Impaired Hand Grip Strength Correlates with Greater Disability and Symptom Severity in Post-COVID Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Hand Grip Strength Measurement
2.3. Questionnaires for Symptom Scoring
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison between Patient Groups
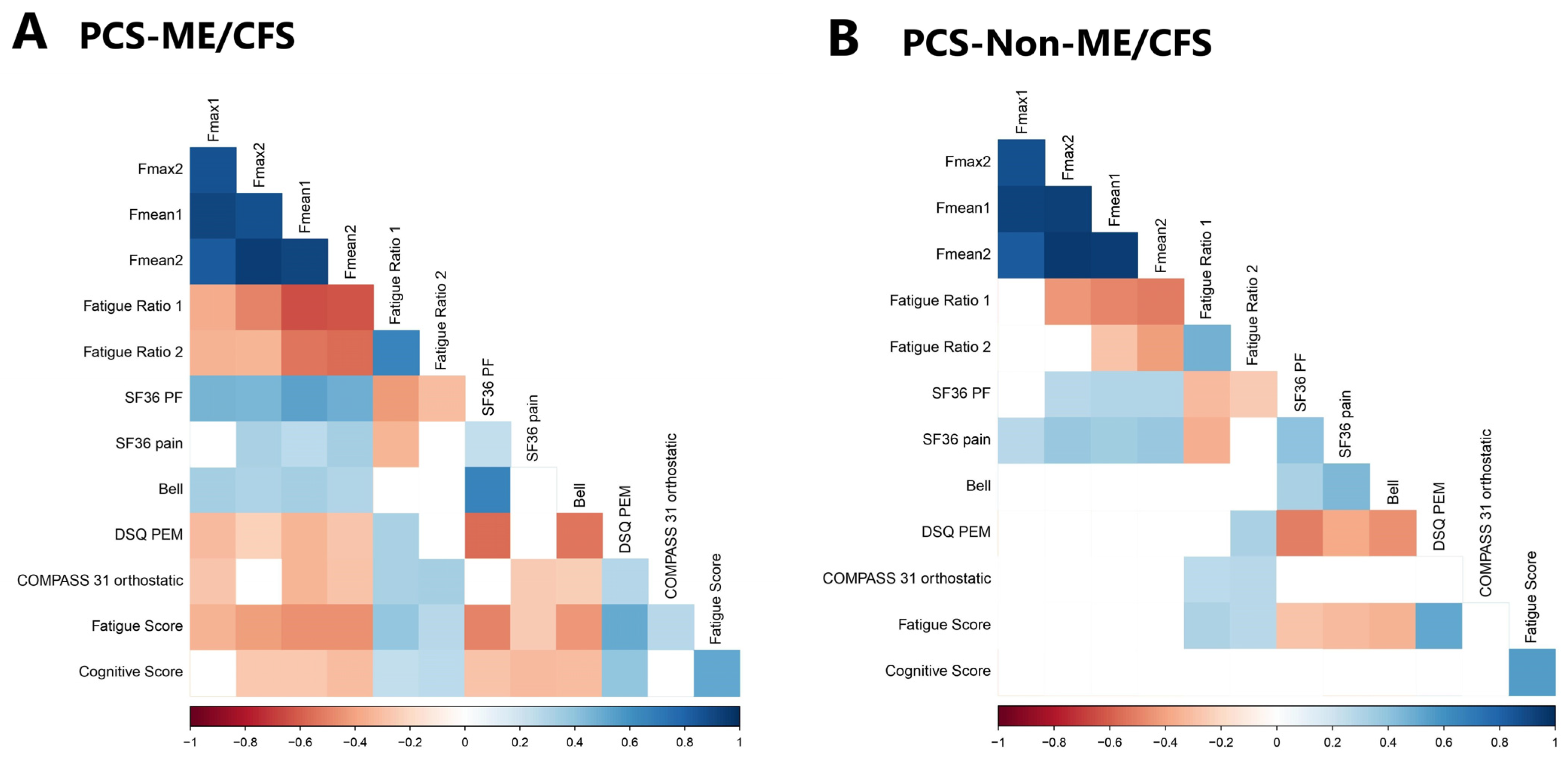
3.3. Hand Grip Strength and Correlation with Disability and Symptom Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Huerne, K.; Filion, K.B.; Grad, R.; Ernst, P.; Gershon, A.S.; Eisenberg, M.J. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open 2023, 9, 100033. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Retornaz, F.; Rebaudet, S.; Stavris, C.; Jammes, Y. Long-term neuromuscular consequences of SARS-CoV-2 and their similarities with myalgic encephalomyelitis/chronic fatigue syndrome: Results of the retrospective CoLGEM study. J. Transl. Med. 2022, 20, 429. [Google Scholar] [CrossRef]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic fatigue syndrome: An emerging sequela in COVID-19 survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef]
- Legler, F.; Meyer-Arndt, L.; Modl, L.; Kedor, C.; Freitag, H.; Stein, E.; Hoppmann, U.; Rust, R.; Wittke, K.; Siebert, N.; et al. Long-term symptom severity and clinical biomarkers in post-COVID-19/chronic fatigue syndrome: Results from a prospective observational cohort. EClinicalMedicine 2023, 63, 102146. [Google Scholar] [CrossRef] [PubMed]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. Mayo Clin. Proc. 2021, 96, 2861–2878. [Google Scholar] [CrossRef]
- Sotzny, F.; Filgueiras, I.S.; Kedor, C.; Freitag, H.; Wittke, K.; Bauer, S.; Sepulveda, N.; Mathias da Fonseca, D.L.; Baiocchi, G.C.; Marques, A.H.C.; et al. Dysregulated autoantibodies targeting vaso- and immunoregulatory receptors in Post COVID Syndrome correlate with symptom severity. Front. Immunol. 2022, 13, 981532. [Google Scholar] [CrossRef] [PubMed]
- Medicine, I.O. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Press: Washington, DC, USA, 2015; p. 304. [Google Scholar]
- Jakel, B.; Kedor, C.; Grabowski, P.; Wittke, K.; Thiel, S.; Scherbakov, N.; Doehner, W.; Scheibenbogen, C.; Freitag, H. Hand grip strength and fatigability: Correlation with clinical parameters and diagnostic suitability in ME/CFS. J. Transl. Med. 2021, 19, 159. [Google Scholar] [CrossRef]
- Nacul, L.C.; Mudie, K.; Kingdon, C.C.; Clark, T.G.; Lacerda, E.M. Hand Grip Strength as a Clinical Biomarker for ME/CFS and Disease Severity. Front. Neurol. 2018, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, G.; Manning, P.; Newton, J.L. Understanding Muscle Dysfunction in Chronic Fatigue Syndrome. J. Aging Res. 2016, 2016, 2497348. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S. The Doctro’s Guide To Chronic Fatigue Syndrome; Da Capo Press Inc.: Cambridge, MA, USA, 1995. [Google Scholar]
- Cella, M.; Chalder, T. Measuring fatigue in clinical and community settings. J. Psychosom. Res. 2010, 69, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fluge, O.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Kristoffersen, E.K.; Sorland, K.; Bruland, O.; Dahl, O.; et al. B-Lymphocyte Depletion in Myalgic Encephalopathy/Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef] [PubMed]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.; Rowe, P.C.; Visser, F.C. Worsening Symptoms Is Associated with Larger Cerebral Blood Flow Abnormalities during Tilt-Testing in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Medicina 2023, 59, 2153. [Google Scholar] [CrossRef] [PubMed]
- Aschman, T.; Wyler, E.; Baum, O.; Hentschel, A.; Rust, R.; Legler, F.; Preusse, C.; Meyer-Arndt, L.; Buttnerova, I.; Forster, A.; et al. Post-COVID exercise intolerance is associated with capillary alterations and immune dysregulations in skeletal muscles. Acta Neuropathol. Commun. 2023, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Petter, E.; Scheibenbogen, C.; Linz, P.; Stehning, C.; Wirth, K.; Kuehne, T.; Kelm, M. Muscle sodium content in patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Transl. Med. 2022, 20, 580. [Google Scholar] [CrossRef] [PubMed]
- Bizjak, D.A.; Ohmayer, B.; Buhl, J.L.; Schneider, E.M.; Walther, P.; Calzia, E.; Jerg, A.; Matits, L.; Steinacker, J.M. Functional and Morphological Differences of Muscle Mitochondria in Chronic Fatigue Syndrome and Post-COVID Syndrome. Int. J. Mol. Sci. 2024, 25, 1675. [Google Scholar] [CrossRef] [PubMed]
- Flaskamp, L.; Roubal, C.; Uddin, S.; Sotzny, F.; Kedor, C.; Bauer, S.; Scheibenbogen, C.; Seifert, M. Serum of Post-COVID-19 Syndrome Patients with or without ME/CFS Differentially Affects Endothelial Cell Function In Vitro. Cells 2022, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Sato, W.; Kimura, Y.; Matsuda, H.; Ota, M.; Maikusa, N.; Suzuki, F.; Amano, K.; Shin, I.; Yamamura, T.; et al. Altered Structural Brain Networks Related to Adrenergic/Muscarinic Receptor Autoantibodies in Chronic Fatigue Syndrome. J. Neuroimaging 2020, 30, 822–827. [Google Scholar] [CrossRef]
- Gravelsina, S.; Vilmane, A.; Svirskis, S.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z.; Vecvagare, K.; Krumina, A.; Leineman, I.; Shoenfeld, Y.; Murovska, M. Biomarkers in the diagnostic algorithm of myalgic encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 2022, 13, 928945. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sato, W.; Maikusa, N.; Ota, M.; Shigemoto, Y.; Chiba, E.; Arizono, E.; Maki, H.; Shin, I.; Amano, K.; et al. Free-water-corrected diffusion and adrenergic/muscarinic antibodies in myalgic encephalomyelitis/chronic fatigue syndrome. J. Neuroimaging 2023, 33, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, V.D.P.; Idavalapati, A.; Tamalapakula, S.S.; Moparthi, V.; Potru, M.; Owolabi, O.J. Depression and Hand-Grip: Unraveling the Association. Cureus 2023, 15, e38632. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Singh, K.; LaMunion, S.R.; Hallett, M.; Jacobson, S.; Chen, K.; Enose-Akahata, Y.; Apps, R.; Barb, J.J.; Bedard, P.; et al. Deep phenotyping of post-infectious myalgic encephalomyelitis/chronic fatigue syndrome. Nat. Commun. 2024, 15, 907. [Google Scholar] [CrossRef] [PubMed]

| PCS-ME/CFS (n = 78, Median with Range) | PCS-Non-ME/CFS (n = 66, Median with Range) | ME/CFS vs. Non-ME/CFS | |
|---|---|---|---|
| Age | 40 (20–61) | 44 (18–67) | p = 0.585 |
| BMI | 23.71 (14.88–37.97) | 24.13 (17.65–41.64) | p = 0.675 |
| Time since COVID-19 (months) | 10 (6–25) | 10.5 (6–23) | p = 0.847 |
| Bell | 40 (20–90) | 50 (20–80) | p < 0.001 *** |
| CFQ | 28.5 (13–33) | 25 (0–33) | p < 0.001 *** |
| PEM-DSQ | 34 (12–46) | 28 (0–44) | p < 0.001 *** |
| SF-36 | |||
| Physical Functioning | 40 (0–95) | 60 (0–100) | p < 0.001 *** |
| Role Limitations | 0 (0–100) | 0 (0–100) | p = 0.107 |
| Energy | 15 (0–80) | 20 (0–60) | p < 0.001 *** |
| Pain | 32.5 (0–100) | 38.75 (0–100) | p = 0.064 |
| Symptom Severity | |||
| Muscle Pain | 6 (1–10) | 6 (1–10) | p = 0.324 |
| Headache | 7 (1–10) | 6 (1–10) | p = 0.115 |
| Joint Pain | 5 (1–10) | 6 (1–10) | p = 0.647 |
| Fatigue Score | 8 (1–10) | 7 (1–10) | p < 0.001 *** |
| Cognitive Score | 6.67 (1.33–10) | 6.5 (1–10) | p = 0.196 |
| Immune Score | 3.33 (1–8.67) | 2.33 (1–9) | p = 0.006 ** |
| COMPASS 31 | 40.80(0–76.23) | 30.64 (0–62.22) | p = 0.002 ** |
| Orthostatic Intolerance | 22 (0–40) | 16 (0–36) | p = 0.006 ** |
| Vasomotor | 0 (0–5) | 0 (0–3.33) | p = 0.003 ** |
| Secretomotor | 4.29 (0–12.86) | 4.29 (0–12.86) | p = 0.485 |
| Gastrointestinal | 6.25 (0–15.18) | 6.25 (0–17.86) | p = 0.187 |
| Bladder | 1.12 (0–7.78) | 1.11 (0–7.78) | p = 0.309 |
| Pupillomotor | 1.67 (0–4) | 1.67 (1–5) | p = 0.634 |
| Hand Grip Strength (in kg) | |||
| Fmax 1 | 20.1 (5.7–43.6) | 19.2 (4.7–33.8) | p = 0.884 |
| Fmax 2 | 18.25 (1.9–41.2) | 17.1 (3.10–34.6) | p = 0.477 |
| Fmean 1 | 16.6 (3.19–38.99) | 16.07 (4.14–31.85) | p = 0.938 |
| Fmean 2 | 15.07 (1.37–39.05) | 14.51 (2.32–30.88) | p = 0.990 |
| Fatigue Ratio 1 | 1.18 (1.01–3.23) | 1.17 (1.03–2.11) | p = 0.642 |
| Fatigue Ratio 2 | 1.2 (1.04–2.24) | 1.17 (1.03–2.0) | p = 0.035 * |
| Recovery Ratio | 0.95 (0.26–1.67) | 0.95 (0.47–1.24) | p = 0.674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paffrath, A.; Kim, L.; Kedor, C.; Stein, E.; Rust, R.; Freitag, H.; Hoppmann, U.; Hanitsch, L.G.; Bellmann-Strobl, J.; Wittke, K.; et al. Impaired Hand Grip Strength Correlates with Greater Disability and Symptom Severity in Post-COVID Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2024, 13, 2153. https://doi.org/10.3390/jcm13072153
Paffrath A, Kim L, Kedor C, Stein E, Rust R, Freitag H, Hoppmann U, Hanitsch LG, Bellmann-Strobl J, Wittke K, et al. Impaired Hand Grip Strength Correlates with Greater Disability and Symptom Severity in Post-COVID Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Journal of Clinical Medicine. 2024; 13(7):2153. https://doi.org/10.3390/jcm13072153
Chicago/Turabian StylePaffrath, Anna, Laura Kim, Claudia Kedor, Elisa Stein, Rebekka Rust, Helma Freitag, Uta Hoppmann, Leif G. Hanitsch, Judith Bellmann-Strobl, Kirsten Wittke, and et al. 2024. "Impaired Hand Grip Strength Correlates with Greater Disability and Symptom Severity in Post-COVID Myalgic Encephalomyelitis/Chronic Fatigue Syndrome" Journal of Clinical Medicine 13, no. 7: 2153. https://doi.org/10.3390/jcm13072153
APA StylePaffrath, A., Kim, L., Kedor, C., Stein, E., Rust, R., Freitag, H., Hoppmann, U., Hanitsch, L. G., Bellmann-Strobl, J., Wittke, K., Scheibenbogen, C., & Sotzny, F. (2024). Impaired Hand Grip Strength Correlates with Greater Disability and Symptom Severity in Post-COVID Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Journal of Clinical Medicine, 13(7), 2153. https://doi.org/10.3390/jcm13072153






