Promoting Artificial Intelligence for Global Breast Cancer Risk Prediction and Screening in Adult Women: A Scoping Review
Abstract
1. Background
2. Methods
2.1. Step 1: Identify Research Questions
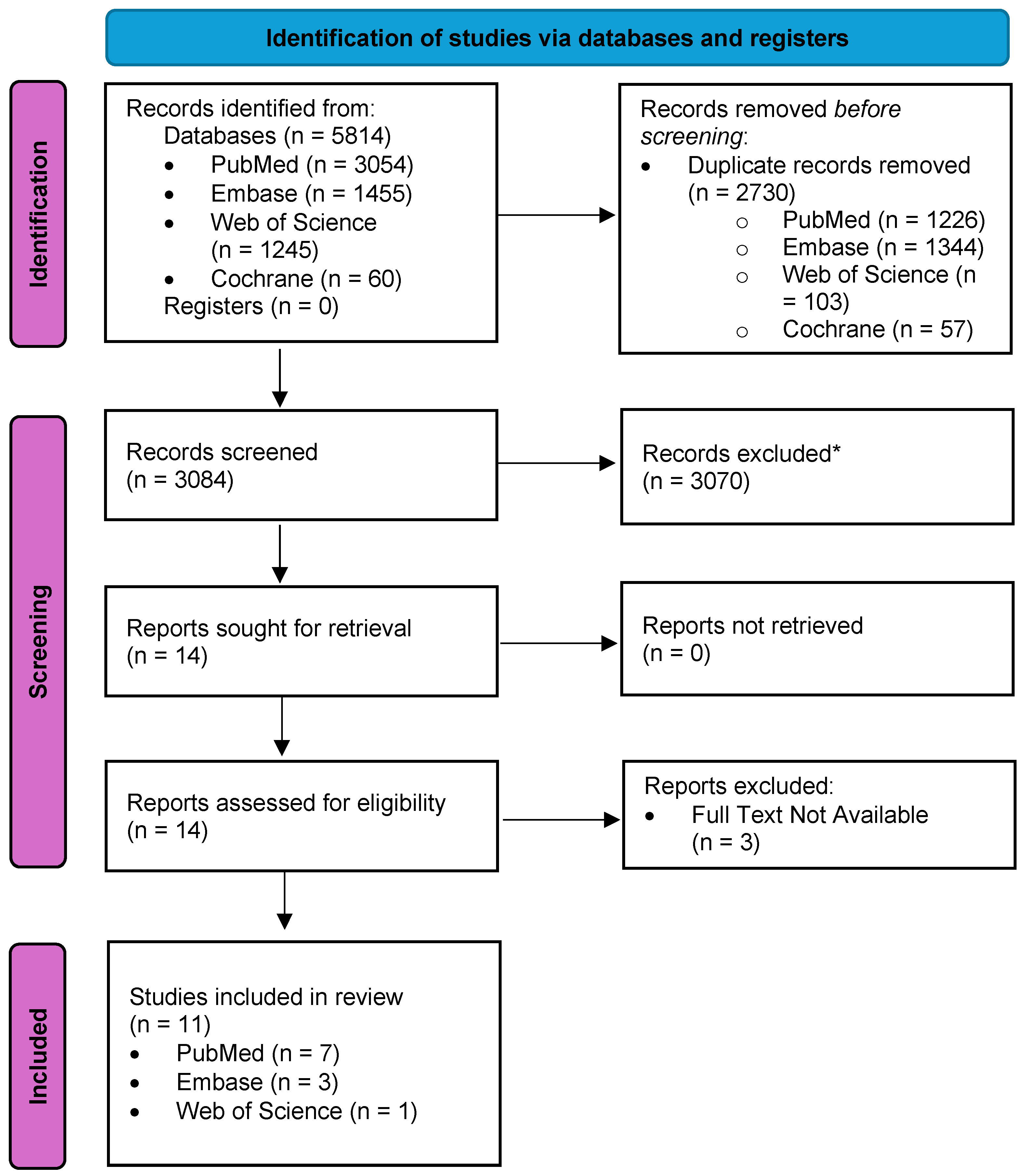
2.2. Step 2: Search for Relevant Studies
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Step 3: Selection of Studies Relevant to the Research Questions
2.4. Steps 4 and 5: Data Charting, Collation, Summarization, and Reporting of Results
3. Results
3.1. Major Outcomes
3.2. Common Barriers in AI Applications for Breast Cancer Prediction and Prevention
3.3. Lessons Learned and Future Directions
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Vargas-Cardona, H.D.; Rodriguez-Lopez, M.; Arrivillaga, M.; Vergara-Sanchez, C.; García-Cifuentes, J.P.; Bermúdez, P.C.; Jaramillo-Botero, A. Artificial intelligence for cervical cancer screening: Scoping review, 2009–2022. Int. J. Gynaecol. Obstet. 2023, 165, 566–578. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef]
- Karanth, S.; Fowler, M.E.; Mao, X.; Wilson, L.E.; Huang, B.; Pisu, M.; Potosky, A.; Tucker, T.; Akinyemiju, T. Race, socioeconomic status, and health-care access disparities in ovarian cancer treatment and mortality: Systematic review and meta-analysis. JNCI Cancer Spectr. 2019, 3, pkz084. [Google Scholar] [CrossRef]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Collin, L.J.; Jiang, R.; Ward, K.C.; Gogineni, K.; Subhedar, P.D.; Sherman, M.E.; Gaudet, M.M.; Breitkopf, C.R.; D’angelo, O.; Gabram-Mendola, S.; et al. Racial disparities in breast cancer outcomes in the metropolitan Atlanta area: New insights and approaches for health equity. JNCI Cancer Spectr. 2019, 3, pkz053. [Google Scholar] [CrossRef]
- Newman, L.A.; Kaljee, L.M. Health disparities and triple-negative breast cancer in African American women: A review: A review. JAMA Surg. 2017, 152, 485–493. [Google Scholar] [CrossRef]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global increase in breast cancer incidence: Risk factors and preventive measures. Biomed Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef]
- Harper, D.M.; Plegue, M.; Jimbo, M.; Sheinfeld Gorin, S.; Sen, A. US women screen at low rates for both cervical and colorectal cancers than a single cancer: A cross-sectional population-based observational study. eLife 2022, 11, e76070. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Gray, E.; Pashayan, N.; Deandrea, S.; Karch, A.; Vale, D.B.; Elder, K.; Procopio, P.; van Ravesteyn, N.T.; Mutabi, M.; et al. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev. Med. 2021, 151, 106585. [Google Scholar] [CrossRef]
- Breast Cancer Screening. Cancer.gov. Available online: https://progressreport.cancer.gov/detection/breast_cancer (accessed on 29 February 2024).
- Breast Cancer: Screening. Uspreventiveservicestaskforce.org. Published January 11, 2016. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening (accessed on 29 February 2024).
- Richman, I.B.; Long, J.B.; Soulos, P.R.; Wang, S.Y.; Gross, C.P. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann. Intern. Med. 2023, 176, 1172–1180. [Google Scholar] [CrossRef]
- Løberg, M.; Lousdal, M.L.; Bretthauer, M.; Kalager, M. Benefits and harms of mammography screening. Breast Cancer Res. 2015, 17, 63. [Google Scholar] [CrossRef]
- Koh, D.-M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial intelligence and machine learning in cancer imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Hunter, B.; Hindocha, S.; Lee, R.W. The role of artificial intelligence in early cancer diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef]
- Can Artificial Intelligence Help See Cancer in New Ways? National Cancer Institute. Published March 22, 2022. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2022/artificial-intelligence-cancer-imaging (accessed on 29 February 2024).
- Carter, S.M. Why does cancer screening persist despite the potential to harm? Sci. Technol. Soc. 2021, 26, 24–40. [Google Scholar] [CrossRef]
- Dembrower, K.; Crippa, A.; Colón, E.; Eklund, M.; Strand, F. Artificial intelligence for breast cancer detection in screening mammography in Sweden: A prospective, population-based, paired-reader, non-inferiority study. Lancet Digit. Health 2023, 5, e703–e711. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine learning and AI in cancer prognosis, prediction, and treatment selection: A critical approach. J. Multidiscip. Healthc. 2023, 16, 1779–1791. [Google Scholar] [CrossRef]
- Badal, K.; Lee, C.M.; Esserman, L.J. Guiding principles for the responsible development of artificial intelligence tools for healthcare. Commun. Med. 2023, 3, 47. [Google Scholar] [CrossRef]
- DeGroff, A.; Miller, J.; Sharma, K.; Sun, J.; Helsel, W.; Kammerer, W.; Rockwell, T.; Sheu, A.; Melillo, S.; Uhd, J.; et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January–June 2020, in the United States. Prev. Med. 2021, 151, 106559. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Akselrod-Ballin, A.; Chorev, M.; Shoshan, Y.; Spiro, A.; Hazan, A.; Melamed, R.; Barkan, E.; Herzel, E.; Naor, S.; Karavani, E.; et al. Predicting breast cancer by applying deep learning to linked health records and mammograms. Radiology 2019, 292, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Arasu, V.A.; Habel, L.A.; Achacoso, N.S.; Buist, D.S.; Cord, J.B.; Esserman, L.J.; Hylton, N.M.; Glymour, M.M.; Kornak, J.; Kushi, L.H.; et al. Comparison of mammography artificial intelligence algorithms for 5-year breast cancer risk prediction. bioRxiv 2022. [Google Scholar] [CrossRef]
- Arasu, V.A.; Habel, L.A.; Achacoso, N.S.; Buist, D.S.M.; Cord, J.B.; Esserman, L.J.; Hylton, N.M.; Glymour, M.M.; Kornak, J.; Kushi, L.H.; et al. Comparison of mammography AI algorithms with a clinical risk model for 5-year breast cancer risk prediction: An observational study. Radiology 2023, 307, e222733. [Google Scholar] [CrossRef] [PubMed]
- Chorev, M.; Barros, V.; Spiro, A.; Evron, E.; Barkan, E.; Kagan, O.; Amit, M.; Ozery-Flato, M.; Akselrod-Balin, A.; Shalev, V.; et al. Leveraging comprehensive health records for breast cancer risk prediction: A binational assessment. AMIA Annu. Symp. Proc. 2022, 2022, 385–394. [Google Scholar] [PubMed]
- Davalagi, S.B.; Palicheralu, B.S.; Murthy, S.S.N.; Hurlihal, S. Acceptance of artificial intelligence (AI)-based screening for breast health in urban slums of central Karnataka, India-SWOC analysis. J. Family Med. Prim. Care 2022, 11, 6023–6028. [Google Scholar] [CrossRef] [PubMed]
- Hersch, J.; Barratt, A.; Jansen, J.; Irwig, L.; McGeechan, K.; Jacklyn, G.; Thornton, H.; Dhillon, H.; Houssami, N.; McCaffery, K. Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: A randomised controlled trial. Lancet 2015, 385, 1642–1652. [Google Scholar] [CrossRef]
- Kukafka, R.; Yi, H.; Xiao, T.; Thomas, P.; Aguirre, A.; Smalletz, C.; David, R.; Crew, K. Why breast cancer risk by the numbers is not enough: Evaluation of a decision aid in multi-ethnic, low-numerate women. J. Med. Internet Res. 2015, 17, e165. [Google Scholar] [CrossRef]
- McGuinness, J.E.; Zhang, T.M.; Cooper, K.; Kelkar, A.; Dimond, J.; Lorenzi, V.; Crew, K.D.; Kukafka, R. Extraction of electronic health record data using Fast Healthcare Interoperability Resources for automated breast cancer risk assessment. AMIA Annu. Symp. Proc. 2021, 2021, 843–852. [Google Scholar] [PubMed]
- Portnoi, T.; Yala, A.; Schuster, T.; Barzilay, R.; Dontchos, B.; Lamb, L.; Lehman, C. Deep learning model to assess cancer risk on the basis of a breast MR image alone. AJR Am. J. Roentgenol. 2019, 213, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Saghatchian, M.; Abehsera, M.; Yamgnane, A.; Geyl, C.; Gauthier, E.; Hélin, V.; Bazire, M.; Villoing-Gaudé, L.; Reyes, C.; Gentien, D.; et al. Feasibility of personalized screening and prevention recommendations in the general population through breast cancer risk assessment: Results from a dedicated risk clinic. Breast Cancer Res. Treat. 2022, 192, 375–383. [Google Scholar] [CrossRef]
- Stark, G.F.; Hart, G.R.; Nartowt, B.J.; Deng, J. Predicting breast cancer risk using personal health data and machine learning models. PLoS ONE 2019, 14, e0226765. [Google Scholar] [CrossRef]
- Elo, S.; Kyngäs, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef]
- Romanov, S.; Howell, S.; Harkness, E.; Bydder, M.; Evans, D.G.; Squires, S.; Fergie, M.; Astley, S. Artificial intelligence for image-based breast cancer risk prediction using attention. Tomography 2023, 9, 2103–2115. [Google Scholar] [CrossRef]
- Yala, A.; Mikhael, P.G.; Strand, F.; Lin, G.; Satuluru, S.; Kim, T.; Banerjee, I.; Gichoya, J.; Trivedi, H.; Lehman, C.D.; et al. Multi-institutional validation of a mammography-based breast cancer risk model. J. Clin. Oncol. 2022, 40, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Balkenende, L.; Teuwen, J.; Mann, R.M. Application of deep learning in breast cancer imaging. Semin. Nucl. Med. 2022, 52, 584–596. [Google Scholar] [CrossRef]
- Mema, E.; McGinty, G. The role of artificial intelligence in understanding and addressing disparities in breast cancer outcomes. Curr. Breast Cancer Rep. 2020, 12, 168–174. [Google Scholar] [CrossRef]
- Manikis, G.C.; Simos, N.J.; Kourou, K.; Kondylakis, H.; Poikonen-Saksela, P.; Mazzocco, K.; Pat-Horenczyk, R.; Sousa, B.; Oliveira-Maia, A.J.; Mattson, J.; et al. Personalized risk analysis to improve the psychological resilience of women undergoing treatment for Breast Cancer: Development of a machine learning–driven clinical decision support tool. J. Med. Internet Res. 2023, 25, e43838. [Google Scholar] [CrossRef]
- Ng, A.Y.; Oberije, C.J.G.; Ambrózay, É.; Szabó, E.; Serfőző, O.; Karpati, E.; Fox, G.; Glocker, B.; Morris, E.A.; Forrai, G.; et al. Prospective implementation of AI-assisted screen reading to improve early detection of breast cancer. Nat. Med. 2023, 29, 3044–3049. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.-M.; Yin, M.; Yu, M.-H.; Yu, J.; Zeng, S.-E.; Lv, W.-Z.; Li, J.; Ye, H.-R.; Cui, X.-W.; Dietrich, C.F. Artificial intelligence in medical imaging of the breast. Front. Oncol. 2021, 11, 600557. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Dell’Aquila, K.; Hodges, L.; Maldjian, T.; Duong, T.Q. Deep learning applications to breast cancer detection by magnetic resonance imaging: A literature review. Breast Cancer Res. 2023, 25, 87. [Google Scholar] [CrossRef] [PubMed]
- Uzun Ozsahin, D.; Ikechukwu Emegano, D.; Uzun, B.; Ozsahin, I. The systematic review of artificial intelligence applications in breast cancer diagnosis. Diagnostics 2022, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Marinovich, M.L.; Wylie, E.; Lotter, W.; Pearce, A.; Carter, S.M.; Lund, H.; Waddell, A.; Kim, J.G.; Pereira, G.F.; Lee, C.I.; et al. Artificial intelligence (AI) to enhance breast cancer screening: Protocol for population-based cohort study of cancer detection. BMJ Open 2022, 12, e054005. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dung, N.T.; Thach, P.N.; Phong, N.T.; Phu, V.D.; Phu, K.D.; Yen, L.M.; Xuan Thy, D.B.; Soltan, A.A.; Thwaites, L.; et al. Generalizability assessment of AI models across hospitals: A comparative study in low-middle income and high income countries. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yang, J.; Soltan, A.A.S.; Clifton, D.A. Machine learning generalizability across healthcare settings: Insights from multi-site COVID-19 screening. NPJ Digit. Med. 2022, 5, 69. [Google Scholar] [CrossRef]
- Segar, M.W.; Hall, J.L.; Jhund, P.S.; Powell-Wiley, T.M.; Morris, A.A.; Kao, D.; Fonarow, G.C.; Hernandez, R.; Ibrahim, N.E.; Rutan, C.; et al. Machine learning-based models incorporating social determinants of health vs traditional models for predicting in-hospital mortality in patients with Heart Failure. JAMA Cardiol. 2022, 7, 844–854. [Google Scholar] [CrossRef]
- Social Determinants of Health. Health.gov. Available online: https://health.gov/healthypeople/objectives-and-data/social-determinants-health (accessed on 29 February 2024).
- Carroll, N.W.; Jones, A.; Burkard, T.; Lulias, C.; Severson, K.; Posa, T. Improving risk stratification using AI and social determinants of health. Am. J. Manag. Care 2022, 28, 582–587. [Google Scholar] [CrossRef]
- Shams, R.A.; Zowghi, D.; Bano, M. AI and the quest for diversity and inclusion: A systematic literature review. AI Ethics 2023. [Google Scholar] [CrossRef]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The future of digital health with federated learning. NPJ Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, C.; Massey, D.; Mahajan, S.; Lu, Y.; Annapureddy, A.R.; Roy, B.; Riley, C.; Murugiah, K.; Valero-Elizondo, J.; Onuma, O.; et al. Racial and ethnic disparities in access to health care among adults in the United States: A 20-year National Health Interview Survey analysis, 1999–2018. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.; Janke, A. Health literacy and access to care. J. Health Commun. 2016, 21 (Suppl. S1), 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Y.; Yang, Y.; Zhang, F. Association between health literacy and mortality: A systematic review and meta-analysis. Arch. Public Health 2021, 79, 119. [Google Scholar] [CrossRef] [PubMed]
- Morris, N.S.; Field, T.S.; Wagner, J.L.; Cutrona, S.L.; Roblin, D.W.; Gaglio, B.; Williams, A.E.; Han, P.J.K.; Costanza, M.E.; Mazor, K.M. The association between health literacy and cancer-related attitudes, behaviors, and knowledge. J. Health Commun. 2013, 18 (Suppl. S1), 223–241. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, D.M.; Larson, J.L.; Zikmund-Fisher, B.J. Associations between health literacy and preventive health behaviors among older adults: Findings from the health and retirement study. BMC Public Health 2016, 16, 596. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Why is health literacy failing so many? Lancet 2022, 400, 1655. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, P. Use of AI-based tools for healthcare purposes: A survey study from consumers’ perspectives. BMC Med. Inform. Decis. Mak. 2020, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Görtz, M.; Baumgärtner, K.; Schmid, T.; Muschko, M.; Woessner, P.; Gerlach, A.; Byczkowski, M.; Sültmann, H.; Duensing, S.; Hohenfellner, M. An artificial intelligence-based chatbot for prostate cancer education: Design and patient evaluation study. Digit. Health 2023, 9, 20552076231173304. [Google Scholar] [CrossRef]
- Wang, A.; Qian, Z.; Briggs, L.; Cole, A.P.; Reis, L.O.; Trinh, Q.D. The use of chatbots in oncological care: A narrative review. Int. J. Gen. Med. 2023, 16, 1591–1602. [Google Scholar] [CrossRef]
- Kirchner, G.J.; Kim, R.Y.; Weddle, J.B.; Bible, J.E. Can artificial intelligence improve the readability of patient education materials? Clin. Orthop. Relat. Res. 2023, 481, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
| Article # | Primary Author/Year | Study Design | Country | Sample Size | Study Population | Study Purpose | Type of AI Model/Technique Applied to Breast Cancer Screening and/or Risk Prediction | Major Outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Akselrod-Ballin et al., 2019 [27] | Retrospective Cohort Study | Israel | n = 13,234 | Women who underwent at least one mammogram between 2013 and 2017 in one of the five Assuta Medical Centers imaging facilities, and who had health records for at least 1 year before undergoing mammography in Maccabi Health Services | To evaluate the accuracy and efficiency of a combined machine and deep learning approach for early breast cancer detection applied to a link dataset of digital mammography images and detailed electronic health records |
|
|
| 2 | Arasu et al., 2022 [28] | Retrospective Case-Cohort Study | USA | n = 13,881 | Women who had a bilateral screening mammogram in 2016 at Kaiser Permanente Northern California, without evidence of cancer on final imaging assessment either at the time of screening or after diagnostic work up of positive screening findings | To examine the ability of 5 artificial intelligence (AI)-based computer vision algorithms, most trained to detect visible breast cancer on mammograms, to predict future risk relative to the Breast Cancer Surveillance Consortium clinical risk prediction model (BCSC v2) |
|
|
| 3 | Arasu et al., 2023 [29] | Retrospective Case-Cohort Study | USA | n = 13,628 | Women who had a bilateral screening mammographic examination in 2016 at Kaiser Permanente NorthernCalifornia that was negative at final imaging assessment, and were followed until 2021 | To compare selected existing mammography artificial intelligence (AI) algorithms and the Breast Cancer Surveillance Consortium (BCSC) risk model for prediction of 5-year risk |
|
|
| 4 | Chorev et al., 2023 [30] | Retrospective Cohort Study | Israel | n = 13786 (Israel); n = 1695 (US) | Israeli and American women who underwent screening mammography | To assess the utility of a personalized breast cancer (BC) risk model using comprehensive health records |
|
|
| 5 | Davalagi et al., 2022 [31] | Mixed-Methods Study | India | n = 768 | Women in the reproductive age group from urban slums of central Karnataka, India | To assess the acceptance and explore challenges for an AI-based screening solution for breast health among the urban slum population |
|
|
| 6 | Hersch et al., 2015 [32] | Randomized Controlled Trial | Australia | n = 879 | Women aged 48–50 years from New South Wales, Australia | To investigate the impact of including information about breast cancer over detection in a decision aid on informed choice in breast screening |
|
|
| 7 | Kukafka et al., 2015 [33] | Mixed-Methods Study | USA | n = 34 | Multi-ethnic women from Upper Manhattan, predominantly Hispanic, with a high proportion of low numeracy | To evaluate a decision aid, RealRisks, in improving breast cancer risk perception and decision-making in low-numerate women |
|
|
| 8 | McGuinness et al., 2022 [34] | Pilot Usability Study | USA | n = 6 | EHR data of 6 patient advocates | To evaluate whether the Fast Healthcare Interoperability Resources (FHIR) standard could support automated breast cancer risk calculations in RealRisks and Breast cancer risk NAVIgation (BNAV) as well as presentation of relevant patient medical history to patients and providers to facilitate shared decision-making |
|
|
| 9 | Portnoi et al., 2019 [35] | Retrospective Cohort Study | USA | n = 1656 | High-risk women who were screened for breast cancer due to risk factors: genetic mutation, chest radiation, family history of breast cancer, or personal history of breast cancer | To develop a deep learning-based model to analyze breast MR and predict 5-year breast cancer risk |
|
|
| 10 | Saghatchian et al., 2022 [36] | Feasibility Study | France | n = 196 | Women aged 40 or older, primarily of Caucasian origin, undergoing breast cancer risk assessment | To assess the feasibility of personalized screening and prevention recommendations in the general population through breast cancer risk assessment at a dedicated risk clinic |
|
|
| 11 | Stark et al., 2019 [37] | Retrospective Cohort Study | USA | n = 64,739 | Study population was derived from the PLCO (Prostate, Lung, Colorectal, and Ovarian) cancer screening trial data, focusing on women who self-identified as White, Black, or Hispanic | To predict breast cancer risk using personal health data and machine learning models |
|
|
| Article # | Primary Author/Year | Barriers | Common Barriers in AI Application for Breast Cancer Screening and Risk Prediction |
|---|---|---|---|
| 1 | Akselrod-Ballin et al., 2019 [27] |
|
|
| 2 | Arasu et al., 2022 [28] |
| |
| 3 | Arasu et al., 2023 [29] |
| |
| 4 | Chorev et al., 2023 [30] |
| |
| 5 | Davalagi et al., 2022 [31] |
| |
| 6 | Hersch et al., 2015 [32] |
| |
| 7 | Kukafka et al., 2015 [33] |
| |
| 8 | McGuiness et al., 2022 [34] |
| |
| 9 | Portnoi et al., 2019 [35] |
| |
| 10 | Saghatchian et al., 2022 [36] |
| |
| 11 | Stark et al., 2019 [37] |
|
| Article # | Primary Author/Year | Recommendations | Recurrent Themes for Future Directions in AI Application for Breast Cancer Screening and Risk Prediction |
|---|---|---|---|
| 1 | Akselrod-Ballin et al., 2019 [27] |
|
|
| 2 | Arasu et al., 2022 [28] |
| |
| 3 | Arasu et al., 2023 [29] |
| |
| 4 | Chorev et al., 2023 [30] |
| |
| 5 | Davalagi et al., 2022 [31] |
| |
| 6 | Hersch et al., 2015 [32] |
| |
| 7 | Kukafka et al., 2015 [33] |
| |
| 8 | McGuiness et al., 2022 [34] |
| |
| 9 | Portnoi et al., 2019 [35] |
| |
| 10 | Saghatchian et al., 2022 [36] |
| |
| 11 | Stark et al., 2019 [37] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacca, L.; Lobaina, D.; Burgoa, S.; Lotharius, K.; Moothedan, E.; Gilmore, N.; Xie, J.; Mohler, R.; Scharf, G.; Knecht, M.; et al. Promoting Artificial Intelligence for Global Breast Cancer Risk Prediction and Screening in Adult Women: A Scoping Review. J. Clin. Med. 2024, 13, 2525. https://doi.org/10.3390/jcm13092525
Sacca L, Lobaina D, Burgoa S, Lotharius K, Moothedan E, Gilmore N, Xie J, Mohler R, Scharf G, Knecht M, et al. Promoting Artificial Intelligence for Global Breast Cancer Risk Prediction and Screening in Adult Women: A Scoping Review. Journal of Clinical Medicine. 2024; 13(9):2525. https://doi.org/10.3390/jcm13092525
Chicago/Turabian StyleSacca, Lea, Diana Lobaina, Sara Burgoa, Kathryn Lotharius, Elijah Moothedan, Nathan Gilmore, Justin Xie, Ryan Mohler, Gabriel Scharf, Michelle Knecht, and et al. 2024. "Promoting Artificial Intelligence for Global Breast Cancer Risk Prediction and Screening in Adult Women: A Scoping Review" Journal of Clinical Medicine 13, no. 9: 2525. https://doi.org/10.3390/jcm13092525
APA StyleSacca, L., Lobaina, D., Burgoa, S., Lotharius, K., Moothedan, E., Gilmore, N., Xie, J., Mohler, R., Scharf, G., Knecht, M., & Kitsantas, P. (2024). Promoting Artificial Intelligence for Global Breast Cancer Risk Prediction and Screening in Adult Women: A Scoping Review. Journal of Clinical Medicine, 13(9), 2525. https://doi.org/10.3390/jcm13092525






