Clinical Trajectory and Risk Stratification for Heart Failure with Preserved Ejection Fraction in a Real-World Cohort of Patients with Suspected Coronary Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Patient Classification
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
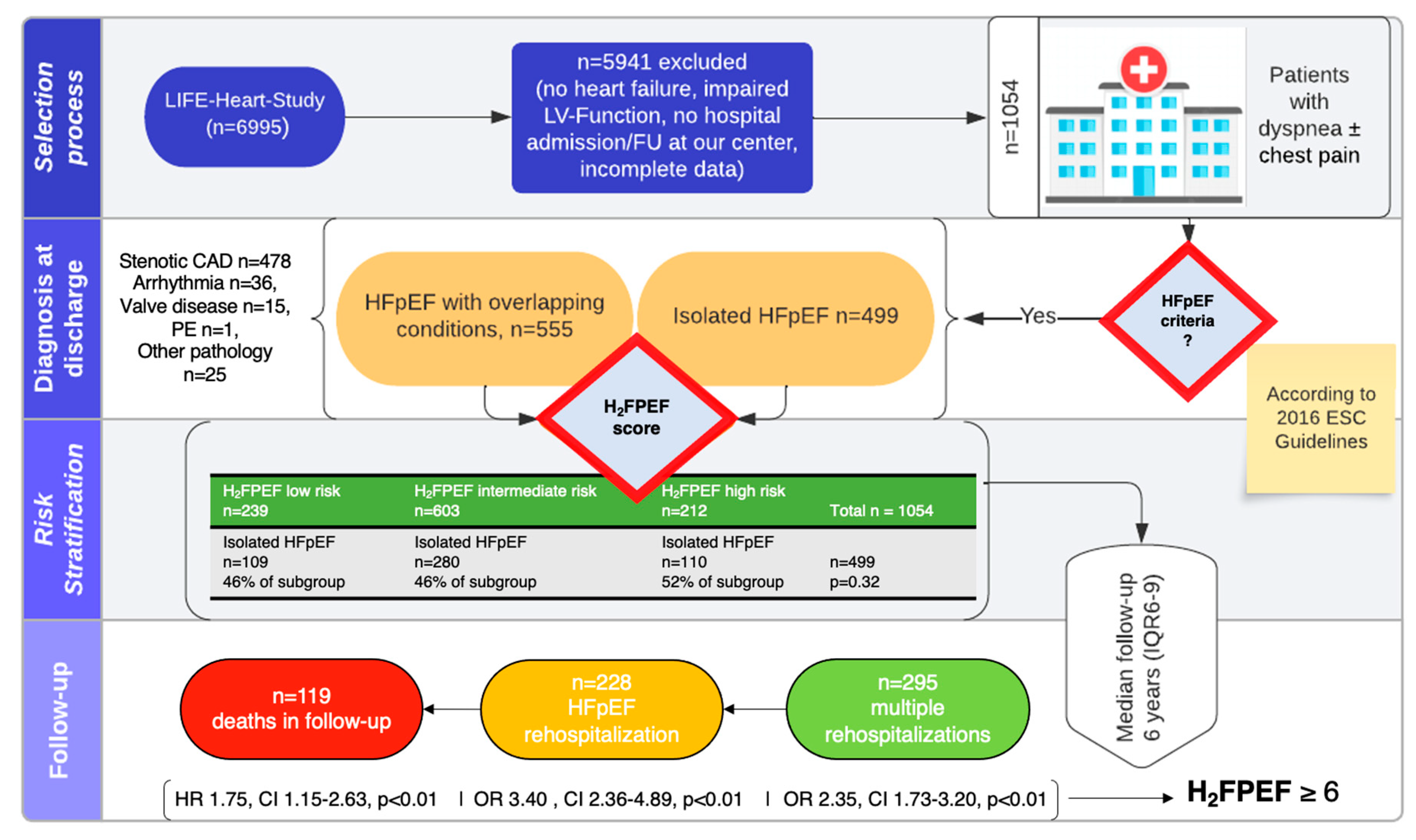
3.1. Patient Population and Definition of Cohorts
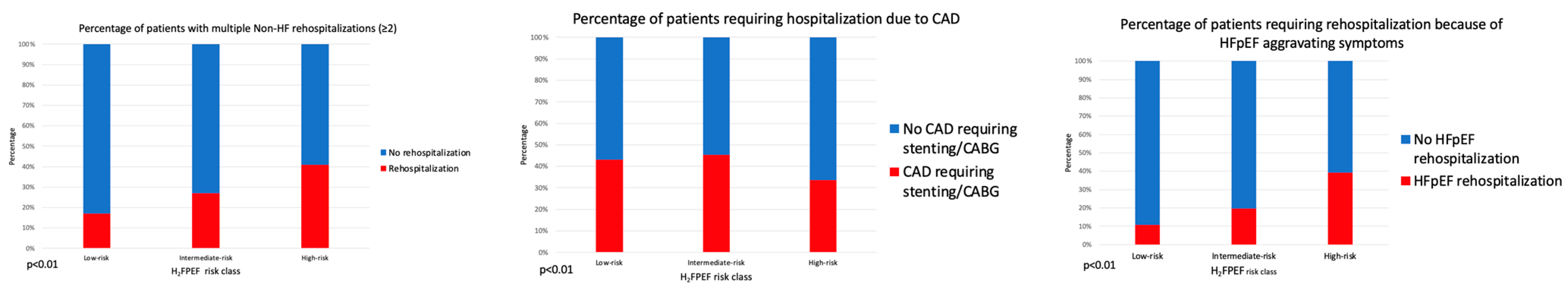
3.2. Rehospitalizations
3.3. Predictors of Rehospitalization
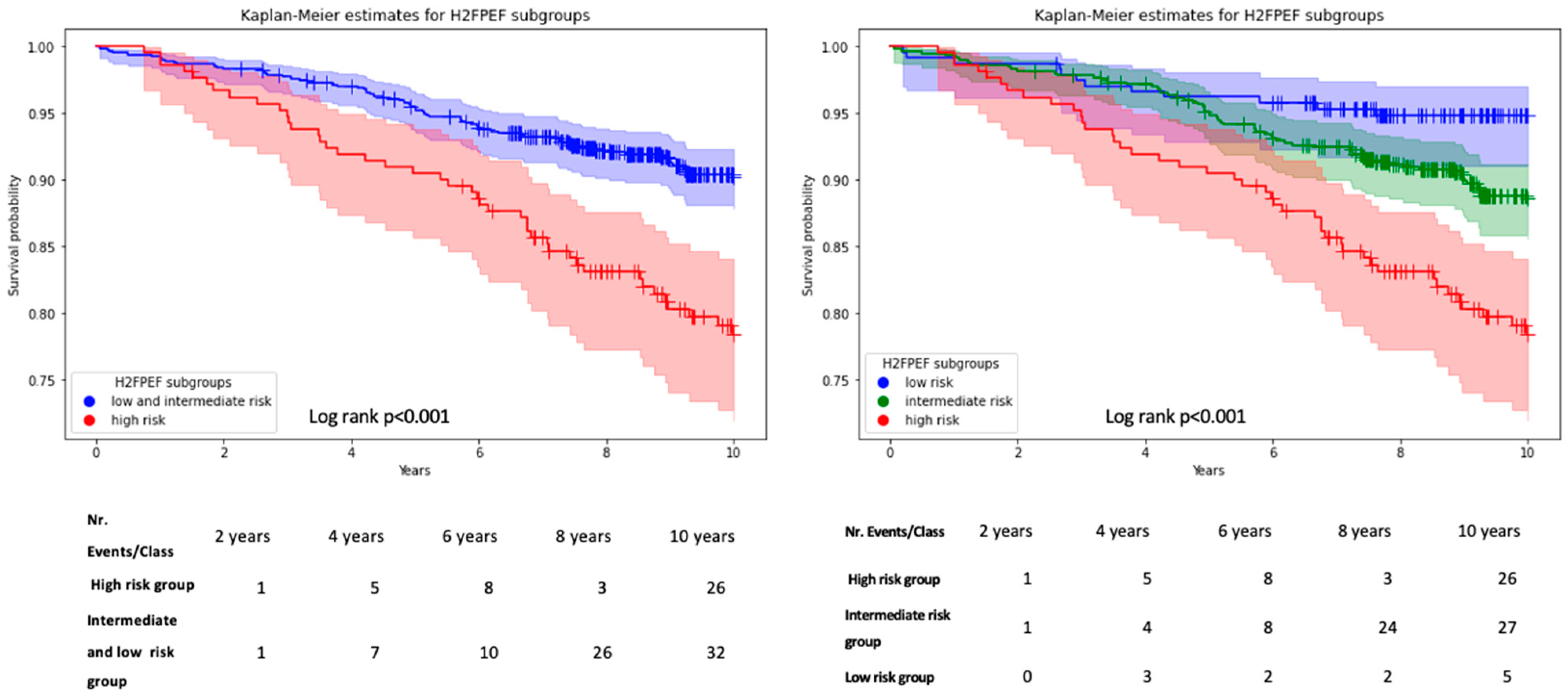
3.4. Mortality
4. Discussion
- Patients presenting with a positive screening for HFpEF while being evaluated for CAD exhibit an important phenotypic heterogeneity with overlapping comorbidities in 53% of patients, and only 20% are classified as high-probability HFpEF based on the H2FPEF score.
- Rehospitalizations were common, but reasons for rehospitalization varied. The H2FPEF score but not the presence of overlapping comorbidities was strongly associated with HF-specific rehospitalizations.
- The H2FPEF score is a potent predictor of mortality in this heterogeneous patient cohort.
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Afib | atrial fibrillation |
| ANOVA | analysis of variance |
| CAD | coronary artery disease |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| ECG | electrocardiogram |
| EDV | end-diastolic volume |
| eGFR | estimated glomerular filtration rate |
| ESC | European Society of Cardiology |
| HF | heart failure |
| HFpEF | heart failure with preserved ejection fraction |
| HR | hazard ratio |
| Hs-troponin | high-sensitivity troponin |
| IQR | interquartile range |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems (WHO version 10) |
| LA | left atrium |
| LV | left ventricle |
| LV-EF | left ventricular systolic ejection fraction |
| NYHA | New York Heart Association |
| NTproBNP | N-terminal-pro hormone B-type natriuretic peptide |
| OR | odds ratio |
| PVI | pulmonary vein ablation |
References
- Sanderson, J.E. Heart Failure with a Normal Ejection Fraction. Heart 2007, 93, 155–158. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16387829 (accessed on 13 June 2016).
- Owan, T.E.; Redfield, M.M. Epidemiology of diastolic heart failure. Prog. Cardiovasc. Dis. 2005, 47, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Nair, N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 531–540. [Google Scholar]
- Borlaug, B.A.; Paulus, W.J. Heart Failure with Preserved Ejection Fraction: Pathophysiology, Diagnosis, and Treatment. Eur. Heart J. 2011, 32, 670–679. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21138935 (accessed on 13 June 2016). [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Sankowski, P.; Zhang, Q. Revenue maximization envy-free pricing for homogeneous resources. IJCAI Int. Jt. Conf. Artif. Intell. 2015, 2015, 90–96. [Google Scholar]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Melenovsky, V.; Borlaug, B.A. Implications of coronary artery disease in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; A de Boer, R.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Rasmussen, S.; Davies, M.; Hovingh, G.K.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2023, 389, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Henger, S.; Beutner, F.; Teren, A.; Baber, R.; Willenberg, A.; Ceglarek, U.; Pott, J.; Burkhardt, R.; Thiery, J. Cohort Profile: The leipzig research center for civilization diseases-Heart study (LIFE-Heart). Int. J. Epidemiol. 2020, 49, 1439–1440. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Glob. Heart 2018, 13, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Kossaify, A.; Nasr, M. Diastolic Dysfunction and the New Recommendations for Echocardiographic Assessment of Left Ventricular Diastolic Function: Summary of Guidelines and Novelties in Diagnosis and Grading. J. Diagn. Med. Sonogr. 2019, 35, 317–325. [Google Scholar] [CrossRef]
- Ckd, D.O.F.; Graded, N. Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Lam, C.S.; Lyass, A.; Kraigher-Krainer, E.; Massaro, J.M.; Lee, D.S.; Ho, J.E.; Levy, D.; Redfield, M.M.; Pieske, B.M.; Benjamin, E.J.; et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 2011, 124, 24–30. [Google Scholar] [CrossRef]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association. Circulation 2023, 147, E93–E621. [Google Scholar] [PubMed]
- Sepehrvand, N.; Alemayehu, W.; Dyck, G.J.; Dyck, J.R.; Anderson, T.; Howlett, J.; Paterson, I.; McAlister, F.A.; Ezekowitz, J.A.; On behalf of the Alberta HEART Investigators. External Validation of the H2F-PEF Model in Diagnosing Patients with Heart Failure and Preserved Ejection Fraction. Circulation 2019, 139, 2377–2379. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Srivaratharajah, K.; Coutinho, T.; Dekemp, R.; Liu, P.; Haddad, H.; Stadnick, E.; Davies, R.A.; Chih, S.; Dwivedi, G.; Guo, A.; et al. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ. Heart Fail. 2016, 9, e002562. [Google Scholar] [CrossRef] [PubMed]
- Bisbal, F.; Baranchuk, A.; Braunwald, E.; Bayés de Luna, A.; Bayés-Genís, A. Atrial Failure as a Clinical Entity: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Sueta, D.; Yamamoto, E.; Nishihara, T.; Tokitsu, T.; Fujisue, K.; Oike, F.; Takae, M.; Usuku, H.; Takashio, S.; Arima, Y.; et al. H2FPEF Score as a Prognostic Value in HFpEF Patients. Am. J. Hypertens. 2019, 32, 1082–1090. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaikita, K.; Yamamoto, E.; Sueta, D.; Yamamoto, M.; Ishii, M.; Ito, M.; Fujisue, K.; Kanazawa, H.; Araki, S.; et al. H2FPEF score for predicting future heart failure in stable outpatients with cardiovascular risk factors. ESC Heart Fail. 2020, 7, 65–74. [Google Scholar] [CrossRef]
| Variables | All Patients n = 1054 | H2FPEF Low-Risk n = 239 | H2FPEF Intermediate-Risk n = 603 | H2FPEF High-Risk n = 212 | p-Value |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, years | 66 ± 10 | 58 ± 10 | 68 ± 9 | 70 ± 7 | <0.01 |
| Female sex, n. (%) | 421 (40%) | 84 (35%) | 256 (42%) | 81 (38%) | 0.13 |
| NYHA class, n. (%) | NYHA II: 977 (93%) NYHA III: 68 (6.5%) NYHA IV: 9 (0.5%) | NYHA II: 233 (97%) NYHA III: 3 (1.5%) NYHA IV: 3 (1.5%) | NYHA II: 568 (94%) NYHA III: 31 (5%) NYHA IV: 4 (1%) | NYHA II: 176 (83%) NYHA III: 34 (16%) NYHA IV: 2 (1%) | <0.01 |
| Chest pain, n. (%) | 445 (42%) | 152 (64%) | 212 (35%) | 81 (38%) | <0.01 |
| BMI, kg/m2 | 30 ± 5 | 28 ± 4 | 31 ± 5 | 31 ± 5 | <0.01 |
| Obesity, n. (%) | 487 (46%) | 35 (15%) | 324 (54%) | 128 (60%) | <0.01 |
| Diabetes, n. (%) | 371 (35%) | 43 (18%) | 217 (36%) | 111 (52%) | <0.01 |
| Arterial hypertension, n. (%) | 656 (62%) | 139 (58%) | 384 (64%) | 133 (63%) | 0.33 |
| Smoking, n. (%) | 368 (35%) | 84 (35%) | 207 (34%) | 77 (36%) | 0.87 |
| Hx CAD intervention, n. (%) | 169 (16%) | 26 (11%) | 107 (18%) | 36 (17%) | 0.05 |
| Dx of CAD, n. (%) | 523 (50%) | 125 (52%) | 311 (52%) | 87 (41%) | 0.02 |
| Atrial fibrillation, n. (%) | 279 (26%) | 0 (0%) | 67 (11%) | 212 (100%) | <0.01 |
| HF hospitalization, n. (%) | 499 (47%) | 109 (46%) | 280 (46%) | 110 (52%) | 0.33 |
| Non-HF hospitalization, n. (%) | 555 (53%) | 130 (54%) | 323 (54%) | 102 (48%) | 0.33 |
| Laboratory Values | |||||
| eGFR, mL/min/1.73 m2 | 66 ± 25 | 76 ± 27 | 63 ± 24 | 63 ± 23 | <0.01 |
| eGFR < 30, n. (%) | 51 (5%) | 5 (2%) | 33 (5%) | 13 (6%) | 0.07 |
| NT-proBNP, ng/L | 540 (178–543) | 217 (164–341) | 280 (177–490) | 413 (242–1112) | 0.05 |
| CRP, mg/L | 5.0 ± 9.8 | 4.5 ± 10.8 | 4.8 ± 9.3 | 6.0 ± 10.1 | 0.02 |
| IL-6, pg/mL | 5.18 ± 12.75 | 5.74 ± 23.93 | 4.96 ± 6.98 | 5.16 ± 4.99 | 0.80 |
| Troponin T, pg/mL | 9.1 (6.0–12.9) | 7.8 (5.1–10.8) | 10.7 (7.0–11.6) | 10.8 (8.7–14.3) | <0.01 |
| Echocardiographic Parameters | |||||
| LV-EF, % | 61 ± 7 | 61 ± 6 | 61 ± 7 | 62 ± 7 | 0.21 |
| E/e’ | 10.4 ± 3.9 | 8.9 ± 3.0 | 10.6 ± 3.8 | 12.8 ± 4.6 | <0.01 |
| LV-EDV index, mL/m2 | 53 ± 18 | 55 ± 18 | 52 ± 17 | 49 ± 18 | 0.01 |
| LV-Mass index, g/m2 | 138 ± 40 | 134 ± 37 | 141 ± 42 | 140 ± 42 | 0.03 |
| LA diameter index, mm/m2 | 24 ± 4 | 23 ± 3 | 24 ± 3 | 26 ± 4 | <0.01 |
| TAPSE, mm | 21 ± 4 | 21 ± 4 | 21 ± 4 | 20 ± 4 | 0.53 |
| TR Vmax, m/s | 2.5 ± 0.8 | 2.5 ± 1.0 | 2.4 ± 0.5 | 2.8 ± 0.6 | <0.01 |
| Moderate valvular disease, n. (%) | 120 (11%) | 13 (5%) | 64 (11%) | 43 (20%) | <0.01 |
| Events during follow-up | |||||
| Follow-up time, years | 6 (IQR 6–9) | 6 (IQR 6–9) | 6 (IQR 6–8) | 6 (IQR 6–8) | 0.72 |
| HF rehospitalization, n. (%) | 228 (22%) | 26 (11%) | 119 (20%) | 83 (39%) | <0.01 |
| Average number of rehospitalizations, n. | 1.15 ± 1.7 | 0.75 ± 1.4 | 1.13 ± 1.7 | 1.77 ± 1.9 | <0.01 |
| All-cause mortality, n. (%) | 119 (11%) | 12 (5%) | 64 (11%) | 43 (20%) | <0.01 |
| Logistic Regression Model (Univariate) | Logistic Regression Model (Multivariable) | |||||||
|---|---|---|---|---|---|---|---|---|
| 95.0% CI for EXP(B) | 95.0% CI for EXP(B) | |||||||
| EXP(B) | Lower | Upper | p-Value | EXP(B) | Lower | Upper | p-Value | |
| Male sex | 1.21 | 0.89 | 1.83 | 0.21 | 1.20 | 0.87 | 1.64 | 0.26 |
| lnNTproBNP | 1.04 | 0.87 | 1.24 | 0.64 | 1.00 | 0.78 | 1.27 | 0.99 |
| NYHA-class | 0.73 | 0.42 | 1.26 | 0.26 | 0.61 | 0.28 | 1.31 | 0.20 |
| H2FPEF high-risk | 3.09 | 2.23 | 4.29 | <0.01 | 3.40 | 2.36 | 4.89 | <0.01 |
| Cox-Proportional Model (Univariate) | Cox-Proportional Model (Multivariable) | |||||||
|---|---|---|---|---|---|---|---|---|
| 95.0% CI for HR | 95.0% CI for HR | |||||||
| HR | Lower | Upper | p-Value | HR | Lower | Upper | p-Value | |
| Age (years) | 1.10 | 1.08 | 1.13 | <0.01 | - | - | - | - |
| Male sex | 1.88 | 1.25 | 2.82 | <0.01 | 1.73 | 1.15 | 2.60 | <0.01 |
| CAD | 0.80 | 0.47 | 1.37 | 0.41 | - | - | - | - |
| Afib | 1.95 | 1.35 | 2.82 | <0.01 | - | - | - | - |
| H2FPEF High-risk | 2.78 | 1.89 | 4.07 | <0.01 | 1.75 | 1.15 | 2.63 | <0.01 |
| lnNT-proBNP | 3.86 | 2.58 | 5.78 | <0.01 | 1.57 | 1.19 | 2.60 | <0.01 |
| NYHA-class | 2.72 | 1.91 | 3.86 | <0.01 | - | - | - | n.s. |
| Diabetes mellitus type 2 | 1.63 | 1.14 | 2.34 | 0.01 | - | - | - | n.s. |
| E/e‘ average | 1.09 | 1.05 | 1.14 | <0.01 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioia, G.; Kresoja, K.-P.; Rosch, S.; Schöber, A.; Harnisch, E.; von Roeder, M.; Scholz, M.; Henger, S.; Isermann, B.; Thiele, H.; et al. Clinical Trajectory and Risk Stratification for Heart Failure with Preserved Ejection Fraction in a Real-World Cohort of Patients with Suspected Coronary Artery Disease. J. Clin. Med. 2024, 13, 2092. https://doi.org/10.3390/jcm13072092
Gioia G, Kresoja K-P, Rosch S, Schöber A, Harnisch E, von Roeder M, Scholz M, Henger S, Isermann B, Thiele H, et al. Clinical Trajectory and Risk Stratification for Heart Failure with Preserved Ejection Fraction in a Real-World Cohort of Patients with Suspected Coronary Artery Disease. Journal of Clinical Medicine. 2024; 13(7):2092. https://doi.org/10.3390/jcm13072092
Chicago/Turabian StyleGioia, Guglielmo, Karl-Patrik Kresoja, Sebastian Rosch, Anne Schöber, Elias Harnisch, Maximilian von Roeder, Markus Scholz, Sylvia Henger, Berend Isermann, Holger Thiele, and et al. 2024. "Clinical Trajectory and Risk Stratification for Heart Failure with Preserved Ejection Fraction in a Real-World Cohort of Patients with Suspected Coronary Artery Disease" Journal of Clinical Medicine 13, no. 7: 2092. https://doi.org/10.3390/jcm13072092
APA StyleGioia, G., Kresoja, K.-P., Rosch, S., Schöber, A., Harnisch, E., von Roeder, M., Scholz, M., Henger, S., Isermann, B., Thiele, H., Lurz, P., & Rommel, K.-P. (2024). Clinical Trajectory and Risk Stratification for Heart Failure with Preserved Ejection Fraction in a Real-World Cohort of Patients with Suspected Coronary Artery Disease. Journal of Clinical Medicine, 13(7), 2092. https://doi.org/10.3390/jcm13072092






