External Scaffold for Venous Graft to Treat Chronic Limb-Threatening Ischemia: Results of the FRAME Vascular Support
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Inclusion Criteria
- (1)
- vein diameter ≥4.5 and ≤8 mm
- (2)
- diffusely varicose vein/severe ectasia in an isolated segment (with reduced thickness)
- (3)
- higher risk of bypass extrinsic compression (e.g., extra-anatomical course).
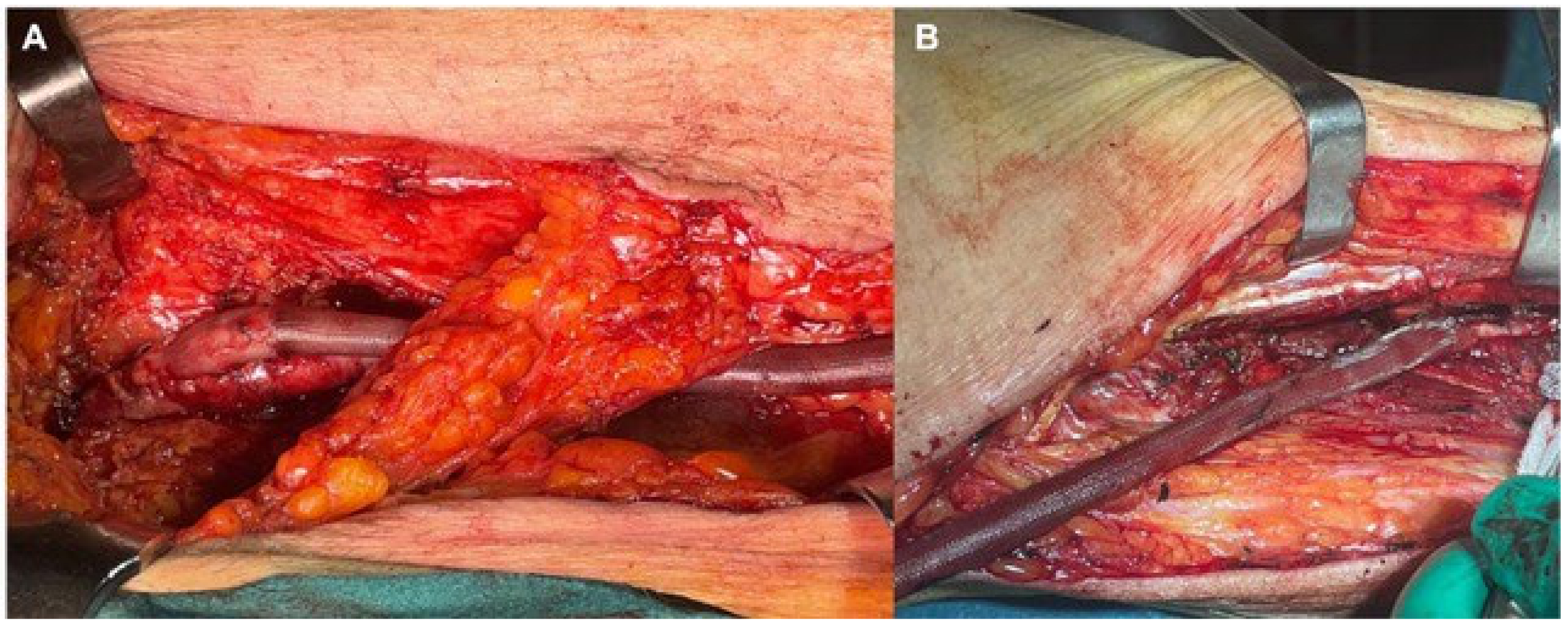
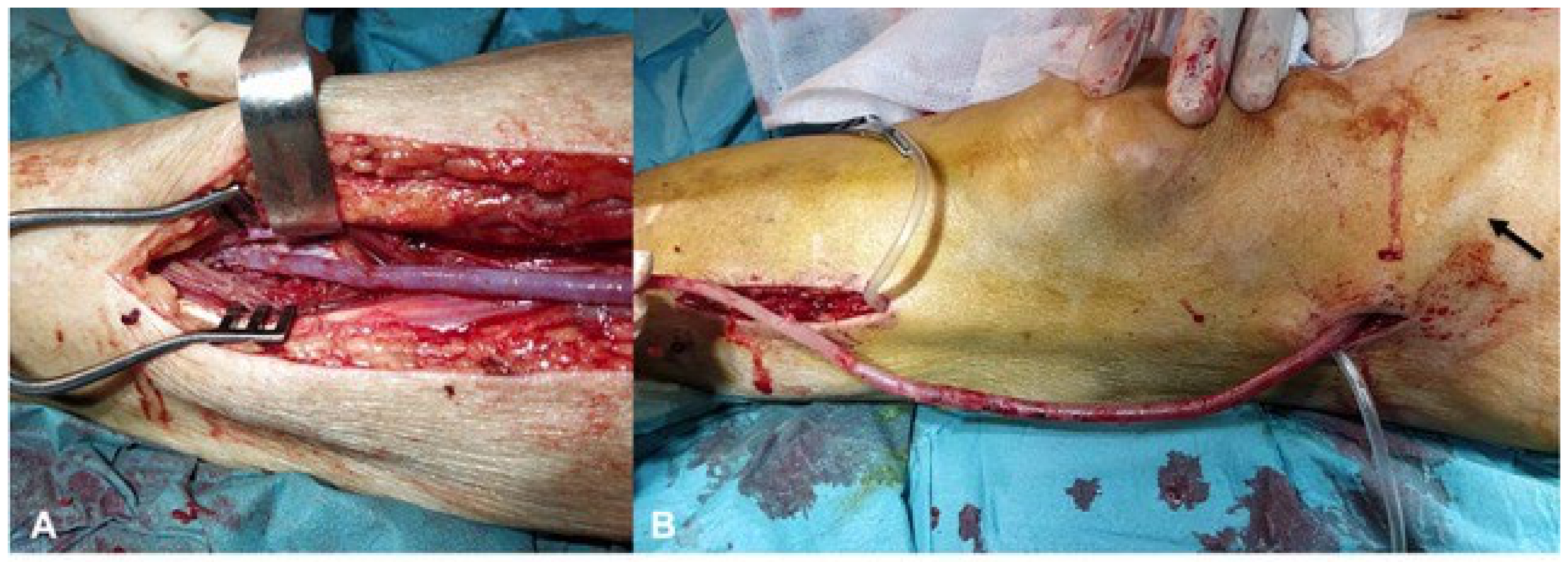
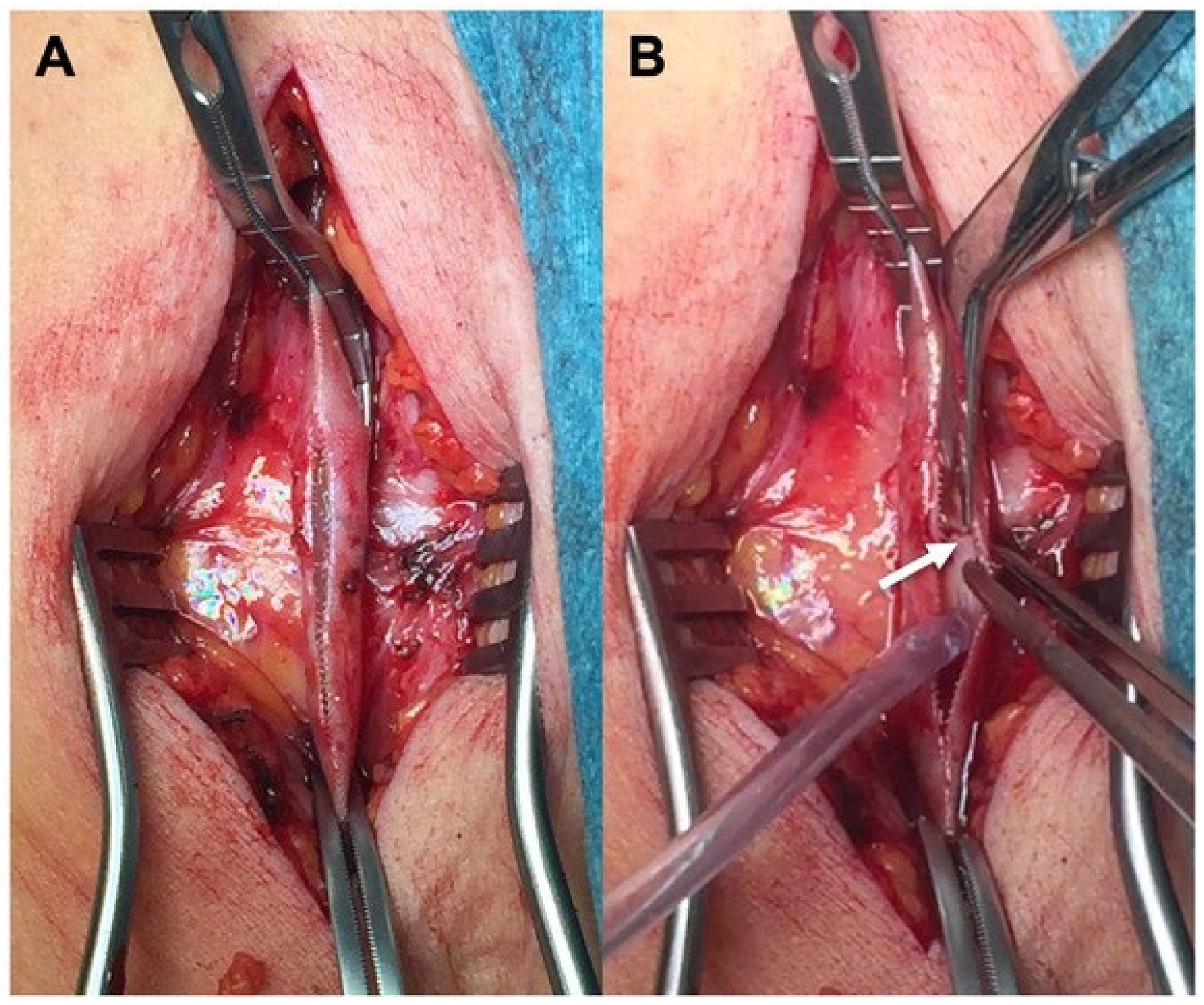
2.3. Material Description
2.4. Description of Variables
2.5. Surgical Preparation
2.6. Outcomes and Follow-Up
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.-B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. New Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.W.; Moakes, C.A.; Popplewell, M.; Meecham, L.; Bate, G.R.; Kelly, L.; Chetter, I.; Diamantopoulos, A.; Ganeshan, A.; Hall, J.; et al. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): An open-label, randomised, multicentre, phase 3 trial. Lancet 2023, 401, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Kutsenko, O.; Sommerset, J.; Chandra, V.; Bryce, Y. Techniques Providing Endpoints for Revascularization in Chronic Limb-Threatening Ischemia. Semin. Interv. Radiol. 2023, 40, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Wang, J.; Westcott, M.J.; Tew, M.; Davies, A.H.; Choong, P.F. A Systematic Review of Cost-Utility Analyses in Chronic Limb-Threatening Ischemia. Ann. Vasc. Surg. 2022, 85, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ucci, A.; Perini, P.; Freyrie, A.; Schreve, M.A.; Unlu, C.; Huizing, E.; Heuvel, D.A.v.D.; Kum, S.; Shishehbor, M.H.; Ferraresi, R. Endovascular and Surgical Venous Arterialization for No-Option Patients with Chronic Limb-Threatening Ischemia: A Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2023. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, P.; Post, P.; Breslau, P.; van Bockel, J. Saphenous Vein Versus PTFE for Above-Knee Femoropopliteal Bypass. A Review of the Literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.R. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef]
- Thomas, J.P.; So, K.L.; Turner, J.T.; Malanowski, A.J.; Colvard, B.D. Optimal conduit choice for open lower extremity bypass in chronic limb-threatening ischemia. Semin. Vasc. Surg. 2022, 35, 172–179. [Google Scholar] [CrossRef]
- Yan, Q.; Prasla, S.; Carlisle, D.C.; Rajesh, A.; Treffalls, J.; Davies, M.G. Deep Venous Arterialization for Chronic Limb Threatening Ischemia in Atherosclerosis Patients—A Meta-Analysis. Ann. Vasc. Surg. 2022, 81, 1–21. [Google Scholar] [CrossRef]
- Faries, P.L.; Arora, S.; Pomposelli, F.B., Jr.; Pulling, M.C.; Smakowski, P.; Rohan, D.I.; Gibbons, G.W.; Akbari, C.M.; Campbell, D.R.; LoGerfo, F.W. The use of arm vein in lower-extremity revascularization: Results of 520 procedures performed in eight years. J. Vasc. Surg. 2000, 31, 50–59. [Google Scholar] [CrossRef]
- Calligaro, K.D.; Syrek, J.R.; Dougherty, M.J.; Rua, I.; Raviola, C.A.; DeLaurentis, D.A. Use of arm and lesser saphenous vein compared with prosthetic grafts for infrapopliteal arterial bypass: Are they worth the effort? J. Vasc. Surg. 1997, 26, 919–924, discussion 917–925. [Google Scholar] [CrossRef] [PubMed]
- Gentile, A.T.; Lee, R.W.; Moneta, G.L.; Taylor, L.M.; Edwards, J.M.; Porter, J.M. Results of bypass to the popliteal and tibial arteries with alternative sources of autogenous vein. J. Vasc. Surg. 1996, 23, 272–279, discussion 279–280. [Google Scholar] [CrossRef][Green Version]
- Chew, D.K.; Conte, M.S.; Donaldson, M.C.; Whittemore, A.D.; Mannick, J.A.; Belkin, M. Autogenous composite vein bypass graft for infrainguinal arterial reconstruction. J. Vasc. Surg. 2001, 33, 259–264, discussion 255–264. [Google Scholar] [CrossRef]
- Davies, A.; Magee, T.; Sheffield, E.; Baird; Horrocks, M. The aetiology of vein graft stenoses. Eur. J. Vasc. Surg. 1994, 8, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, R.; Kruse, R.; Fritschy, W.; Moll, F. Treatment of Dilated Venous Bypass Grafts with an Expanded Polytetrafluoroethylene-covered Nitinol Endoprosthesis. Vasc. Endovasc. Surg. 2009, 43, 190–192. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef]
- Armstrong, P.A.; Bandyk, D.F.; Wilson, J.S.; Shames, M.L.; Johnson, B.L.; Back, M.R. Optimizing infrainguinal arm vein bypass patency with duplex ultrasound surveillance and endovascular therapy. J. Vasc. Surg. 2004, 40, 724–730, discussion 721–730. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, S.; Fujimura, N.; Utsunomiya, M.; Bolstad, F.; Nakai, T.; Iwakoshi, S.; Tanaka, T. Hemodynamic evaluation of lower limbs in patients with chronic limb-threatening ischemia. Cardiovasc. Interv. Ther. 2022, 37, 635–640. [Google Scholar] [CrossRef]
- Panetta, T.F.; Marin, M.L.; Veith, F.J.; Goldsmith, J.; Gordon, R.E.; Jones, A.M.; Schwartz, M.L.; Gupta, S.K.; Wengerter, K.R. Unsuspected preexisting saphenous vein disease: An unrecognized cause of vein bypass failure. J. Vasc. Surg. 1992, 15, 102–110, discussion 102–110. [Google Scholar] [CrossRef][Green Version]
- Jeremy, J.Y.; Gadsdon, P.; Shukla, N.; Vijayan, V.; Wyatt, M.; Newby, A.C.; Angelini, G.D. On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials 2007, 28, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Smith, F.; Angelini, G.; Bulbulia, R.; Jeremy, J. External Supports and the Prevention of Neointima Formation in Vein Grafts. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Violaris, A.G.; Newby, A.C.; Angelini, G.D. Effects of external stenting on wall thickening in arteriovenous bypass grafts. Ann. Thorac. Surg. 1993, 55, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, M.; Angelini, G.; Wan, S.; Yim, A.; Mehta, D.; Izzat, M.; Jeremy, J. Does External Stenting Reduce Porcine Vein-Graft Occlusion Via an Action on Vascular Nerves? J. Card. Surg. 2002, 17, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Arvela, E.; Kauhanen, P.; Albäck, A.; Lepäntalo, M.; Neufang, A.; Adili, F.; Schmitz-Rixen, T. Initial Experience with a New Method of External Polyester Scaffolding for Infrainguinal Vein Grafts. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Moritz, A.; Grabenwöger, F.; Raderer, F.; Ptakovsky, H.; Staudacher, M.; Magometschnigg, H.; Ullrich, R.; Wolner, E. Mesh Tube—Constricted Varicose Veins Used as Bypass Grafts for Infrainguinal Arterial Reconstruction. Arch. Surg. 1992, 127, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Neufang, A.; Espinola-Klein, C.; Savvidis, S.; Schmiedt, W.; Poplawski, A.; Vahl, C.F.; Dorweiler, B. External polytetrafluoroethylene reinforcement of varicose autologous vein grafts in peripheral bypass surgery produces durable bypass function. J. Vasc. Surg. 2018, 67, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Mellière, D.; Desgrange, P.; Allaire, E.; Becquemin, J.-P. Long-Term Results of Venous Bypass for Lower Extremity Arteries with Selective Short Segment Prosthetic Reinforcement of Varicose Dilatations. Ann. Vasc. Surg. 2007, 21, 45–49. [Google Scholar] [CrossRef]
- Carella, G.S.; Stilo, F.; Benedetto, F.; David, A.; Risitano, D.C.; Buemi, M.; Spinelli, F. Femoro-Distal Bypass with Varicose Veins Covered by Prosthetic Mesh. J. Surg. Res. 2011, 168, e189–e194. [Google Scholar] [CrossRef]
- Vigliotti, R.C.; Montelione, N.; Franceschi, F.; Franceschini, E.; Zardi, E.; Spinelli, F.; Stilo, F. Externally Supported Extra-anatomical Venous Bypass to Treat Upper Limb Ischemia with Shoulder Prosthetic Infection. Ann. Vasc. Surg. 2020, 69, 453.e5–453.e10. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Gasbarrini, A.; Massetti, M.; Flex, A. Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies. Int. J. Mol. Sci. 2021, 22, 2002. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerli, C.; Habrina, D.; Puchner, S.; Laminger, F.; Werzowa, J.; Roka, S. Primary External Stenting of an Autogenous Brachial-Basilic Upper Arm Transposition. Ann. Vasc. Surg. 2020, 65, 288.e1–288.e4. [Google Scholar] [CrossRef] [PubMed]
- Klesius, A.; Konerding, M.A.; Knez, P.; Dzemali, O.; Schmitz-Rixen, T.; Ackermann, H.; Moritz, A.; Kleine, P. External Stenting with a New Polyester Mesh Reduces Neointimal Hyperplasia of Vein Grafts in a Sheep Model. Int. J. Artif. Organs 2007, 30, 930.e8. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.H.; Hawdon, A.J.; Sydes, M.R.; Thompson, S.G.; on behalf of the VGST participants. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the vein graft surveillance randomised trial (VGST). Circulation 2005, 112, 1985.e91. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L. Mechanisms of vein graft failure: The location, distribution and characteristics of lesions that predispose to graft failure. Semin. Vasc. Surg. 1993, 6, 78–91. [Google Scholar] [PubMed]
- Huerta, C.T.; Voza, F.A.; Ortiz, Y.Y.; Liu, Z.-J.; Velazquez, O.C. Mesenchymal stem cell-based therapy for non-healing wounds due to chronic limb-threatening ischemia: A review of preclinical and clinical studies. Front. Cardiovasc. Med. 2023, 10, 1113982. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Duran, M.; Garabet, W.; Schelzig, H.; Jacobs, M.; Gombert, A. Gene Therapy of Chronic Limb-Threatening Ischemia: Vascular Medical Perspectives. J. Clin. Med. 2022, 11, 1282. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, G.; Cusumano, G.; Mariotta, L.; Canevascini, R.; Gola, M.; Gornati, R.; Soldati, G. Upgrading Monocytes Therapy for Critical Limb Ischemia Patient Treatment: Pre-Clinical and GMP-Validation Aspects. Int. J. Mol. Sci. 2022, 23, 12669. [Google Scholar] [CrossRef]
- Kim, T.I.; Schneider, P.A. New Innovations and Devices in the Management of Chronic Limb-Threatening Ischemia. J. Endovasc. Ther. 2020, 27, 524–539. [Google Scholar] [CrossRef]
- Ventoruzzo, G.; Mazzitelli, G.; Ruzzi, U.; Liistro, F.; Scatena, A.; Martelli, E. Limb Salvage and Survival in Chronic Limb-Threatening Ischemia: The Need for a Fast-Track Team-Based Approach. J. Clin. Med. 2023, 12, 6081. [Google Scholar] [CrossRef]
- Castro-Dominguez, Y.; Shishehbor, M.H. Team-Based Care in Patients with Chronic Limb-Threatening Ischemia. Curr. Cardiol. Rep. 2022, 24, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Goodney, P.; Shah, S.; Hu, Y.D.; Suckow, B.; Kinlay, S.; Armstrong, D.G.; Geraghty, P.; Patterson, M.; Menard, M.; Patel, M.R.; et al. A systematic review of patient-reported outcome measures patients with chronic limb-threatening ischemia. J. Vasc. Surg. 2022, 75, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Cimaglia, P.; Manfrini, M.; Fortini, F.; Marracino, L.; Bernucci, D.; Pompei, G.; Scala, A.; Trichilo, M.; De Carolis, B.; et al. Circulating Biomarkers of Endothelial Dysfunction and Inflammation in Predicting Clinical Outcomes in Diabetic Patients with Critical Limb Ischemia. Int. J. Mol. Sci. 2022, 23, 10641. [Google Scholar] [CrossRef] [PubMed]


| Age, Median (Range) | 74.5 | (65–91) |
|---|---|---|
| Male (n, %) | 13 | 81% |
| Smoking (n, %) | 11 | 69% |
| Hypertension (n, %) | 12 | 75% |
| Diabetes (n, %) | 7 | 43% |
| Hyperlipidemia (n, %) | 8 | 50% |
| Coronary Artery Disease (n, %) | 3 | 19% |
| Chronic Kidney Disease stage III + (n, %) | 1 | 6% |
| Indication for Surgery | Anesthesia | Inflow Artery | Outflow Artery | Vein Graft Details |
|---|---|---|---|---|
| CLTI | GA | CFA | POP | LSV |
| CLTI | SA | CFA | PER | GSV + LSV |
| CLTI | SA | CFA | PTA | GSV |
| CLTI | GA | DFA | PTA | GSV |
| CLTI | SA + LRA | CFA | PTA | GSV |
| CLTI | SA | CFA | ATA | GSV |
| CLTI | SA + LRA | CFA | ATA | GSV |
| CLTI | SA + LRA | SFA | PTA | GSV |
| CLTI and Infection | GA | EIA | DFA | GSV |
| ALI | GA | Axillary A | Brachial A | GSV |
| CLTI | SA + LRA | SFA | PTA | GSV |
| CLTI | GA | CFA | ATA | Cephalic V |
| CLTI | SA + LRA | CFA | PTA | GSV |
| CLTI | SA + LRA | SFA | PER | GSV |
| CLTI | SA + LRA | CFA | PTA | GSV |
| CLTI | SA + LRA | CFA | ATA | GSV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montelione, N.; Catanese, V.; Nenna, A.; Gabellini, T.; Ferrisi, C.; Paolini, J.; Ciolli, A.; Barillà, D.; Loreni, F.; Chello, M.; et al. External Scaffold for Venous Graft to Treat Chronic Limb-Threatening Ischemia: Results of the FRAME Vascular Support. J. Clin. Med. 2024, 13, 2095. https://doi.org/10.3390/jcm13072095
Montelione N, Catanese V, Nenna A, Gabellini T, Ferrisi C, Paolini J, Ciolli A, Barillà D, Loreni F, Chello M, et al. External Scaffold for Venous Graft to Treat Chronic Limb-Threatening Ischemia: Results of the FRAME Vascular Support. Journal of Clinical Medicine. 2024; 13(7):2095. https://doi.org/10.3390/jcm13072095
Chicago/Turabian StyleMontelione, Nunzio, Vincenzo Catanese, Antonio Nenna, Teresa Gabellini, Chiara Ferrisi, Julia Paolini, Alessandro Ciolli, David Barillà, Francesco Loreni, Massimo Chello, and et al. 2024. "External Scaffold for Venous Graft to Treat Chronic Limb-Threatening Ischemia: Results of the FRAME Vascular Support" Journal of Clinical Medicine 13, no. 7: 2095. https://doi.org/10.3390/jcm13072095
APA StyleMontelione, N., Catanese, V., Nenna, A., Gabellini, T., Ferrisi, C., Paolini, J., Ciolli, A., Barillà, D., Loreni, F., Chello, M., Spinelli, F., & Stilo, F. (2024). External Scaffold for Venous Graft to Treat Chronic Limb-Threatening Ischemia: Results of the FRAME Vascular Support. Journal of Clinical Medicine, 13(7), 2095. https://doi.org/10.3390/jcm13072095








