure: The Past, Present, and Future. Rev. Cardiovasc. Med. 2021, 22, 1095–1113. [Google Scholar] [CrossRef]
- Ski, C.F.; Thompson, D.R.; Brunner-La Rocca, H.-P. Putting AI at the Centre of Heart Failure Care. ESC Heart Fail. 2020, 7, 3257–3258. [Google Scholar] [CrossRef]
- Khan, M.S.; Arshad, M.S.; Greene, S.J.; Van Spall, H.G.C.; Pandey, A.; Vemulapalli, S.; Perakslis, E.; Butler, J. Artificial Intelligence and Heart Failure: A State-of-the-Art Review. Eur. J. Heart Fail. 2023, 25, 1507–1525. [Google Scholar] [CrossRef]
- Haq, I.U.; Chhatwal, K.; Sanaka, K.; Xu, B. Artificial Intelligence in Cardiovascular Medicine: Current Insights and Future Prospects. Vasc. Health Risk Manag. 2022, 18, 517–528. [Google Scholar] [CrossRef]
- Golas, S.B.; Shibahara, T.; Agboola, S.; Otaki, H.; Sato, J.; Nakae, T.; Hisamitsu, T.; Kojima, G.; Felsted, J.; Kakarmath, S.; et al. A Machine Learning Model to Predict the Risk of 30-Day Readmissions in Patients with Heart Failure: A Retrospective Analysis of Electronic Medical Records Data. BMC Med. Inform. Decis. Mak. 2018, 18, 44. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, K.-H.; Jeon, K.-H.; Lee, S.E.; Lee, H.-Y.; Cho, H.-J.; Choi, J.O.; Jeon, E.-S.; Kim, M.-S.; Kim, J.-J.; et al. Artificial Intelligence Algorithm for Predicting Mortality of Patients with Acute Heart Failure. PLoS ONE 2019, 14, e0219302. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial Intelligence in Cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Sloane, E.B.; Silva, R.J. Chapter 83—Artificial Intelligence in Medical Devices and Clinical Decision Support Systems. In Clinical Engineering Handbook, 2nd ed.; Iadanza, E., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 556–568. ISBN 978-0-12-813467-2. [Google Scholar]
- Gupta, M.D.; Kunal, S.; Girish, M.P.; Gupta, A.; Yadav, R. Artificial Intelligence in Cardiology: The Past, Present and Future. Indian Heart J. 2022, 74, 265–269. [Google Scholar] [CrossRef]
- Labovitz, D.L.; Shafner, L.; Reyes Gil, M.; Virmani, D.; Hanina, A. Using Artificial Intelligence to Reduce the Risk of Nonadherence in Patients on Anticoagulation Therapy. Stroke 2017, 48, 1416–1419. [Google Scholar] [CrossRef]
- Bekfani, T.; Fudim, M.; Cleland, J.G.F.; Jorbenadze, A.; von Haehling, S.; Lorber, A.; Rothman, A.M.K.; Stein, K.; Abraham, W.T.; Sievert, H.; et al. A Current and Future Outlook on Upcoming Technologies in Remote Monitoring of Patients with Heart Failure. Eur. J. Heart Fail. 2021, 23, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Steventon, A.; Bardsley, M.; Billings, J.; Dixon, J.; Doll, H.; Hirani, S.; Cartwright, M.; Rixon, L.; Knapp, M.; Henderson, C.; et al. Effect of Telehealth on Use of Secondary Care and Mortality: Findings from the Whole System Demonstrator Cluster Randomised Trial. BMJ 2012, 344, e3874. [Google Scholar] [CrossRef] [PubMed]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Raisaro, A.; Curti, M.; Regoli, F.; Auricchio, A. Economic Impact of Remote Patient Monitoring: An Integrated Economic Model Derived from a Meta-Analysis of Randomized Controlled Trials in Heart Failure. Eur. J. Heart Fail. 2011, 13, 450–459. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; McAlister, F.A.; Stewart, S.; Cleland, J.G.F. Which Components of Heart Failure Programmes Are Effective? A Systematic Review and Meta-Analysis of the Outcomes of Structured Telephone Support or Telemonitoring as the Primary Component of Chronic Heart Failure Management in 8323 Patients: Abridged Cochrane Review. Eur. J. Heart Fail. 2011, 13, 1028–1040. [Google Scholar] [CrossRef]
- Grustam, A.S.; Severens, J.L.; De Massari, D.; Buyukkaramikli, N.; Koymans, R.; Vrijhoef, H.J.M. Cost-Effectiveness Analysis in Telehealth: A Comparison between Home Telemonitoring, Nurse Telephone Support, and Usual Care in Chronic Heart Failure Management. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 2018, 21, 772–782. [Google Scholar] [CrossRef]
- Dávalos, M.E.; French, M.T.; Burdick, A.E.; Simmons, S.C. Economic Evaluation of Telemedicine: Review of the Literature and Research Guidelines for Benefit-Cost Analysis. Telemed. J. E-Health Off. J. Am. Telemed. Assoc. 2009, 15, 933–948. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial | Device Type | Sample Size (n) | Primary Endpoint | Comparator | Results |
|---|---|---|---|---|---|
| TRUST [61] | ICD | 1339 |
| Conventional ICD follow-up | Telemonitoring in patient care cut in-hospital device checks by 45%, significantly sped up arrhythmic event assessments (p < 0.001), and swiftly identified those needing urgent care. It proved as safe as traditional monitoring, showing no safety differences between groups. |
| CONNECT [62] | ICD, CRTD | 1997 |
| Standard in-office care |
|
| EVOLVO [63] | ICD, CRTD | 200 |
| Remote transmission off |
|
| ECOST [64] | ICD | 433 |
| Ambulatory follow-ups |
|
| IN-TIME [56] | ICD, CRTD | 664 |
| Standard care without telemonitoring for 12 months |
|
| IMPACT [65] | ICD, CRTD | 2718 |
| Standard follow-up and anticoagulat |
|
| OptiLink HF [52] | ICD, CRTD | 1002 |
| No transmitted alerts |
|
| REMOTE-CIED [66] | ICD | 595 |
| In-clinic group |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, A.; Palazzini, M.; Trimarchi, G.; Conti, N.; Di Spigno, F.; Gentile, P.; D’Angelo, L.; Garascia, A.; Ammirati, E.; Morici, N.; et al. Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. J. Clin. Med. 2024, 13, 2592. https://doi.org/10.3390/jcm13092592
Tedeschi A, Palazzini M, Trimarchi G, Conti N, Di Spigno F, Gentile P, D’Angelo L, Garascia A, Ammirati E, Morici N, et al. Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. Journal of Clinical Medicine. 2024; 13(9):2592. https://doi.org/10.3390/jcm13092592
Chicago/Turabian StyleTedeschi, Andrea, Matteo Palazzini, Giancarlo Trimarchi, Nicolina Conti, Francesco Di Spigno, Piero Gentile, Luciana D’Angelo, Andrea Garascia, Enrico Ammirati, Nuccia Morici, and et al. 2024. "Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts" Journal of Clinical Medicine 13, no. 9: 2592. https://doi.org/10.3390/jcm13092592
APA StyleTedeschi, A., Palazzini, M., Trimarchi, G., Conti, N., Di Spigno, F., Gentile, P., D’Angelo, L., Garascia, A., Ammirati, E., Morici, N., & Aschieri, D. (2024). Heart Failure Management through Telehealth: Expanding Care and Connecting Hearts. Journal of Clinical Medicine, 13(9), 2592. https://doi.org/10.3390/jcm13092592





