Risk of Mortality and Cardiovascular Events in Patients with Chronic Obstructive Pulmonary Disease Treated with Azithromycin, Roxithromycin, Clarithromycin, and Amoxicillin
Abstract
1. Introduction
1.1. Background
1.2. Objective
2. Method
2.1. Data Sources and Covariates
- (1)
- The Danish Register of Chronic Obstructive Pulmonary Disease DrCOPD-Data were collected from all COPD outpatients across Denmark. Numerous beneficial variables are available. These variables include the Medical Research Council Dyspnea Scale (MRC), which measures respiratory distress; Forced Expired Volume in the first second (FEV1), a critical measure of lung function; Body Mass Index (BMI), reflecting patients’ nutritional and health status; along with the dates of outpatient visits, providing insights into healthcare utilization patterns. Additionally, demographic details such as age and gender, alongside crucial life events such as the date of death, were also captured. The Danish Register of Chronic Obstructive Pulmonary Disease (DrCOPD) is an important resource, meticulously crafted to strengthen the capacities of healthcare professionals, researchers, and policymakers in their quest to comprehend and tackle COPD with greater efficacy in the Danish context. Within this rich database lie invaluable variables, each offering a unique lens through which the complexities and severity of COPD can be discerned and categorized. The integrity and depth of this data repository are upheld through the diligent contributions of physicians and nurses operating within outpatient clinics, ensuring a robust and reliable foundation for advancing COPD care and research initiatives.
- (2)
- The Danish National Patient Registry (DNPR)—we had access to all of the patient’s hospital admissions in Denmark registered with ICD-10. They are divided into A and B diagnoses. We used the A diagnosis to find the cardiovascular event admissions registered after each patient study entry. We used the B diagnosis to find the patient’s comorbidities. We looked at the 10-year prior baseline for the comorbidities. The Danish National Patient Registry is a comprehensive medical database that collects data on all hospital admissions and outpatient visits in Denmark, offering invaluable insights for healthcare research and policy-making by tracking patient diagnoses, treatments, and outcomes since its inception in 1977. The outpatient visits have been recorded since 1995.
- (3)
- The Danish National Health Service Prescription Database (DNHSPD)- we obtained all of the patient’s prescriptions. Each prescription is named by its Anatomical Therapeutic Chemical ATC) classification system. The following ATC codes were used for the four groups: amoxicillin ‘J01CA04’, azithromycin ‘J01FA10’, clarithromycin ‘J01FA09’, and roxithromycin ‘J01FA06’. The Danish National Health Service Prescription Database meticulously records all prescriptions dispensed at Danish pharmacies, providing a detailed overview of medication use patterns across the population. This extensive database is instrumental in pharmaceutical research and healthcare policy development, enabling the study of prescription trends, drug safety, and adherence. Data have been registered since 2004.
- (4)
- With the Danish National Death Registry, we can now identify the reason for death for each patient. The Danish National Death Registry is a resource that compiles information on all deaths occurring within Denmark, including causes and dates. This registry plays a vital role in epidemiological studies and public health planning, offering insights into mortality trends, life expectancy, and the impact of specific health interventions.
2.2. Study Design
2.3. Exposure to Antibiotics
2.4. Outcome
2.5. Statistical Analysis
2.6. Patient and Public Involvement
3. Results
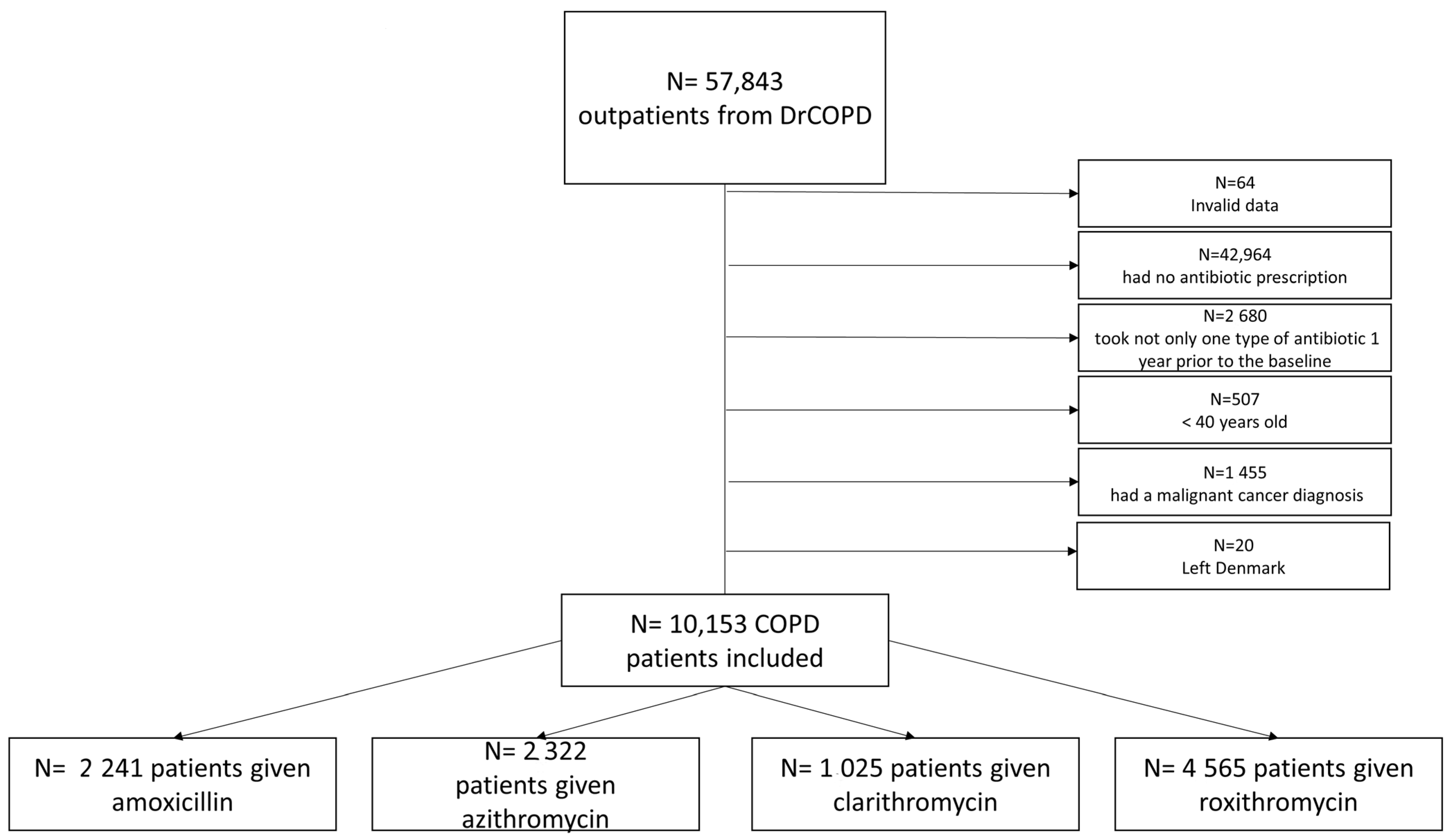
3.1. Participants
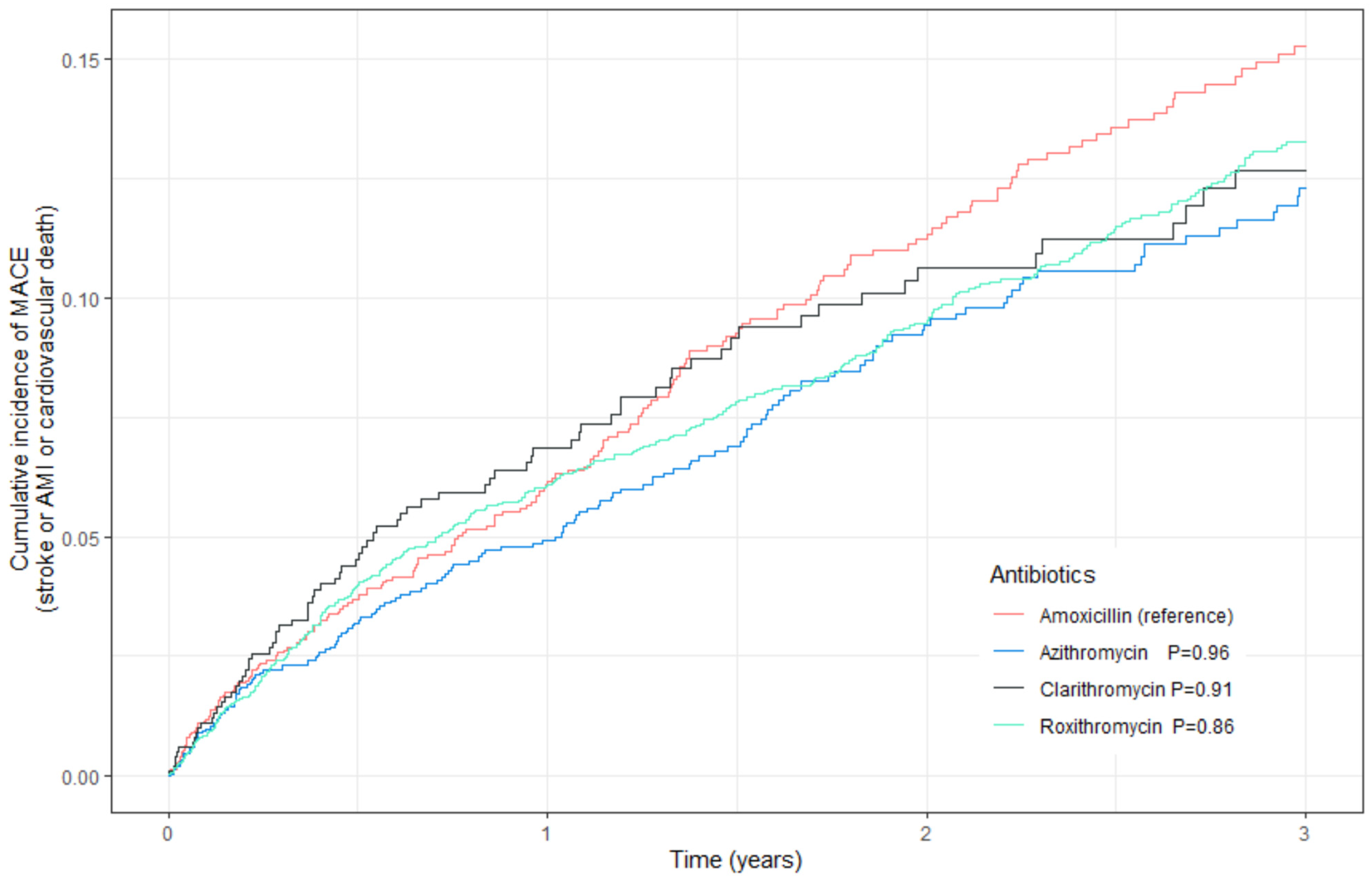
3.1.1. Primary Outcome
3.1.2. Secondary Outcome
4. Discussion
Strengths and Limitations
5. Conclusions
6. Future Perspectives
7. Ethical Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Eddine Aichour, M.T.; Alam, K.; et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Respirology 2017, 22, 575–601. [Google Scholar] [CrossRef] [PubMed]
- Müllerova, H.; Chigbo, C.; Hagan, G.W.; Woodhead, M.A.; Miravitlles, M.; Davis, K.J.; Wedzicha, J.A. The natural history of community-acquired pneumonia in COPD patients: A population database analysis. Respir. Med. 2012, 106, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Holguin, F.; Folch, E.; Redd, S.C.; Mannino, D.M. Comorbidity and mortality in COPD-related hospitalizations in the United States, 1979 to 2001. Chest 2005, 128, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Prina, E.; Ranzani, O.T.; Torres, A. Community-acquired pneumonia. Lancet 2015, 386, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the Risk of Cardiovascular Death. N. Engl. J. Med. 2012, 366, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Svanström, H.; Pasternak, B.; Hviid, A. Use of Azithromycin and Death from Cardiovascular Causes. N. Engl. J. Med. 2013, 368, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Winkel, P.; Hilden, J.; Hansen, J.F.; Kastrup, J.; Kolmos, H.J.; Kjøller, E.; Jensen, G.B.; Skoog, M.; Lindschou, J.; Gluud, C. Clarithromycin for stable coronary heart disease increases all-cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. Int. J. Cardiol. 2015, 182, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Mosholder, A.D.; Lee, J.Y.; Zhou, E.H.; Kang, E.M.; Ghosh, M.; Izem, R.; Major, J.M.; Graham, D.J. Long-Term Risk of Acute Myocardial Infarction, Stroke, and Death with Outpatient Use of Clarithromycin: A Retrospective Cohort Study. Am. J. Epidemiol. 2018, 187, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Schembri, S.; Williamson, P.A.; Short, P.M.; Singanayagam, A.; Akram, A.; Taylor, J.; Singanayagam, A.; Hill, A.T.; Chalmers, J.D. Cardiovascular events After clarithromycin use in lower respiratory tract infections: Analysis of two prospective cohort studies. BMJ 2013, 346, f1235. [Google Scholar] [CrossRef] [PubMed]
- Dansk Lungemedicinsk Selskab. KOL Exacerbation og NIV; DLS: Vejle, Denmark, 2021. [Google Scholar]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- COP:TRIN [Internet]. Available online: http://coptrin.dk/steering-committee-members/ (accessed on 1 January 2024).
- Mathioudakis, A.G.; Abroug, F.; Agusti, A.; Ananth, S.; Bakke, P.; Bartziokas, K.; Beghe, B.; Bikov, A.; Bradbury, T.; Brusselle, G.; et al. ERS statement: A core outcome set for clinical trials evaluating the management of COPD exacerbations. Eur. Respir. J. 2022, 59, 2102006. [Google Scholar] [CrossRef] [PubMed]
- Lunge.dk [Internet]. Available online: www.lunge.dk (accessed on 1 January 2024).
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Grayston, J.T.; Muhlestein, B.; Giugliano, R.P.; Cairns, R.; Skene, A.M. Antibiotic Treatment of Chlamydia pneumoniae after Acute Coronary Syndrome. N. Engl. J. Med. 2005, 352, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Cercek, B.; Shah, P.K.; Noc, M.; Zahger, D.; Zeymer, U.; Matetzky, S.; Maurer, G.; Mahrer, P. Effect of short-term treatment with azithromycin on recurrent ischaemic events in patients with acute coronary syndrome in the Azithromycin in Acute Coronary Syndrome (AZACS) trial: A randomised controlled trial. Lancet 2003, 361, 809–813. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.M.; Dunne, M.W.; Pfeffer, M.A.; Muhlestein, J.B.; Yao, L.; Gupta, S.; Benner, R.J.; Fisher, M.R.; Cook, T.D. Azithromycin for the Secondary Prevention of Coronary Heart Disease Events: The WIZARD Study: A Randomized Controlled Trial. JAMA 2003, 290, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Andraws, R.; Berger, J.S.; Brown, D.L. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: A meta-analysis of randomized controlled trials. JAMA 2005, 14, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J. Antibiotics in the prevention of heart attacks. Lancet 2005, 365, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.S.; Root, A.; Douglas, I.J.; Chui, C.S.L.; Chan, E.W.; Ghebremichael-Weldeselassie, Y.; Siu, C.-W.; Smeeth, L.; Wong, I.C.K. Cardiovascular outcomes associated with use of clarithromycin: Population based study. BMJ 2016, 352, h6926. [Google Scholar] [CrossRef] [PubMed]
| All | Amoxicillin | Azithromycin | Clarithromycin | Roxithromycin | |
|---|---|---|---|---|---|
| Number of subjects, n (%) | 10,153 (100.0) | 2241 (22.1) | 2322 (22.9) | 1025 (10.1) | 4565 (45.0) |
| Females, n (%) | 5843 (57.5) | 1158 (51.7) | 1366 (58.8) | 596 (58.1) | 2723 (59.6) |
| FEV1, % of expected, median (IQR) | 48.0 (35.0–63.0) | 47.0 (34.0–62.0) | 45.0 (32.0–60.0) | 49.0 (36.0–63.0) | 50.0 (36.0–64.0) |
| Age | 69.69 (40–98) | 70.85 (40–98) | 68.96 (40–92) | 69.39 (40–93) | 69.55 (40–96) |
| Asprin | 2989 | 722 | 646 | 305 | 1316 |
| Renin-Angiotension-System (RAS)-inhibitors | 2954 | 698 | 604 | 300 | 1352 |
| Novel oral anticoagulants (NOAC) | 451 | 98 | 115 | 47 | 191 |
| Beta-blockers, n (%) | 2604 | 675 | 523 | 258 | 1148 |
| Amount prescriptions of antibiotic courses: | |||||
| ≤2 | 8621 | 1998 | 1677 | 925 | 4021 |
| >2 | 1532 | 243 | 645 | 100 | 544 |
| MRC, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) |
| BMI, n (%), kg/m² | 25.0 (21.0–29.0) | 25.0 (21.0–29.0) | 24.8 (21.0–28.4) | 25.0 (21.2–29.0) | 25.0 (22.0–29.0) |
| Peripheral vascular disease, n (%) | 1149 (11.3) | 284 (12.7) | 233 (10.0) | 118 (11.5) | 514 (11.3) |
| Ischaemic heart disease, n (%) | 902 (8.9) | 241 (10.8) | 186 (8.0) | 98 (9.6) | 377 (8.3) |
| Heart failure, n (%) | 1430 (14.1) | 375 (16.7) | 285 (12.3) | 139 (13.6) | 631 (13.8) |
| Diabetes without complications, n (%) | 1266 (12.5) | 278 (12.4) | 261 (11.2) | 132 (12.9) | 595 (13.0) |
| Diabetes with complications, n (%) | 411 (4.0) | 92 (4.1) | 72 (3.1) | 45 (4.4) | 202 (4.4) |
| Stroke, n (%) | 1115 (11.0) | 266 (11.9) | 214 (9.2) | 126 (12.3) | 509 (11.2) |
| Renal disease, n (%) | 415 (4.1) | 115 (5.1) | 74 (3.2) | 52 (5.1) | 174 (3.8) |
| Rheumatological disease, n (%) | 460 (4.5) | 99 (4.4) | 108 (4.7) | 50 (4.9) | 203 (4.4) |
| Paraplegia, n (%) | 46 (0.5) | 12 (0.5) | 12 (0.5) | 7 (0.7) | 15 (0.3) |
| Antibiotic Groups | End of Follow-Up N (%) | Stroke N (%) | AMI ** N (%) | Cardiovascular Death N (%) | Cause of Death * N (%) | Antibiotic Switch N (%) | Total (n) |
|---|---|---|---|---|---|---|---|
| Amoxicillin | 1092 (48.73) | 74 (3.30) | 37 (1.65) | 84 (3.75) | 502 (22.40) | 452 (20.17) | 2241 |
| Azithromycin | 1329 (57.24) | 58 (2.50) | 41 (1.77) | 63 (2.71) | 473 (20.37) | 358 (15.42) | 2322 |
| Clarithromycin | 553 (53.95) | 29 (2.83) | 13 (1.27) | 38 (3.71) | 183 (17.85) | 209 (20.39) | 1025 |
| Roxithromycin | 2568 (56.25) | 128 (2.80) | 78 (1.71) | 174 (3.81) | 1007 (22.06) | 610 (13.36) | 4565 |
| Total | 5542 (54.58) | 289 (2.85) | 169 (1.66) | 359 (3.54) | 2165 (21.32) | 1629 (16.04) | 10,153 |
| Adjusted | IPTW | |||||
|---|---|---|---|---|---|---|
| Treatment Groups | Hazard Ratio | 95% Confidential Interval | p Value | Hazard Ratio | 95% Confidential Interval | p Value |
| Azithromycin | 1.01 | 0.81–1.25 | 0.96 | 0.94 | 0.76–1.16 | 0.54 |
| Clarithromycin | 0.99 | 0.75–1.30 | 0.91 | 1.03 | 0.79–1.35 | 0.82 |
| Roxithromycin | 1.02 | 0.85–1.22 | 0.86 | 1.00 | 0.83–1.19 | 0.97 |
| Amoxicillin | 1.00 | ref | ref | 1.00 | ref | ref |
| Adjusted | IPTW | |||||
|---|---|---|---|---|---|---|
| Treatment Groups | Hazard Ratio | 95% Confidential Interval | p Value | Hazard Ratio | 95% Confidential Interval | p Value |
| Azithromycin | 1.06 | 0.94–1.19 | 0.37 | 0.98 | 0.88–1.11 | 0.78 |
| Clarithromycin | 0.95 | 0.81–1.11 | 0.51 | 0.93 | 0.80–1.08 | 0.31 |
| Roxithromycin | 0.98 | 0.89–1.09 | 0.70 | 0.98 | 0.88–1.08 | 0.63 |
| Amoxicillin | 1.00 | ref | ref | 1.00 | ref | ref |
| Adjusted | IPTW | |||||
|---|---|---|---|---|---|---|
| Treatment Groups | Hazard Ratio | 95% Confidential Interval | p Value | Hazard Ratio | 95% Confidential Interval | p Value |
| Azithromycin | 0.96 | 0.70–1.33 | 0.82 | 0.87 | 0.64–1.19 | 0.40 |
| Clarithromycin | 1.16 | 0.79–1.69 | 0.45 | 1.22 | 0.85–1.74 | 0.27 |
| Roxithromycin | 1.12 | 0.87–1.45 | 0.37 | 1.1 | 0.85–1.40 | 0.48 |
| Amoxicillin | 1.00 | ref | ref | 1.00 | ref | Ref |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alispahic, I.A.; Eklöf, J.; Sivapalan, P.; Jordan, A.R.; Harboe, Z.B.; Biering-Sørensen, T.; Jensen, J.-U.S. Risk of Mortality and Cardiovascular Events in Patients with Chronic Obstructive Pulmonary Disease Treated with Azithromycin, Roxithromycin, Clarithromycin, and Amoxicillin. J. Clin. Med. 2024, 13, 1987. https://doi.org/10.3390/jcm13071987
Alispahic IA, Eklöf J, Sivapalan P, Jordan AR, Harboe ZB, Biering-Sørensen T, Jensen J-US. Risk of Mortality and Cardiovascular Events in Patients with Chronic Obstructive Pulmonary Disease Treated with Azithromycin, Roxithromycin, Clarithromycin, and Amoxicillin. Journal of Clinical Medicine. 2024; 13(7):1987. https://doi.org/10.3390/jcm13071987
Chicago/Turabian StyleAlispahic, Imane Achir, Josefin Eklöf, Pradeesh Sivapalan, Alexander Ryder Jordan, Zitta Barrella Harboe, Tor Biering-Sørensen, and Jens-Ulrik Stæhr Jensen. 2024. "Risk of Mortality and Cardiovascular Events in Patients with Chronic Obstructive Pulmonary Disease Treated with Azithromycin, Roxithromycin, Clarithromycin, and Amoxicillin" Journal of Clinical Medicine 13, no. 7: 1987. https://doi.org/10.3390/jcm13071987
APA StyleAlispahic, I. A., Eklöf, J., Sivapalan, P., Jordan, A. R., Harboe, Z. B., Biering-Sørensen, T., & Jensen, J.-U. S. (2024). Risk of Mortality and Cardiovascular Events in Patients with Chronic Obstructive Pulmonary Disease Treated with Azithromycin, Roxithromycin, Clarithromycin, and Amoxicillin. Journal of Clinical Medicine, 13(7), 1987. https://doi.org/10.3390/jcm13071987










