An International, Multidisciplinary Consensus Set of Patient-Centered Outcome Measures for Substance-Related and Addictive Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Project Team
2.2. Working Group
2.3. Procedure
2.3.1. Modified Delphi Process
2.3.2. Open Review
3. Results
3.1. Recommended Outcomes and Measures
3.1.1. Population
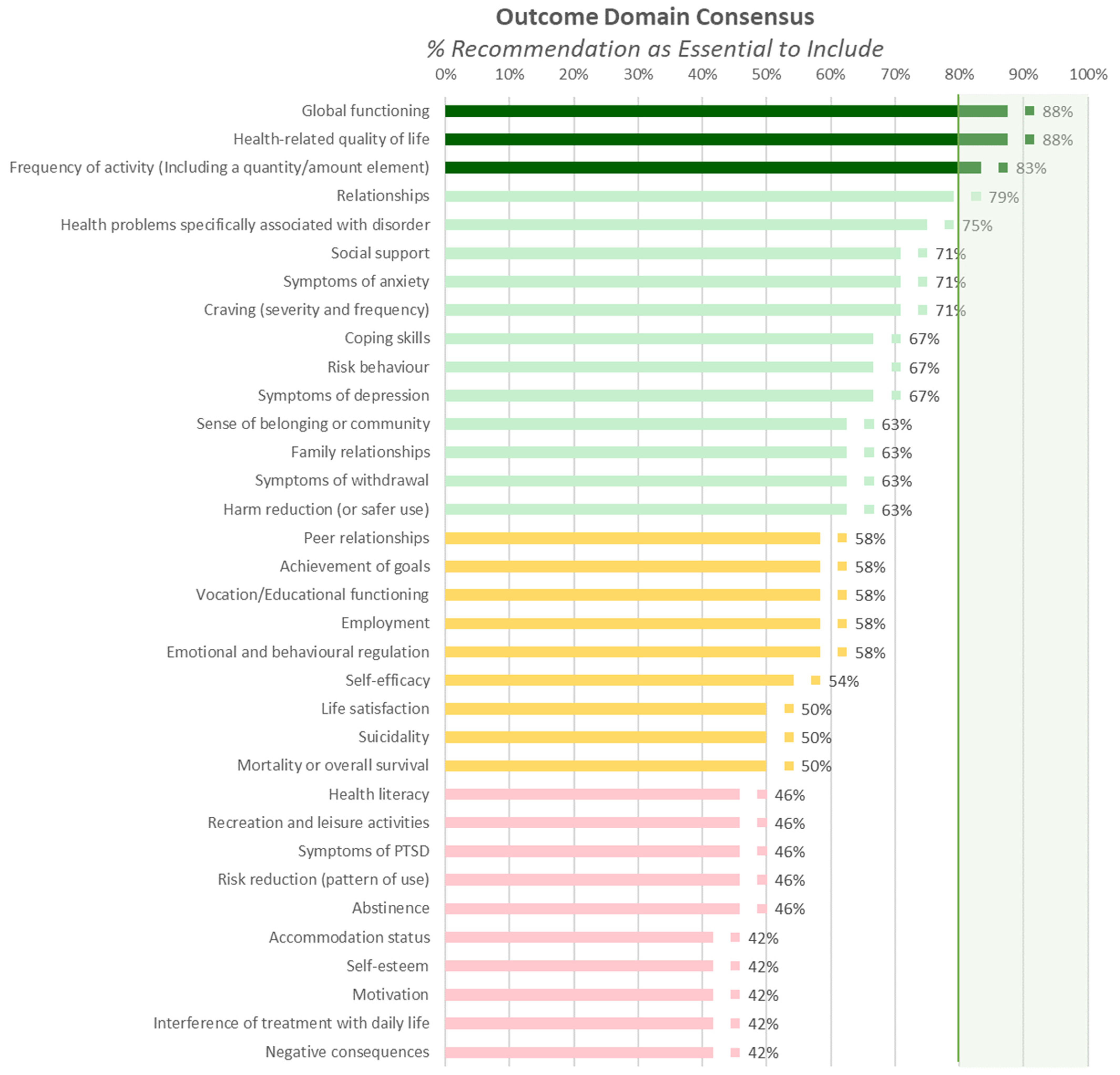
3.1.2. Outcome Domains
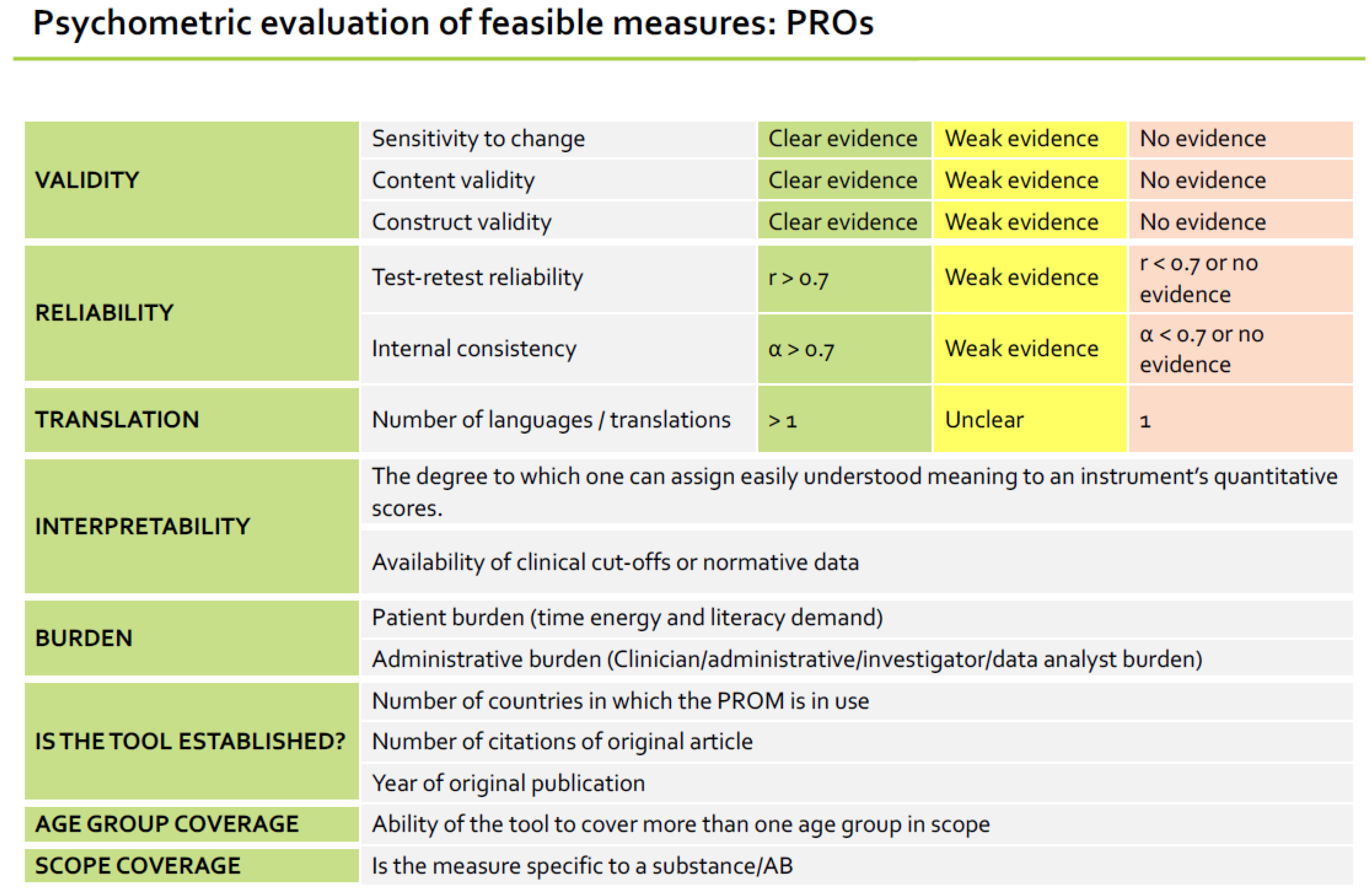
3.1.3. Outcome Measures
| Outcomes | Validity 3 | Reliability 4 | ||||||
|---|---|---|---|---|---|---|---|---|
| Areas 1 * | Measure | Primary Domain 2 | Content | Construct | Change * | Countries with Validation Studies * | Test–Retest | Internal * |
| All Disorders * | Treatment Outcome Profile (TOP), with recommended additions | FQ | Australia, Chile, China, UK | |||||
| Alcohol | PROMIS Alcohol Use 7a | SB | USA | |||||
| Drugs | PROMIS Severity of Substance Use past 30 days 7a | SB | USA | |||||
| Smoking | PROMIS Nicotine Dependence for All Smokers 8a | SB | USA | |||||
| Smoking–1-item | Heaviness of Smoking Index | SB | Australia, Brazil, Canada, France, Germany, Malaysia, Spain, Switzerland, Taiwan, UK, USA | |||||
| Gambling | Problem Gambling Severity Index (PGSI) | SB | Australia, Canada, Italy, South Korea, Spain, Sweden, Taiwan, USA | |||||
| Gaming | Internet Gaming Disorder Test (IGDT-10) | SB | Australia, Belgium, Canada, Czech Republic, Finland, France, Hungary, Iran, Italy, Norway, Peru, Slovakia, Slovenia, South Korea, Taiwan, Turkey, UK, USA | |||||
| GF:Adults and Adolescents | World Health Organisation Disability Assessment Schedule (WHODAS) | QoL | Canada, China, Poland, Rwanda, “International” [35] | |||||
| GF: Adolescents | KIDSCREEN-10 for services that exclusively treat adolescents | QoL | Austria, Belgium, Bulgaria, Czech Republic, France, Germany, Greece, Greenland, Hungary, Iran, Ireland, Japan, Luxembourg, Macedonia, The Netherlands, Poland, Portugal, Romania, Russia, Slovenia, Spain, Sweden, Switzerland, Turkey, UK | |||||
| Disorder specific * | Substance Use Recovery Evaluator (SURE) | QoL | UK | |||||
| Physical Health * | 1 item from PROMIS Global Health: “In general, how would you rate your physical health?” | PH | Brazil, USA | |||||
| Mental Health * | 2 items from PROMIS Global Health: “In general, how would you rate your mental health, including your mood and your ability to think?”; “How often have you been bothered by emotional problems such as feeling anxious, depressed or irritable?” | MH | Brazil, USA | |||||
 | ||||||||
3.2. Recommended Case-Mix Factors
3.3. Recommended Measurement Time-Points
4. Discussion
4.1. Strengths of the Set
4.2. Limitations and Areas of Future Research
4.3. Implementation
5. Conclusions and Call to Action
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gakidou, E.; Afshin, A.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1345–1422. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. Substance use disorders: A comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention. World Psychiatry 2023, 22, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, L.; Charlson, F.; Ferrari, A.; Santomauro, D.; Erskine, H.; Mantilla-Herrara, A.; Whiteford, H.; Leung, J.; Naghavi, M.; Griswold, M.; et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Alcohol. Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 12 December 2023).
- UNODC. World Drug Report 2023; UNODC: Vienna, Austria, 2023. [Google Scholar]
- World Health Organization. International Standards for the Treatment of Drug Use Disorders: Revised Edition Incorporating Results of Field-Testing; UNODC: Vienna, Austria, 2020. [Google Scholar]
- Calado, F.; Griffiths, M.D. Problem gambling worldwide: An update and systematic review of empirical research (2000–2015). J. Behav. Addict. 2016, 5, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.W.; Dorstyn, D.; Delfabbro, P.H.; King, D.L. Global prevalence of gaming disorder: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2021, 55, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.J.; Sellman, J.D.; Frampton, C.M. Patient predictors of alcohol treatment outcome: A systematic review. J. Subst. Abus. Treat. 2009, 36, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Drake, R.E.; O’Neal, E.L.; Wallach, M.A. A systematic review of psychosocial research on psychosocial interventions for people with co-occurring severe mental and substance use disorders. J. Subst. Abus. Treat. 2008, 34, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Merkouris, S.S.; Thomas, S.A.; Browning, C.J.; Dowling, N.A. Predictors of outcomes of psychological treatments for disordered gambling: A systematic review. Clin. Psychol. Rev. 2016, 48, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Johnston, M.; Michie, S.; Hartmann-Boyce, J.; West, R.; Viechtbauer, W.; Eisma, M.C.; Scott, C.; de Bruin, M. Behaviour change techniques associated with smoking cessation in intervention and comparator groups of randomized controlled trials: A systematic review and meta-regression. Addiction 2020, 115, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Boswell, J.F.; Kraus, D.R.; Miller, S.D.; Lambert, M.J. Implementing routine outcome monitoring in clinical practice: Benefits, challenges, and solutions. Psychother. Res. 2015, 25, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Wiessing, L.; Ferri, M.; Darke, S.; Simon, R.; Griffiths, P. Large variation in measures used to assess outcomes of opioid dependence treatment: A systematic review of longitudinal observational studies. Drug Alcohol. Rev. 2018, 37 (Suppl. 1), S323–S338. [Google Scholar] [CrossRef] [PubMed]
- Shorter, G.W.; Bray, J.W.; Giles, E.L.; O’Donnell, A.J.; Berman, A.H.; Holloway, A.; Heather, N.; Barbosa, C.; Stockdale, K.J.; Scott, S.J.; et al. The Variability of Outcomes Used in Efficacy and Effectiveness Trials of Alcohol Brief Interventions: A Systematic Review. J. Stud. Alcohol. Drugs 2019, 80, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Shorter, G.W.; Bray, J.W.; Heather, N.; Berman, A.H.; Giles, E.L.; Clarke, M.; Barbosa, C.; O’Donnell, A.J.; Holloway, A.; Riper, H.; et al. The “Outcome Reporting in Brief Intervention Trials: Alcohol”(ORBITAL) core outcome set: International consensus on outcomes to measure in efficacy and effectiveness trials of alcohol brief interventions. J. Stud. Alcohol Drugs 2021, 82, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Shorter, G.W.; Heather, N.; Bray, J.W.; Berman, A.H.; Giles, E.L.; O’Donnell, A.; Barbosa, C.; Clarke, M.; Holloway, A.; Birch, D.N. Prioritization of Outcomes in Efficacy and Effectiveness of Alcohol Brief Intervention Trials: International Multi-Stakeholder e-Delphi Consensus Study to Inform a Core Outcome Set. J. Stud. Alcohol Drugs 2019, 80, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Toneatto, T.; Potenza, M.N.; Petry, N.; Ladouceur, R.; Hodgins, D.C.; el-Guebaly, N.; Echeburua, E.; Blaszczynski, A. A framework for reporting outcomes in problem gambling treatment research: The Banff, Alberta Consensus. Addiction 2006, 101, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Hall, W.; Wodak, A.; Heather, N.; Ward, J. Development and validation of a multi-dimensional instrument for assessing outcome of treatment among opiate users: The Opiate Treatment Index. Br. J. Addict. 1992, 87, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Marsden, J.; Farrell, M.; Bradbury, C.; Dale-Perera, A.; Eastwood, B.; Roxburgh, M.; Taylor, S. Development of the Treatment Outcomes Profile. Addiction 2008, 103, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Lintzeris, N.; Mammen, K.; Holmes, J.; Deacon, R.; Mills, L.; Black, E.; Gardner, L.; Dunlop, A. Australian Treatment Outcomes Profile (ATOP) Manual 1: Using the ATOP with Individual Clients; AoD Treatment Clinical Outcomes and Quality Indicators Program: Sydney, Australia, 2020. [Google Scholar]
- Migchels, C.; Zerrouk, A.; Crunelle, C.L.; Matthys, F.; Gremeaux, L.; Fernandez, K.; Antoine, J.; van den Brink, W.; Vanderplasschen, W. Patient Reported Outcome and Experience Measures (PROMs and PREMs) in substance use disorder treatment services: A scoping review. Drug Alcohol. Depend. 2023, 253, 111017. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Drug Abuse (US). Common Data Elements [Internet]. Available online: http://cde.drugabuse.gov/ (accessed on 15 December 2023).
- Hamilton, C.M.; Strader, L.C.; Pratt, J.G.; Maiese, D.; Hendershot, T.; Kwok, R.K.; Hammond, J.A.; Huggins, W.; Jackman, D.; Pan, H.; et al. The PhenX Toolkit: Get the most from your measures. Am. J. Epidemiol. 2011, 174, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Ghitza, U.E.; Gore-Langton, R.E.; Lindblad, R.; Shide, D.; Subramaniam, G.; Tai, B. Common data elements for substance use disorders in electronic health records: The NIDA Clinical Trials Network experience. Addiction 2013, 108, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ghitza, U.E.; Gore-Langton, R.E.; Lindblad, R.; Tai, B. NIDA Clinical Trials Network Common Data Elements Initiative: Advancing Big-Data Addictive-Disorders Research. Front. Psychiatry 2015, 6, 125732. [Google Scholar] [CrossRef] [PubMed]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.M.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET Handbook: Version 1.0. Trials 2017, 18, 280. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Sandford, B.A. The Delphi technique: Making sense of consensus. Pract. Assess. Res. Eval. 2019, 12, 10. [Google Scholar]
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Reeve, B.B.; Wyrwich, K.W.; Wu, A.W.; Velikova, G.; Terwee, C.B.; Snyder, C.F.; Schwartz, C.; Revicki, D.A.; Moinpour, C.M.; McLeod, L.D.; et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual. Life Res. 2013, 22, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
- Pilkonis, P.A.; Yu, L.; Colditz, J.; Dodds, N.; Johnston, K.L.; Maihoefer, C.; Stover, A.M.; Daley, D.C.; McCarty, D. Item banks for alcohol use from the Patient-Reported Outcomes Measurement Information System (PROMIS): Use, consequences, and expectancies. Drug Alcohol. Depend. 2013, 130, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Pilkonis, P.A.; Yu, L.; Dodds, N.E.; Johnston, K.L.; Lawrence, S.M.; Hilton, T.F.; Daley, D.C.; Patkar, A.A.; McCarty, D. Item banks for substance use from the Patient-Reported Outcomes Measurement Information System (PROMIS®): Severity of use and positive appeal of use. Drug Alcohol. Depend. 2015, 156, 184–192. [Google Scholar] [CrossRef]
- Edelen, M.O.; Stucky, B.D.; Hansen, M.; Tucker, J.S.; Shadel, W.G.; Cai, L. The PROMIS Smoking Initiative: Initial validity evidence for six new smoking item banks. Nicotine Tob. Res. 2014, 16 (Suppl. 3), S250–S260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferris, J.A.; Wynne, H.J. The Canadian Problem Gambling Index; Canadian Centre on Substance Abuse: Ottawa, ON, Canada, 2001. [Google Scholar]
- Király, O.; Sleczka, P.; Pontes, H.M.; Urbán, R.; Griffiths, M.D.; Demetrovics, Z. Validation of the Ten-Item Internet Gaming Disorder Test (IGDT-10) and evaluation of the nine DSM-5 Internet Gaming Disorder criteria. Addict. Behav. 2017, 64, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Heatherton, T.F.; Kozlowski, L.T.; Frecker, R.C.; Rickert, W.; Robinson, J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br. J. Addict. 1989, 84, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Neale, J.; Vitoratou, S.; Finch, E.; Lennon, P.; Mitcheson, L.; Panebianco, D.; Rose, D.; Strang, J.; Wykes, T.; Marsden, J. Development and validation of ‘sure’: A patient reported outcome measure (prom) for recovery from drug and alcohol dependence. Drug Alcohol. Depend. 2016, 165, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ustün, T.B.; Chatterji, S.; Kostanjsek, N.; Rehm, J.; Kennedy, C.; Epping-Jordan, J.; Saxena, S.; von Korff, M.; Pull, C. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull. World Health Organ. 2010, 88, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Ravens-Sieberer, U.; Erhart, M.; Rajmil, L.; Herdman, M.; Auquier, P.; Bruil, J.; Power, M.; Duer, W.; Abel, T.; Czemy, L.; et al. Reliability, construct and criterion validity of the KIDSCREEN-10 score: A short measure for children and adolescents’ well-being and health-related quality of life. Qual. Life Res. 2010, 19, 1487–1500. [Google Scholar] [CrossRef] [PubMed]
- Migchels, C.; Zerrouk, A.; Matthys, F.; van den Brink, W.; Fernandez, K.; Antoine, J.; Vanderplasschen, W.; Crunelle, C.L. Outcome Measurement and Evaluation as a Routine practice in alcohol and other drug services in Belgium (OMER-BE). Eur. Psychiatry 2023, 66, S141–S142. [Google Scholar] [CrossRef]
- Boness, C.L.; Carlos Gonzalez, J.; Sleep, C.; Venner, K.L.; Witkiewitz, K. Evidence-Based Assessment of Substance Use Disorder. Assessment 2024, 31, 168–190. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, I.N.; Cavka, B.; Lippa, J.; Bucknill, A. The feasibility of implementing the ICHOM Standard Set for Hip and Knee Osteoarthritis: A mixed-methods evaluation in public and private hospital settings. J. Patient Rep. Outcomes 2017, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; Ropp, C.; Sousa, S.; Woo, W.; Vedelago, H.; Rush, B. The development and implementation of an outcome monitoring system for addiction treatment. Can. J. Addict. 2016, 7, 15–24. [Google Scholar] [CrossRef]
- Lu, S.; Costello, J.; Rush, B.; Taha, S.; MacKillop, J.; Corace, K. Building the Foundation for a Standardized Approach to Progress and Outcome Monitoring across Substance Use and Addiction Services; Homewood Research Institute: Guelph, ON, Canada, 2022. [Google Scholar]
- Damschroder, L.J.; Reardon, C.M.; Widerquist, M.A.O.; Lowery, J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement. Sci. 2022, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Mauluddin, A.; Rush, B.R.; Corace, K.; MacKillop, J. Measurement-based care for substance use disorders. Spec. Issue Psynopsis Subst. Use Health Addict. 2023, 3, 20–23. [Google Scholar]
| Alcohol | Drugs | Tobacco | Gambling | Gaming | |
|---|---|---|---|---|---|
| Frequency and quantity | Treatment Outcome Profile (TOP), with additions | ||||
| Symptom burden | PROMIS Alcohol Use 7a | PROMIS Severity of Substance Use past 30 days 7a | PROMIS Nicotine Dependence for All Smokers 8a and Heaviness of Smoking Index | Problem Gambling Severity Index (PGSI) | Internet Gaming Disorder Test (IGDT-10) |
| Health related quality of life | Generic: World Health Organisation Disability Assessment Schedule (WHODAS) for services treating adults and services that follow adolescents into adulthood; KIDSCREEN-10 for services that exclusively treat adolescents Condition specific: Also administer the Substance Use Recovery Evaluator (SURE) if problem relates to alcohol or drugs | ||||
| Global functioning | |||||
| Psychosocial functioning | |||||
| Overall physical health and wellbeing | Single item from PROMIS Global Health: “In general, how would you rate your physical health?” | ||||
| Overall mental health and wellbeing | Two items from PROMIS Global Health: “In general, how would you rate your mental health, including your mood and your ability to think?”; “How often have you been bothered by emotional problems such as feeling anxious, depressed or irritable?” | ||||
| Sub-Criterion | SF-36 | EQ5D | WHO QOL | WHO DAS-12 | PROMIS Profile | PROMIS GH | |
|---|---|---|---|---|---|---|---|
| Validitiy | Substance use and/or addictive behaviours | Drugs, Alcohol | Drugs, Alcohol | Drugs, Alcohol | Drugs, Alcohol | Some items | No |
| Sensitivity to change | |||||||
| Content validity | |||||||
| Construct validity | |||||||
| Reliability | Test–retest | ||||||
| Internal consistency | |||||||
| Translation | Number available | >170 | >170 | >9 | >47 | >2 | >2 |
| Interpretability | Response scale | Mix | 3 or 5 | 5 | 4 | 5 | 5 |
| Recall | 7 or 30 days | Today | 14 days | 30 days | 7 days | General/7 days | |
| Burden | Items | 20–36 | 6 | 26 | 12 | 29–57 | 10 |
| Tool established? | Citations | 3901 | 2939 | 3333 | 618 | 125 | 125 |
| Year published | 1992 | 1990 | 1998 | 2010 | 2005 | 2004 | |
| Reporters | Self/Interview | Self | Self | Self | Self | Self | Self |
| Age Coverage | Validated 13+ | Youth version (12+) | Older adult version, validated to 11+ | Older adult version, validated to 11+ | Older adult version, validated to 11+ | Older adult version, validated to 11+ | |
 | |||||||
| Category | Variable | Definition | Source |
|---|---|---|---|
| Demographic | Age | Year of birth | Self-report |
| Sex | “What sex were you assigned at birth?” [ ] Male [ ] Female | Self-report | |
| Gender identity | “Do you identify yourself as…?” [ ] Boy/Man [ ] Girl/Woman [ ] Non-binary [ ] Trans man/Transgender man/FTM [ ] Trans woman/Transgender woman/MTF [ ] None of these describe me, and I’d like to specify_________ [ ] Prefer not to answer | Self-report | |
| Sexual orientation | “Do you identify yourself as…?” [ ] Straight or heterosexual [ ] Gay or lesbian or homosexual [ ] Bisexual [ ] None of these describe me, and I’d like to specify_________ [ ] I don’t know right now [ ] Prefer not to answer | Self-report | |
| Socioeconomic status | Adults: highest level of education completed. Adolescents: proxy to be used: highest level of education completed by parents. | Self-report | |
| Work status | [ ] “What is your work status?” [ ] Unable to work [ ] Not working by choice (retired, homemaker) [ ] Seeking employment (I consider myself able to work but cannot find a job) [ ] Part-time work, school, or vocational training [ ] Full-time work, school, or vocational training | Self-report | |
| Accommodation or homelessness status | SURE: “I have had stable housing” [Past week] Treatment Outcome Profile/Clinician report: “At risk of eviction” [Yes/No] “Acute housing problem” [Yes/No] | Self- and clinician-report | |
| Clinical | Genetic disposition | “Have either of your biological parents or siblings had an alcohol problem?” [Yes/No/Don’t know] “Have either of your biological parents or siblings used non-prescribed drugs?” [Yes/No/Don’t know] | Self-report |
| Environmental exposure | “Do you live with anyone who has a current alcohol problem?” [Yes/No] “Do you live with anyone who currently uses non-prescribed drugs?” [Yes/No] | Self-report | |
| Exposure to negative life events and their impact | Primary Care PTSD Screen (PC-PTSD-5) Sometimes things happen to people that are unusually or especially frightening, horrible, or traumatic. For example, a serious accident or fire; a physical or sexual assault or abuse; an earthquake or flood; a war; seeing someone be killed or seriously injured; having a loved one die through homicide or suicide. 1. Have you ever experienced this kind of event? [Yes/No] If no, screen total = 0. Please stop here. If yes, please answer the questions below. In the past month, have you… 2. had nightmares about the event(s) or thought about the event(s) when you did not want to? [Yes/No] 3. tried hard not to think about the event(s) or went out of your way to avoid situations that reminded you of the event(s)? [Yes/No] 4. been constantly on guard, watchful, or easily startled? [Yes/No] 5. felt numb or detached from people, activities, or your surroundings? [Yes/No] 6. felt guilty or unable to stop blaming yourself or others for the event(s) or any problems the event(s) may have caused? [Yes/No] | Self-report | |
| Intervention | Intervention setting | “Please indicate in which setting an intervention has taken place. Please check all that apply” [ ] Residential or inpatient treatment [ ] Non-residential or outpatient treatment [ ] Day treatment [ ] Digital [ ] Other | Clinician-report |
| Intervention type | “Please indicate the type of intervention. Please check all that apply” [ ] Medication for substance use (including agonist treatment) [ ] Counselling or psychotherapy [ ] Other | Clinician-report |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, N.; Chung, S.; Tisdale, C.; Fialho, L.S.; Aramrattana, A.; Assanangkornchai, S.; Blaszczynski, A.; Bowden-Jones, H.; van den Brink, W.; Brown, A.; et al. An International, Multidisciplinary Consensus Set of Patient-Centered Outcome Measures for Substance-Related and Addictive Disorders. J. Clin. Med. 2024, 13, 2154. https://doi.org/10.3390/jcm13072154
Black N, Chung S, Tisdale C, Fialho LS, Aramrattana A, Assanangkornchai S, Blaszczynski A, Bowden-Jones H, van den Brink W, Brown A, et al. An International, Multidisciplinary Consensus Set of Patient-Centered Outcome Measures for Substance-Related and Addictive Disorders. Journal of Clinical Medicine. 2024; 13(7):2154. https://doi.org/10.3390/jcm13072154
Chicago/Turabian StyleBlack, Nicola, Sophie Chung, Calvert Tisdale, Luz Sousa Fialho, Apinun Aramrattana, Sawitri Assanangkornchai, Alex Blaszczynski, Henrietta Bowden-Jones, Wim van den Brink, Adrian Brown, and et al. 2024. "An International, Multidisciplinary Consensus Set of Patient-Centered Outcome Measures for Substance-Related and Addictive Disorders" Journal of Clinical Medicine 13, no. 7: 2154. https://doi.org/10.3390/jcm13072154
APA StyleBlack, N., Chung, S., Tisdale, C., Fialho, L. S., Aramrattana, A., Assanangkornchai, S., Blaszczynski, A., Bowden-Jones, H., van den Brink, W., Brown, A., Brown, Q. L., Cottler, L. B., Elsasser, M., Ferri, M., Florence, M., Gueorguieva, R., Hampton, R., Hudson, S., Kelly, P. J., ... Farrell, M. (2024). An International, Multidisciplinary Consensus Set of Patient-Centered Outcome Measures for Substance-Related and Addictive Disorders. Journal of Clinical Medicine, 13(7), 2154. https://doi.org/10.3390/jcm13072154










