Visuospatial Function in Women with Premenstrual Dysphoric Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Study Measures
2.3. Data Analysis
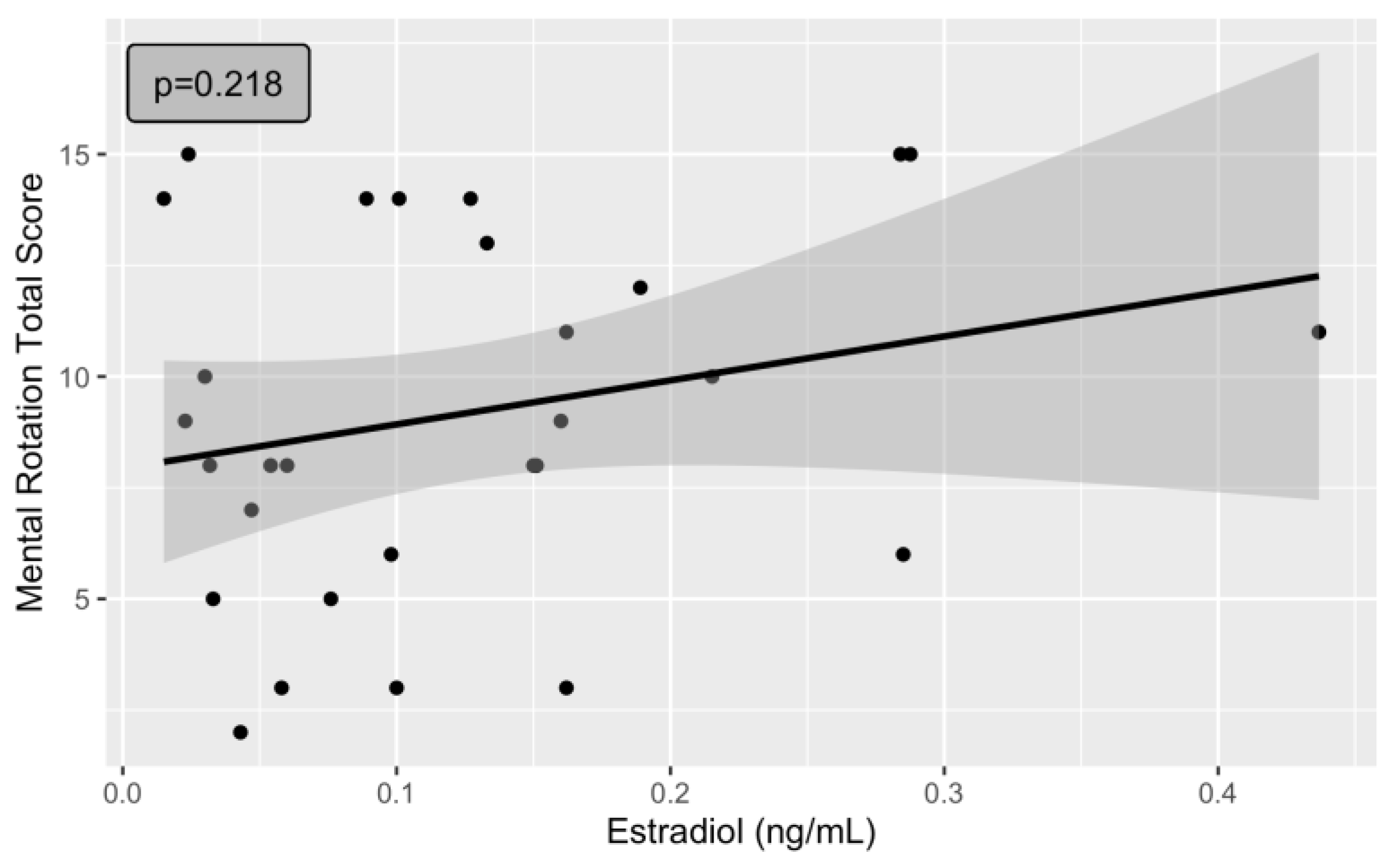
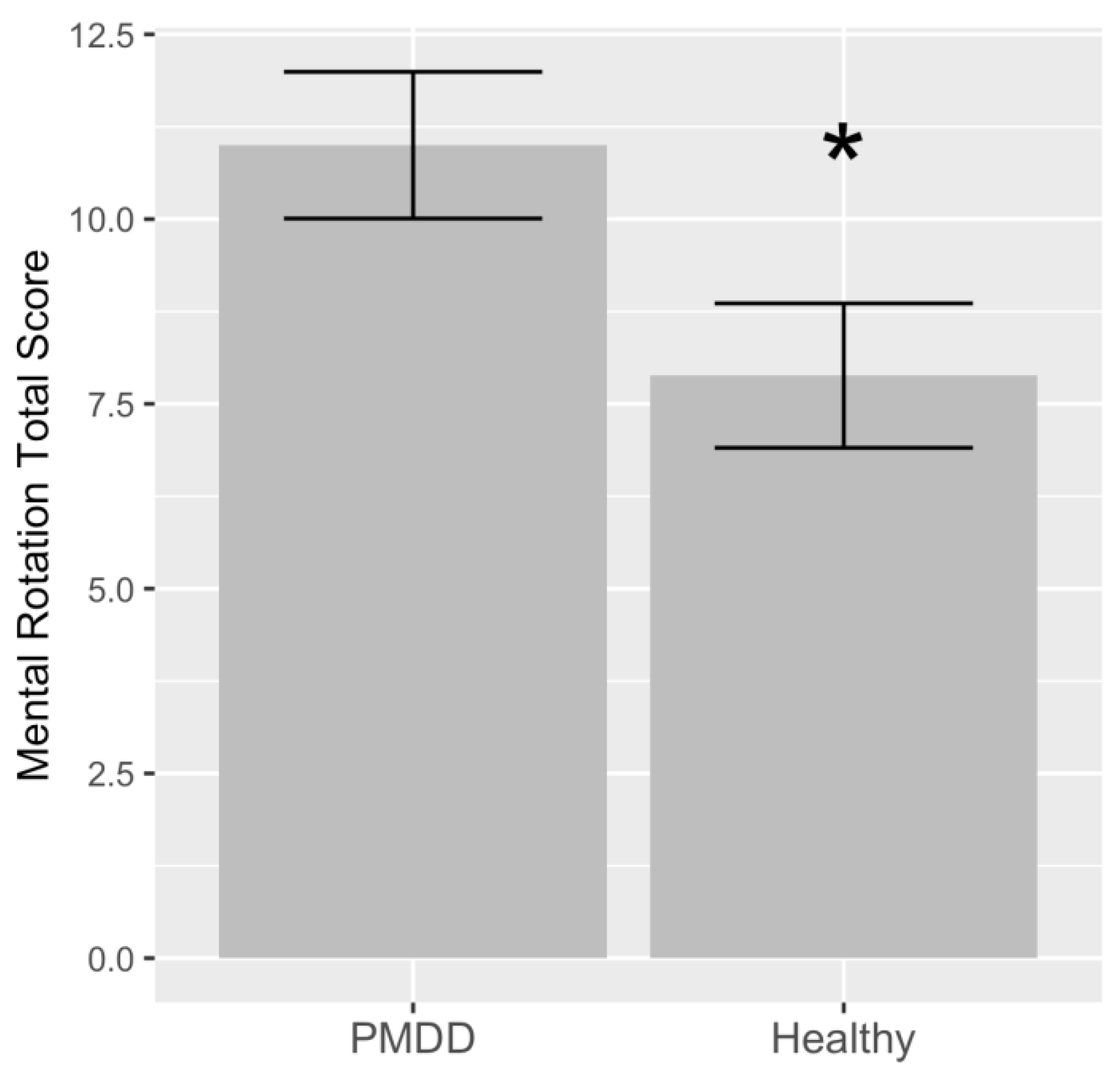
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehlert, S.; Song, I.H.; Chang, C.-H.; Hartlage, S.A. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol. Med. 2008, 39, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Macdougall, M.; Brown, E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch. Womens Ment. Health 2003, 6, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Hylan, T.R.; Sundell, K.; Judge, R. The impact of premenstrual symptomatology on functioning and treatment-seeking behavior: Experience from the United States, United Kingdom, and France. J. Womens Health Gend. Based Med. 1999, 8, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Thomas, N.; Gurvich, C. Cognition, the Menstrual Cycle, and Premenstrual Disorders: A Review. Brain Sci. 2020, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Brinton, R.D.; Thompson, R.F.; Foy, M.R.; Baudry, M.; Wang, J.; Finch, C.E.; Morgan, T.E.; Pike, C.J.; Mack, W.J.; Stanczyk, F.Z.; et al. Progesterone receptors: Form and function in brain. Front. Neuroendocr. 2008, 29, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Luine, V.; Arellanos, A.; Frankfurt, M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006, 1126, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Engler-Chiurazzi, E.B.; Singh, M.; Simpkins, J.W. Reprint of: From the 90’s to now: A brief historical perspective on more than two decades of estrogen neuroprotection. Brain Res. 2016, 1645, 79–82. [Google Scholar] [CrossRef]
- Woolley, C.S.; McEwen, B.S. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993, 336, 293–306. [Google Scholar] [CrossRef]
- Sundström Poromaa, I.; Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 2014, 8, 380. [Google Scholar]
- Voyer, D.; Voyer, S.; Bryden, M.P. Magnitude of sex differences in spatial abilities: A meta-analysis and consideration of critical variables. Psychol. Bull. 1995, 117, 250–270. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Krings, T.; Neulen, J.; Willmes, K.; Erberich, S.; Thron, A.; Sturm, W. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. NeuroImage 2001, 13, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Halari, R.; Hines, M.; Kumari, V.; Mehrotra, R.; Wheeler, M.; Ng, V.; Sharma, T. Sex differences and individual differences in cognitive performance and their relationship to endogenous gonadal hormones and gonadotropins. Behav. Neurosci. 2005, 119, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, T.; Yasukouchi, A. Sex differences on components of mental rotation at different menstrual phases. Int. J. Neurosci. 2009, 119, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Schisterman, E.F.; Gaskins, A.J.; Pollack, A.Z.; Perkins, N.J.; Whitcomb, B.W.; Ye, A.; Wactawski-Wende, J. Realignment and multiple imputation of longitudinal data: An application to menstrual cycle data. Paediatr. Perinat. Epidemiol. 2011, 25, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Conklin, S.E.; Knezevic, C.E. Advancements in the gold standard: Measuring steroid sex hormones by mass spectrometry. Clin. Biochem. 2020, 82, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J.; Sun, J.; Dang, N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites 2022, 12, 941. [Google Scholar] [CrossRef] [PubMed]
- Endicott, J.; Nee, J.; Harrison, W. Daily Record of Severity of Problems (DRSP): Reliability and validity. Arch. Womens Ment. Health 2005, 9, 41–49. [Google Scholar] [CrossRef]
- Hamidovic, A.; Davis, J.; Soumare, F.; Naveed, A.; Ghani, Y.; Semiz, S.; Khalil, D.; Wardle, M. Allopregnanolone Is Associated with a Stress-Induced Reduction of Heart Rate Variability in Premenstrual Dysphoric Disorder. J. Clin. Med. 2023, 12, 1553. [Google Scholar] [CrossRef]
- Li, H.J.; Goff, A.; Rudzinskas, S.A.; Jung, Y.; Dubey, N.; Hoffman, J.; Hipolito, D.; Mazzu, M.; Rubinow, D.R.; Schmidt, P.J.; et al. Altered estradiol-dependent cellular Ca2+ homeostasis and endoplasmic reticulum stress response in Premenstrual Dysphoric Disorder. Mol. Psychiatry 2021, 26, 6963–6974. [Google Scholar] [CrossRef] [PubMed]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J. Mid-Luteal Progesterone Is Inversely Associated with Premenstrual Food Cravings. Nutrients 2023, 15, 1097. [Google Scholar] [CrossRef]
- Hampson, E.; Levy-Cooperman, N.; Korman, J.M. Estradiol and mental rotation: Relation to dimensionality, difficulty, or angular disparity? Horm. Behav. 2014, 65, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, S.G.; Kuse, A.R. Mental rotations, a group test of three-dimensional spatial visualization. Percept. Mot. Skills 1978, 47, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, M.; Slabbekoorn, D.; Van Goozen, S.H.; Cohen-Kettenis, P.T.; Güntürkün, O. Sex hormones affect spatial abilities during the menstrual cycle. Behav. Neurosci. 2000, 114, 1245–1250. [Google Scholar] [CrossRef]
- Peters, M.; Laeng, B.; Latham, K.; Jackson, M.; Zaiyouna, R.; Richardson, C. A redrawn Vandenberg and Kuse mental rotations test: Different versions and factors that affect performance. Brain Cogn. 1995, 28, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Jansen, P.; Heil, M. Gender differences in mental rotation across adulthood. Exp. Aging Res. 2009, 36, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Courvoisier, D.S.; Renaud, O.; Geiser, C.; Paschke, K.; Gaudy, K.; Jordan, K. Sex hormones and mental rotation: An intensive longitudinal investigation. Horm. Behav. 2013, 63, 345–351. [Google Scholar] [CrossRef]
- Maki, P.M.; Rich, J.B.; Rosenbaum, R.S. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia 2002, 40, 518–529. [Google Scholar] [CrossRef]
- Leeners, B.; Kruger, T.H.C.; Geraedts, K.; Tronci, E.; Mancini, T.; Ille, F.; Egli, M.; Röblitz, S.; Saleh, L.; Spanaus, K.; et al. Lack of Associations between Female Hormone Levels and Visuospatial Working Memory, Divided Attention and Cognitive Bias across Two Consecutive Menstrual Cycles. Front. Behav. Neurosci. 2017, 11, 120. [Google Scholar] [CrossRef]
- Ghazal, K.; Brabant, S.; Prie, D.; Piketty, M.-L. Hormone Immunoassay Interference: A 2021 Update. Ann. Lab. Med. 2022, 42, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Demers, L.M. Testosterone and estradiol assays: Current and future trends. Steroids 2008, 73, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, I.; Hayward, W.G.; Tarr, M.J.; Anderson, A.W.; Skudlarski, P.; Gore, J.C. BOLD activity during mental rotation and viewpoint-dependent object recognition. Neuron 2002, 34, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.M.; Egan, G.F.; Sonkkila, C.; Tochon-Danguy, H.J.; Paxinos, G.; Watson, J.D. Selective right parietal lobe activation during mental rotation: A parametric PET study. Brain J. Neurol. 2000, 123 Pt 1, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zacks, J.M. Neuroimaging studies of mental rotation: A meta-analysis and review. J. Cogn. Neurosci. 2008, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Epperson, C.N.; Haga, K.; Mason, G.F.; Sellers, E.; Gueorguieva, R.; Zhang, W.; Krystal, J.H. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: A proton magnetic resonance spectroscopy study. Arch. Gen. Psychiatry 2002, 59, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Manyukhina, V.O.; Orekhova, E.V.; Prokofyev, A.O.; Obukhova, T.S.; Stroganova, T.A. Altered visual cortex excitability in premenstrual dysphoric disorder: Evidence from magnetoencephalographic gamma oscillations and perceptual suppression. PLoS ONE 2022, 17, e0279868. [Google Scholar] [CrossRef]
- Christophel, T.B.; Cichy, R.M.; Hebart, M.N.; Haynes, J.-D. Parietal and early visual cortices encode working memory content across mental transformations. NeuroImage 2015, 106, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.; Mills, E.D.; Conrad, A.L.; Koscik, T.; Andreasen, N.C.; Nopoulos, P. Sex differences in parietal lobe structure and development. Gend. Med. 2012, 9, 44–55. [Google Scholar] [CrossRef]
- Koscik, T.; O’Leary, D.; Moser, D.J.; Andreasen, N.C.; Nopoulos, P. Sex differences in parietal lobe morphology: Relationship to mental rotation performance. Brain Cogn. 2009, 69, 451–459. [Google Scholar] [CrossRef][Green Version]
- Studholme, C.; Kroenke, C.D.; Dighe, M. Motion corrected MRI differentiates male and female human brain growth trajectories from mid-gestation. Nat. Commun. 2020, 11, 3038. [Google Scholar] [CrossRef] [PubMed]
- Dubol, M.; Stiernman, L.; Wikström, J.; Lanzenberger, R.; Epperson, C.N.; Sundström-Poromaa, I.; Bixo, M.; Comasco, E. Differential grey matter structure in women with premenstrual dysphoric disorder: Evidence from brain morphometry and data-driven classification. Transl. Psychiatry 2022, 12, 250. [Google Scholar] [CrossRef] [PubMed]
| Demographic Variable | Category | Diagnostic Category | ||
|---|---|---|---|---|
| Healthy (n = 17) | PMDD (n = 12) | p-Value | ||
| Age | 26.23 (5.27) | 24.75 (4.95) | 0.45 | |
| Race | White | 5 | 5 | 0.73 |
| Black or African American | 4 | 1 | ||
| American Indian or Alaska Native | 0 | 1 | ||
| Asian | 5 | 3 | ||
| Native Hawaiian or Other Pacific Islander | 0 | 0 | ||
| More than one race | 1 | 0 | ||
| Unknown/do not want to specify | 2 | 2 | ||
| Ethnicity | Hispanic | 4 | 5 | 0.42 |
| Non-Hispanic | 11 | 7 | ||
| Unknown/do not want to specify | 2 | 0 | ||
| Student status | Yes | 7 | 5 | 0.14 |
| No | 5 | 12 | ||
| Age of menarche | 12.25 (1.57) | 12.08 (1.164) | 0.76 | |
| BMI | 24.17 (4.81) | 26.18 (4.76) | 0.28 | |
| Model | Variable | Adjusted R2 | Estimate | SE | t Value | Pr(>|t|) | Significance |
|---|---|---|---|---|---|---|---|
| 1 | Diagnosis (PMDD) | 0.1177 | 3.118 | 1.433 | 2.17 | 0.0385 | * |
| 2 | Diagnosis (PMDD) | 0.1492 | −3.455 | 1.424 | 2.42 | 0.0228 | * |
| Current age | 0.156 | 0.139 | 1.12 | 0.2723 | |||
| Age of menarche | 0.693 | 0.517 | 1.34 | 0.1923 | |||
| 3 | Diagnosis (PMDD) | 0.3308 | 4.552 | 1.321 | 3.44 | 0.0023 | ** |
| Current age | 0.283 | 0.133 | 2.13 | 0.0441 | * | ||
| Age of menarche | 0.367 | 0.500 | 0.73 | 0.4705 | |||
| BMI | −2.196 | 1.437 | −1.52 | 0.1407 | |||
| Estradiol | 10.10 | 6.372 | 1.58 | 0.1271 | |||
| 4 | Diagnosis (PMDD) | 0.4537 | 5.566 | 1.275 | 4.36 | 0.0004 | *** |
| Current age | 0.127 | 0.144 | 0.88 | 0.3889 | |||
| Age of menarche | 0.726 | 0.540 | 1.34 | 0.1965 | |||
| BMI | −2.019 | 1.402 | −1.44 | 0.1681 | |||
| Estradiol | 13.24 | 7.121 | 1.86 | 0.0802 | . | ||
| Race (African American) | 2.594 | 1.792 | 1.44 | 0.1660 | |||
| Race (American Indian/Alaska Native) | −5.829 | 3.540 | −1.64 | 0.1179 | |||
| Race (Asian) | −1.135 | 1.849 | −0.61 | 0.5474 | |||
| Race (more than one race) | 4.603 | 3.550 | 1.29 | 0.2120 | |||
| Race (unknown/do not want to specify) | −2.600 | 1.977 | −1.31 | 0.2060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Cho, S.; Smadi, S.; Davis, J. Visuospatial Function in Women with Premenstrual Dysphoric Disorder. J. Clin. Med. 2024, 13, 2004. https://doi.org/10.3390/jcm13072004
Hamidovic A, Cho S, Smadi S, Davis J. Visuospatial Function in Women with Premenstrual Dysphoric Disorder. Journal of Clinical Medicine. 2024; 13(7):2004. https://doi.org/10.3390/jcm13072004
Chicago/Turabian StyleHamidovic, Ajna, Soojeong Cho, Shahd Smadi, and John Davis. 2024. "Visuospatial Function in Women with Premenstrual Dysphoric Disorder" Journal of Clinical Medicine 13, no. 7: 2004. https://doi.org/10.3390/jcm13072004
APA StyleHamidovic, A., Cho, S., Smadi, S., & Davis, J. (2024). Visuospatial Function in Women with Premenstrual Dysphoric Disorder. Journal of Clinical Medicine, 13(7), 2004. https://doi.org/10.3390/jcm13072004








