Data-Driven Surveillance Protocol for Patients at Risk for Peritoneal Recurrence of Primary Colon Cancer: Surveillance for Peritoneal Carcinomatosis †
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient and Disease Characteristics
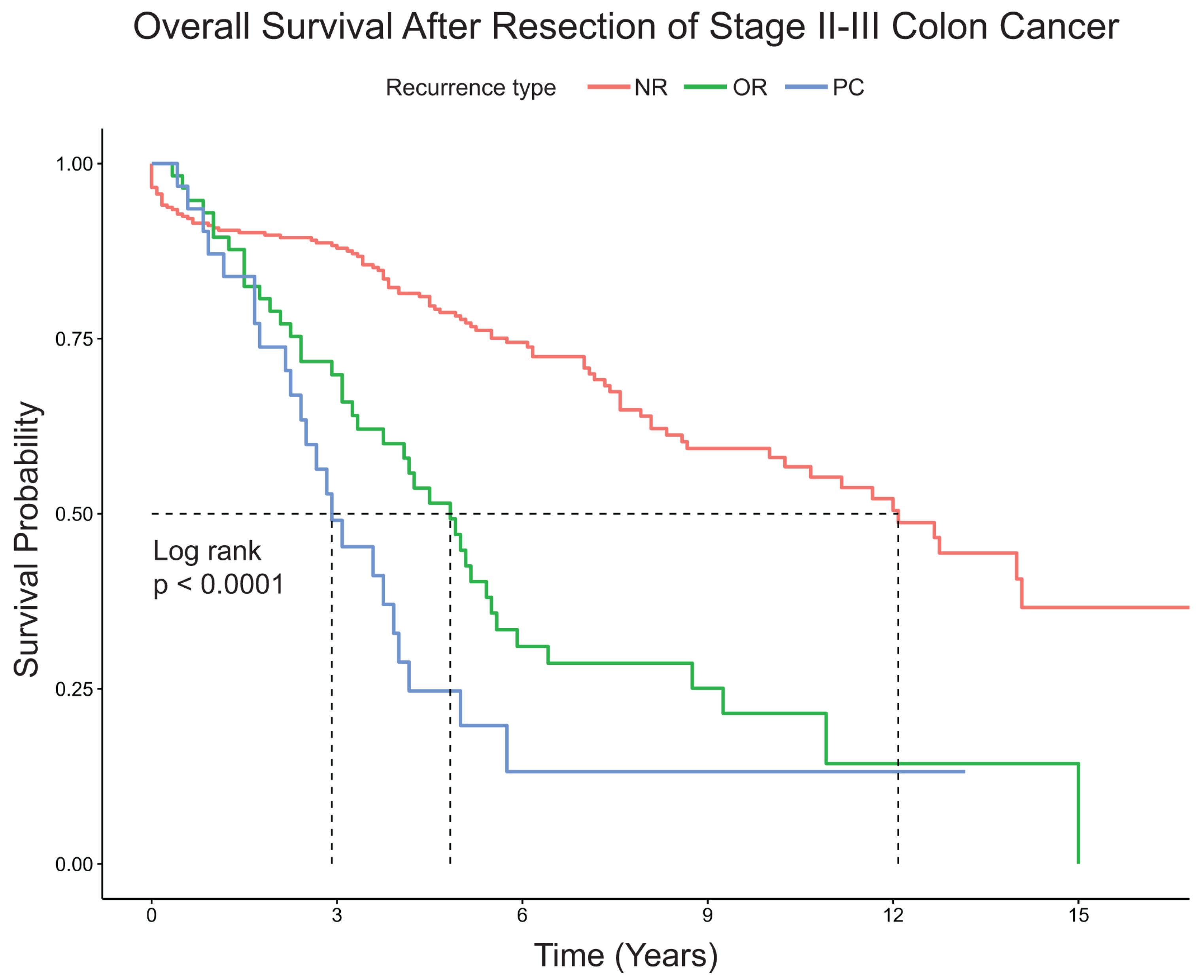
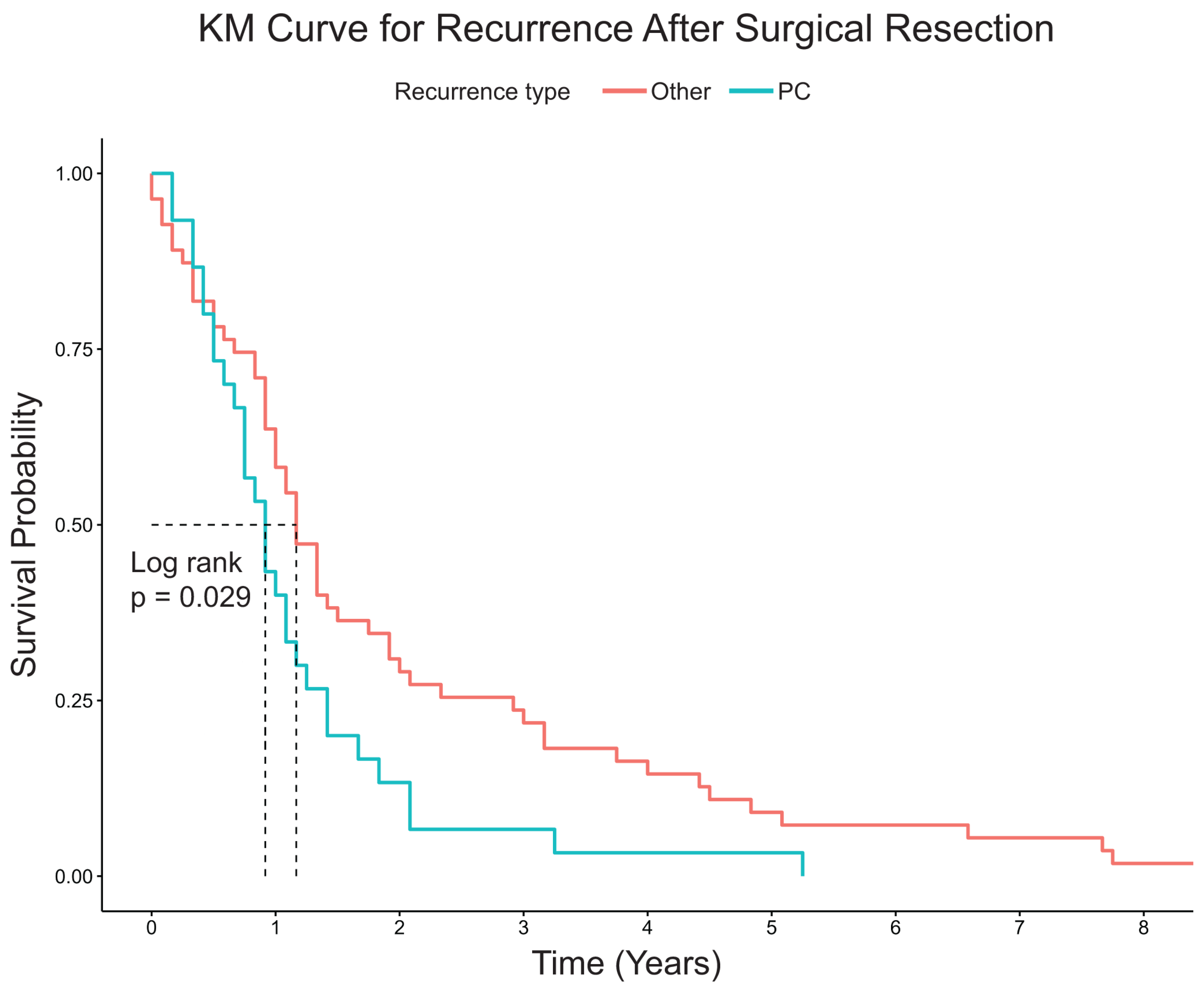
3.2. Recurrence and Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, V.; Huang, N.; Liauw, W.; Morris, D.L. High risk features of primary colorectal carcinomas which subsequently undergo peritonectomy. Eur. J. Surg. Oncol. 2016, 42, 836–840. [Google Scholar] [CrossRef]
- Kerscher, A.G.; Chua, T.C.; Gasser, M.; Maeder, U.; Kunzmann, V.; Isbert, C.; Germer, C.T.; Pelz, J.O.W. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer 2013, 108, 1432–1439. [Google Scholar] [CrossRef]
- Kanda, M.; Kodera, Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J. Gastroenterol. 2016, 22, 6829–6840. [Google Scholar] [CrossRef]
- Elias, D.; Honoré, C.; Dumont, F.; Ducreux, M.; Boige, V.; Malka, D.; Burtin, P.; Dromain, C.; Goéré, D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann. Surg. 2011, 254, 289–293. [Google Scholar] [CrossRef]
- Le, V.H.; Thornblade, L.; Ituarte, P.H.G.; Lai, L.L.; Melstrom, K.A. Metachronous peritoneal metastases following curative resection for colon cancer: Understanding risk factors and patterns of recurrence. J. Surg. Oncol. 2021, 123, 622–629. [Google Scholar] [CrossRef]
- Mayanagi, S.; Kashiwabara, K.; Honda, M.; Oba, K.; Aoyama, T.; Kanda, M.; Maeda, H.; Hamada, C.; Sadahiro, S.; Sakamoto, J.; et al. Risk Factors for Peritoneal Recurrence in Stage II to III Colon Cancer. Dis. Colon Rectum 2018, 61, 803–808. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.-M.; Bereder, J.-M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.-J.; et al. Second-Look Surgery Plus Hyperthermic Intraperitoneal Chemotherapy versus Surveillance in Patients 1 at High Risk of Developing Colorectal Peritoneal Metastases (PROPHYLOCHIP-PRODIGE 15): A 2 Randomised, Phase 3 Study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Bastiaenen, V.P.; Klaver, C.E.L.; Kok, N.F.M.; De Wilt, J.H.W.; De Hingh, I.H.J.T.; Aalbers, A.G.J.; Boerma, D.; Bremers, A.J.A.; Burger, J.W.A.; Van Duyn, E.B.; et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; The COLOPEC 2 randomized multicentre trial. BMC Cancer 2019, 19, 254. [Google Scholar] [CrossRef]
- Provenzale, D.; Gupta, S.; Ahnen, D.J.; Markowitz, A.J.; Chung, D.C.; Mayer, R.J.; Regenbogen, S.E.; Blanco, A.M.; Bray, T.; Cooper, G.; et al. NCCN Guidelines Insights: Colorectal Cancer Screening, Version 1.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 939–949. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Mo, T.W.; Zhang, Z.J.; Chen, Y.L.; Huang, J.; Su, D.; Song, W.; Hu, J.; He, X. Risk factors for metachronous peritoneal carcinomatosis after radical resection for patients with nonmetastatic pT3-4 colon cancer. J. Surg. Oncol. 2022, 126, 757–771. [Google Scholar] [CrossRef]
- Aiken, T.; Hu, C.Y.; Uppal, A.; Francescatti, A.B.; Fournier, K.F.; Chang, G.J.; Zafar, S.N. Peritoneal recurrence after resection for Stage I–III colorectal cancer: A population analysis. J. Surg. Oncol. 2023, 127, 678–687. [Google Scholar] [CrossRef]
- Kanda, M.; Oba, K.; Aoyama, T.; Kashiwabara, K.; Mayanagi, S.; Maeda, H.; Honda, M.; Hamada, C.; Sadahiro, S.; Sakamoto, J.; et al. Clinical signatures of mucinous and poorly differentiated subtypes of colorectal adenocarcinomas by a propensity score analysis of an independent patient database from three phase III trials. Dis. Colon Rectum 2018, 61, 461–471. [Google Scholar] [CrossRef]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Marmor, R.A.; Kelly, K.J.; Lowy, A.M.; Baumgartner, J.M. Laparoscopy is Safe and Accurate to Evaluate Peritoneal Surface Metastasis Prior to Cytoreductive Surgery. Ann. Surg. Oncol. 2016, 23, 1461–1467. [Google Scholar] [CrossRef]
- Attiyeh, F.; Stearns, M. Second-look laparotomy based on CEA elevations in colorectal cancer. Cancer 1981, 47, 2119–2125. [Google Scholar] [CrossRef]
- Cortes-Guiral, D.; Elias, D.; Cascales-Campos, P.A.; Badía Yébenes, A.; Guijo Castellano, I.; León Carbonero, A.I.; Martín Valadés, J.I.; Garcia-Foncillas, J.; Garcia-Olmo, D. Second-look surgery plus hyperthermic intraperitoneal chemotherapy for patients with colorectal cancer at high risk of peritoneal carcinomatosis: Does it really save lives? World J. Gastroenterol. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Dromain, C.; Leboulleux, S.; Auperin, A.; Goere, D.; Malka, D.; Lumbroso, J.; Schumberger, M.; Sigal, R.; Elias, D. Staging of peritoneal carcinomatosis: Enhanced CT vs. PET/CT. Abdom. Imaging 2008, 33, 87–93. [Google Scholar] [CrossRef]
- De Bree, E.; Koops, W.; Kröger, R.; Van Ruth, S.; Witkamp, A.J.; Zoetmulder, F.A.N. Peritoneal Carcinomatosis from Colorectal or Appendiceal Origin: Correlation of Preoperative CT with Intraoperative Findings and Evaluation of Interobserver Agreement. J. Surg. Oncol. 2004, 86, 64–73. [Google Scholar] [CrossRef]
- Elias, D.; Glehen, O.; Pocard, M.; Quenet, F.; Goéré, D.; Arvieux, C.; Rat, P.; Gilly, F. A comparative study of complete cytoreductive surgery plus intraperitoneal chemotherapy to treat peritoneal dissemination from colon, rectum, small bowel, and nonpseudomyxoma appendix. Ann. Surg. 2010, 251, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Nakamura, Y.; Taniguchi, H.; Shiozawa, M.; Yasui, H.; Esaki, T.; Kagawa, Y.; Denda, T.; Satoh, T.; Yamazaki, K.; et al. Effects of Metastatic Sites on Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. JCO Precis. Oncol. 2022, 6, e2100535. [Google Scholar] [CrossRef] [PubMed]
| PC (n = 32) | OTR (n = 57) | NR (n = 323) | p-Value | |
|---|---|---|---|---|
| Age, median (IQR) | 62 (50.5–73.5) | 63 (53–71) | 69 (58–78) | 0.003 |
| Sex, N (%) | 0.864 | |||
| Male | 15 (46.9) | 26 (45.6) | 159 (49.2) | |
| Race, N (%) | 0.752 | |||
| Black | 2 (6.2) | 2 (3.5) | 8 (2.5) | |
| Other | 1 (3.1) | 3 (5.3) | 12 (3.7) | |
| White | 29 (90.6) | 52 (91.2) | 303 (93.8) | |
| Non-Hispanic, N (%) | 32 (100.0) | 57 (100.0) | 317 (98.1) | 0.432 |
| BMI, median (IQR) | 26.98 (22.95–33.11) | 27.05 (24.40–31.91) | 28.00 (23.99–32.98) | 0.914 |
| Smoking history, N (%) | 12 (37.5) | 20 (35.1) | 131 (40.6) | 0.716 |
| CCI, median (IQR) | 4 (3–6) | 5 (4–6) | 5 (4–6) | 0.271 |
| IBD | 3 (9.4) | 0 (0.0) | 13 (4.0) | 0.086 |
| Polyposis, N (%) | 4 (12.5) | 1 (1.8) | 11 (3.4) | 0.027 |
| PC (n = 32) | OTR (n = 57) | NR (n = 323) | p-Value | |
|---|---|---|---|---|
| Surgery to Recurrence, months, median (IQR) | 11.0 (6.3–16.50 | 14.0 (9.0–31.5) | NA | 0.064 |
| Neoadjuvant Systemic Therapy, N (%) | 1 (3.1) | 4 (7.0) | 8 (2.5) | 0.195 |
| Adjuvant Systemic Therapy, N (%) | 28 (87.5) | 43 (75.4) | 158 (48.9) | <0.001 |
| Open Surgical Approach, N (%) | 18 (56.2) | 37 (64.9) | 175 (54.5) | 0.346 |
| Bowel Diversion, N (%) | 8 (25.0) | 11 (19.3) | 68 (21.1) | 0.817 |
| Blood Transfusion, N (%) | 2 (6.2) | 1 (1.8) | 12 (3.7) | 0.548 |
| Right-sided tumor, N (%) | 24 (75.0) | 33 (57.9) | 189 (58.5) | 0.185 |
| Obstruction, N (%) | 8 (25.0) | 9 (15.8) | 42 (13.0) | 0.171 |
| Gross perforation, N (%) | 8 (25.0) | 6 (10.5) | 17 (5.3) | <0.001 |
| Microscopic tumor perforation, N (%) | 8 (25.0) | 5 (8.8) | 22 (6.8) | 0.002 |
| Tumor Deposits, N (%) | 12 (37.5) | 14 (24.6) | 59 (18.3) | 0.027 |
| Margins involved, N (%) | 8 (25.0) | 5 (8.8) | 15 (4.6) | <0.001 |
| Mucinous component > 50%, N (%) | 8 (25.0) | 8 (14.0) | 57 (17.6) | 0.428 |
| Microsatellite instability, N (%) | 4 (12.9) | 15 (34.9) | 44 (15.7) | 0.007 |
| Lymphovascular invasion, N (%) | 18 (56.2) | 19 (33.3) | 79 (24.5) | <0.001 |
| Perineural invasion, N (%) | 9 (28.1) | 9 (15.8) | 37 (11.5) | 0.026 |
| Tumor Grade, N (%) | 0.205 | |||
| well | 2 (6.2) | 2 (3.5) | 10 (3.1) | |
| moderate | 22 (68.8) | 43 (75.4) | 270 (83.6) | |
| poor | 2 (6.2) | 2 (3.5) | 16 (5.0) | |
| undifferentiated | 2 (7.1) | 1 (2.1) | 14 (7.1) | |
| T stage, N (%) | <0.001 | |||
| 1 | 0 (0.0) | 1 (1.8) | 6 (1.9) | |
| 2 | 0 (0.0) | 1 (1.8) | 13 (4.0) | |
| 3 | 9 (28.1) | 35 (61.4) | 241 (74.6) | |
| 4 | 23 (71.9) | 20 (35.1) | 63 (19.5) | |
| N stage, N (%) | 0.006 | |||
| 0 | 9 (28.1) | 20 (35.1) | 166 (51.4) | |
| 1 | 12 (37.5) | 20 (35.1) | 111 (34.4) | |
| 2 | 11 (34.4) | 17 (29.8) | 45 (13.9) | |
| 3 | 0 (0.0) | 0 (0.0) | 1 (0.3) |
| Variable | HR | 95% CI | p-Value |
|---|---|---|---|
| Type (no recurrence, reference) | |||
| Other recurrence | 3.72 | (2.30, 6.01) | <0.001 |
| PC | 4.79 | (2.61, 8.76) | <0.001 |
| Age at Diagnosis | 1.06 | (1.04, 1.08) | <0.001 |
| Polyposis (None, ref) | |||
| Yes | 0.59 | (0.17, 2.05) | 0.4 |
| Side (Left, ref) | |||
| Right | 1.19 | (0.76, 1.87) | 0.44 |
| Neoadjuvant therapy (No, ref) | |||
| Yes | 1.5 | (0.57, 4.01) | 0.41 |
| Microscopic Tumor Perforation (None, ref) | |||
| Yes | 1.75 | (0.73,4.18) | 0.21 |
| Mucin (None, ref) | |||
| Yes | 1.18 | (0.78, 1.80) | 0.43 |
| Gross Perforation (None, ref) | |||
| Yes | 1.34 | (0.73, 2.45) | 0.34 |
| Grade (Moderate, ref) | |||
| Poor | 1.32 | (0.71, 2.42) | 0.38 |
| Undifferentiated | 1.4 | (0.69, 2.82) | 0.35 |
| Well | 1.69 | (0.55, 5.16) | 0.36 |
| Margins (None, ref) | |||
| Yes | 1.67 | (0.88, 3.20) | 0.12 |
| Lymphovascular invasion (None, ref) | |||
| Yes | 1.46 | (0.92, 2.31) | 0.1 |
| Perineural invasion (None, ref) | |||
| Yes | 0.71 | (0.40, 1.27) | 0.25 |
| Tumor Deposit (None, ref) | |||
| Yes | 1.38 | (0.84, 2.28) | 0.21 |
| Stage (0, ref) | |||
| 1 | 1.35 | (0.84, 2.16) | 0.21 |
| 2 | 1.61 | (0.91, 2.86) | 0.1 |
| 3 | 0 | (0.00, Inf) | 1 |
| Microsatellite instability (No, ref) | |||
| Yes | 0.7 | (0.41, 1.18) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoskins, M.A.; Finkelstein, A.; Rashid, A.; Ziegler, O.; Mankarious, M.M.; Benavides, J.V.; Pameijer, C.R. Data-Driven Surveillance Protocol for Patients at Risk for Peritoneal Recurrence of Primary Colon Cancer: Surveillance for Peritoneal Carcinomatosis. J. Clin. Med. 2024, 13, 2358. https://doi.org/10.3390/jcm13082358
Hoskins MA, Finkelstein A, Rashid A, Ziegler O, Mankarious MM, Benavides JV, Pameijer CR. Data-Driven Surveillance Protocol for Patients at Risk for Peritoneal Recurrence of Primary Colon Cancer: Surveillance for Peritoneal Carcinomatosis. Journal of Clinical Medicine. 2024; 13(8):2358. https://doi.org/10.3390/jcm13082358
Chicago/Turabian StyleHoskins, Meloria A., Adam Finkelstein, Aisha Rashid, Olivia Ziegler, Marc M. Mankarious, Jorge V. Benavides, and Colette R. Pameijer. 2024. "Data-Driven Surveillance Protocol for Patients at Risk for Peritoneal Recurrence of Primary Colon Cancer: Surveillance for Peritoneal Carcinomatosis" Journal of Clinical Medicine 13, no. 8: 2358. https://doi.org/10.3390/jcm13082358
APA StyleHoskins, M. A., Finkelstein, A., Rashid, A., Ziegler, O., Mankarious, M. M., Benavides, J. V., & Pameijer, C. R. (2024). Data-Driven Surveillance Protocol for Patients at Risk for Peritoneal Recurrence of Primary Colon Cancer: Surveillance for Peritoneal Carcinomatosis. Journal of Clinical Medicine, 13(8), 2358. https://doi.org/10.3390/jcm13082358






