The Relationship between Selected Parameters and the Occurrence of Premyopia in a Group of 1155 Children Aged 8 in Northwestern Poland
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutti, D.O. Vitamin D May Reduce the Prevalence of Myopia in Korean Adolescents. Investig. Opthalmol. Vis. Sci. 2014, 55, 2048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, P.C.; Tsai, C.L.; Wu, H.L.; Yang, Y.H.; Kuo, H.K. Outdoor Activity during Class Recess Reduces Myopia Onset and Progression in School Children. Ophthalmology 2013, 120, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Tkatchenko, A.V. A Review of Current Concepts of the Etiology and Treatment of Myopia. Eye Contact Lens Sci. Clin. Pract. 2018, 44, 231–247. [Google Scholar] [CrossRef]
- Rose, K.A.; Morgan, I.G.; Ip, J.; Kifley, A.; Huynh, S.; Smith, W. Outdoor Activity Reduces the Prevalence of Myopia in Children. Ophthalmology 2008, 115, 1279–1285. [Google Scholar] [CrossRef]
- Guggenheim, J.A.; Northstone, K.; McMahon, G.; Ness, A.R.; Deere, K.; Mattocks, C. Time Outdoors and Physical Activity as Predictors of Incident Myopia in Childhood: A Prospective Cohort Study. Investig. Opthalmol. Vis. Sci. 2012, 53, 2856. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sinnott, L.T.; Mutti, D.O.; Mitchell, G.L.; Moeschberger, M.L.; Zadnik, K. Parental History of Myopia, Sports and Outdoor Activities, and Future Myopia. Investig. Opthalmol. Vis. Sci. 2007, 48, 3524. [Google Scholar] [CrossRef]
- Deng, L.; Gwiazda, J.; Thorn, F. Children’s Refractions and Visual Activities in the School Year and Summer. Optom. Vis. Sci. 2010, 87, 406–413. [Google Scholar] [CrossRef]
- Rose, K.A.; French, A.N.; Morgan, I.G. Environmental Factors and Myopia: Paradoxes and Prospects for Prevention. Asia-Pac. J. Ophthalmol. 2016, 5, 403–410. [Google Scholar] [CrossRef]
- Wong, Y.L.; Sabanayagam, C.; Ding, Y.; Wong, C.W.; Yeo, A.C.H.; Cheung, Y.B. Prevalence, Risk Factors, and Impact of Myopic Macular Degeneration on Visual Impairment and Functioning Among Adults in Singapore. Investig. Opthalmol. Vis. Sci. 2018, 59, 4603. [Google Scholar] [CrossRef]
- Brennan, N.A.; Toubouti, Y.M.; Cheng, X.; Bullimore, M.A. Efficacy in myopia control. Prog. Retin. Eye Res. 2021, 83, 100923. [Google Scholar] [CrossRef]
- Bullimore, M.A.; Brennan, N.A. Myopia Control: Why Each Diopter Matters. Optom. Vis. Sci. 2019, 96, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Ip, J.M.; Saw, S.M.; Rose, K.A.; Morgan, I.G.; Kifley, A.; Wang, J.J. Role of Near Work in Myopia: Findings in a Sample of Australian School Children. Investig. Opthalmol. Vis. Sci. 2008, 49, 2903. [Google Scholar] [CrossRef]
- Saw, S.M.; Tong, L.; Chua, W.H.; Chia, K.S.; Koh, D.; Tan, D.T.H. Incidence and Progression of Myopia in Singaporean School Children. Investig. Opthalmol. Vis. Sci. 2005, 46, 51. [Google Scholar] [CrossRef]
- Logan, N.S.; Radhakrishnan, H.; Cruickshank, F.E.; Allen, P.M.; Bandela, P.K.; Davies, L.N. IMI Accommodation and Binocular Vision in Myopia Development and Progression. Investig. Opthalmol. Vis. Sci. 2021, 62, 4. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, J.; Brennan, N.A. Accommodation and its role in myopia progression and control with soft contact lenses. Ophthalmic Physiol. Opt. 2019, 39, 162–171. [Google Scholar] [CrossRef]
- Wagner, S.; Zrenner, E.; Strasser, T. Emmetropes and myopes differ little in their accommodation dynamics but strongly in their ciliary muscle morphology. Vis. Res. 2019, 163, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Rose, K.A. Myopia and international educational performance. Ophthalmic Physiol. Opt. 2013, 33, 329–338. [Google Scholar] [CrossRef]
- Straßer, T.; Wagner, S. Performance of the Deep Neural Network Ciloctunet, Integrated with Open-Source Software for Ciliary Muscle Segmentation in Anterior Segment OCT Images, Is on Par with Experienced Examiners. Diagnostics 2022, 12, 3055. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Park, K. Recent trend of increasing myopia can be traced to infancy. Med. Hypotheses 2019, 128, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Donders, F.C. An Essay on the Nature and Consequences of Anomalies of Refraction. JAMA J. Am. Med. Assoc. 1899, 33, 1307. [Google Scholar]
- Yu, C.B.O. Changing patterns of strabismus: A decade of experience in Hong Kong. Br. J. Ophthalmol. 2002, 86, 854–856. [Google Scholar] [CrossRef]
- Quek, T.P.L.; Chua, C.G.; Chong, C.S.; Chong, J.H.; Hey, H.W.; Lee, J. Prevalence of refractive errors in teenage high school students in Singapore. Ophthalmic Physiol. Opt. 2004, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Szaflik, J.; Prost, M.; Zaleska-Zmijewska, A.; Hapunik, A.; Wójcik, A. The analysis of refractive error in children and adolescents from 6-15 years of age based on 1000 examinations in two major Polish regions. Klin. Oczna 2004, 106 (Suppl. 3), 471–473. [Google Scholar] [PubMed]
- Monika, M.; Durajczyk, M. Evaluation of the Prevalence of Refractive Defects and Ocular Function in a Group of 1518 Children Aged 8 Years in Northwestern Poland—A Retrospective Study. J. Clin. Med. 2023, 12, 2880. [Google Scholar] [CrossRef] [PubMed]
- Tasci, Y.; Yesilirmak, N.; Yuzbasioglu, S.; Ozdas, D.; Temel, B. Comparison of effects of mydriatic drops (1% cyclopentolate and 0.5% tropicamide) on anterior segment parameters. Indian. J. Ophthalmol. 2021, 69, 1802. [Google Scholar] [PubMed]
- Lim, J.; Chia, A.; Saffari, S.E.; Handa, S. Factors Affecting Pupil Reactivity After Cycloplegia in Asian Children. Asia-Pac. J. Ophthalmol. 2019, 8, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S. IMI—Interventions for Controlling Myopia Onset and Progression Report. Investig. Opthalmol. Vis. Sci. 2019, 60, M106. [Google Scholar] [CrossRef] [PubMed]
- Horner, D.G.; Salmon, T.; Sarita, P. Borish’s Clinical Refraction; Elsevier: Amsterdam, The Netherlands, 2006; Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780750675246X50017 (accessed on 23 August 2023).
- Ong, E.; Ciuffreda, K.J. Accomodation, Nearwork, and Myopia; Optometric Extension Program: Santa Ana, CA, USA, 1997; 211p. [Google Scholar]
- Bullimore, M.A.; Gilmartin, B. Tonic Accommodation, Cognitive Demand, and Ciliary Muscle Innervation. Optom. Vis. Sci. 1987, 64, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Woo, G.C.; Schmid, K.L. Bifocal lens control of myopic progression in children. Clin. Exp. Optom. 2011, 94, 24–32. [Google Scholar] [CrossRef]
- García-Montero, M.; Felipe-Márquez, G.; Arriola-Villalobos, P.; Garzón, N. Pseudomyopia: A Review. Vision 2022, 6, 17. [Google Scholar] [CrossRef]
- Sun, W.; Yu, M.; Wu, J.; Han, X.; Jan, C.; Song, J. Pseudomyopia as an independent risk factor for myopia onset: A prospective cohort study among school-aged children. Br. J. Ophthalmol. 2023. [Google Scholar] [CrossRef]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Opthalmol. Vis. Sci. 2019, 60, M20. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Yu, S.; Ma, N.; Huang, C.; Wang, M. Prevention of myopia shift and myopia onset using 0.01% atropine in premyopic children—A prospective, randomized, double-masked, and crossover trial. Eur. J. Pediatr. 2023, 182, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
- Nadell, M.C.; Weymouth, F.W.; Hirsch, M.J. The relationship of frequency of use of the eyes in close work to the distribution of refractive error in a selected sample. Optom. Vis. Sci. 1957, 34, 523–537. [Google Scholar] [CrossRef]
- Goss, D.A.; Winkler, R.L. Progression of Myopia in Youth: Age of Cessation. Optom. Vis. Sci. 1983, 60, 651–658. [Google Scholar] [CrossRef]
- Drobe, B.; De Saint-André, R. The pre-myopic syndrome. Ophthalmic Physiol. Opt. 1995, 15, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Mum, D.O.; Zadnik, K. The utility of three predictors of childhood myopia: A Bayesian analysis. Vis. Res. 1995, 35, 1345–1352. [Google Scholar] [CrossRef]
- Millodot, M. Dictionary of Optometry and Visual Science, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2009. [Google Scholar]
- Rosenfield, M.; Gilmartin, B. Effect of Target Proximity on the Open-Loop Accommodative Response. Optom. Vis. Sci. 1990, 67, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Wick, B.; Currie, D. Dynamic Demonstration of Proximal Vergence and Proximal Accommodation. Optom. Vis. Sci. 1991, 68, 163–167. [Google Scholar] [CrossRef]
- Rosenfield, M.; Ciuffreda, K.J. Effect of surround propinquity on the open-loop accommodative response. Investig. Ophthalmol. Vis. Sci. 1991, 32, 142–147. [Google Scholar]
- Ting, P.W.K.; Schmid, K.L.; Lam, C.S.Y.; Edwards, M.H. Objective real-time measurement of instrument myopia in microscopists under different viewing conditions. Vis. Res. 2006, 46, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.E.; Smith, E.L. Long-term adaptational aftereffects of accommodation are associated with distal dark focus and not with late onset myopia. Investig. Ophthalmol. Vis. Sci. 1993, 34, 1308. [Google Scholar]
- Mather, G.; Smith, D.R.R. Blur Discrimination and its Relation to Blur-Mediated Depth Perception. Perception 2002, 31, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Gray, L.S.; Heron, G. The Effect of Monocular and Binocular Viewing on the Accommodation Response to Real Targets in Emmetropia and Myopia. Optom. Vis. Sci. 2005, 82, 279–285. [Google Scholar] [CrossRef]
- Vera-Diaz, F.A.; Bex, P.J.; Ferreira, A.; Kosovicheva, A. Binocular temporal visual processing in myopia. J. Vis. 2018, 18, 17. [Google Scholar] [CrossRef]
- Jorge, J.; de Almeida, J.B.; Parafita, M.A. Binocular Vision Changes in University Students: A 3-Year Longitudinal Study. Optom. Vis. Sci. 2008, 85, E999–E1006. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, V.; Irving, E.L.; Bobier, W.R. Effect of heterophoria type and myopia on accommodative and vergence responses during sustained near activity in children. Vis. Res. 2012, 57, 9–17. [Google Scholar] [CrossRef]
- Gifford, K.L.; Gifford, P.; Hendicott, P.L.; Schmid, K.L. Zone of Clear Single Binocular Vision in Myopic Orthokeratology. Eye Contact Lens Sci. Clin. Pract. 2020, 46, 82–90. [Google Scholar] [CrossRef]
- Kaphle, D.; Schmid, K.L.; Davies, L.N.; Suheimat, M.; Atchison, D.A. Ciliary Muscle Dimension Changes with Accommodation Vary in Myopia and Emmetropia. Investig. Opthalmol. Vis. Sci. 2022, 63, 24. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Thibos, L.N.; Bradley, A. Effects of refractive error on detection acuity and resolution acuity in peripheral vision. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2134–2143. [Google Scholar]
- Hoffman, D.M.; Banks, M.S. Focus information is used to interpret binocular images. J. Vis. 2010, 10, 13. [Google Scholar] [CrossRef]
- Mather, G. The Use of Image Blur as a Depth Cue. Perception 1997, 26, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Domaradzki, J.; Modrzejewska, M.; Koźlenia, D.; Zwierko, T. Assessment of the Functional Form of the Relationship between Balance Control and Physical Activity Regarding Demographic, Anthropometrical, and Eye Impairment Explanatory Covariates in 9- to 11-Year-Old Children: Results of Polynomial and Cluster Analyses. Biology 2022, 11, 1663. [Google Scholar] [CrossRef]
- Gifford, K.L.; Richdale, K.; Kang, P.; Aller, T.A.; Lam, C.S.; Liu, Y.M. IMI—Clinical Management Guidelines Report. Investig. Opthalmol. Vis. Sci. 2019, 60, M184. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Alcocer, J.; Madrid-Costa, D.; Radhakrishnan, H.; Ferrer-Blasco, T.; Montés-Micó, R. Changes in Accommodation and Ocular Aberration with Simultaneous Vision Multifocal Contact Lenses. Eye Contact Lens Sci. Clin. Pract. 2012, 38, 288–294. [Google Scholar] [CrossRef]
- Tarrant, J.; Severson, H.; Wildsoet, C.F. Accommodation in emmetropic and myopic young adults wearing bifocal soft contact lenses. Ophthalmic Physiol. Opt. 2008, 28, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Wildsoet, C.F. Acute and short-term changes in visual function with multifocal soft contact lens wear in young adults. Contact Lens Anterior Eye 2016, 39, 133–140. [Google Scholar] [CrossRef]
- Ozkan, J.; Fedtke, C.; Chung, J.; Thomas, V.; Bakaraju, R.C. Short-Term Adaptation of Accommodative Responses in Myopes Fitted with Multifocal Contact Lenses. Eye Contact Lens Sci. Clin. Pract. 2018, 44, S30–S37. [Google Scholar] [CrossRef]

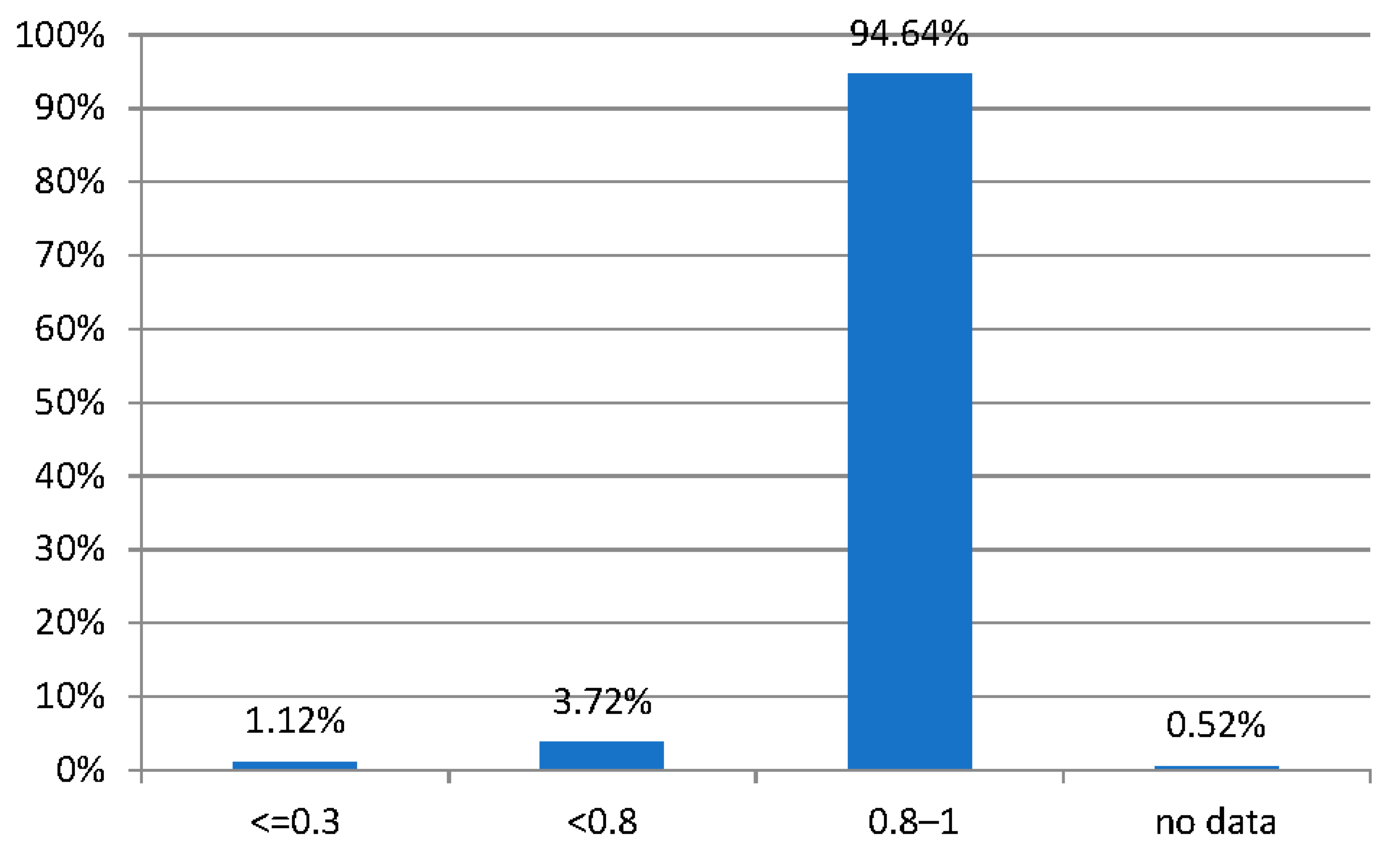
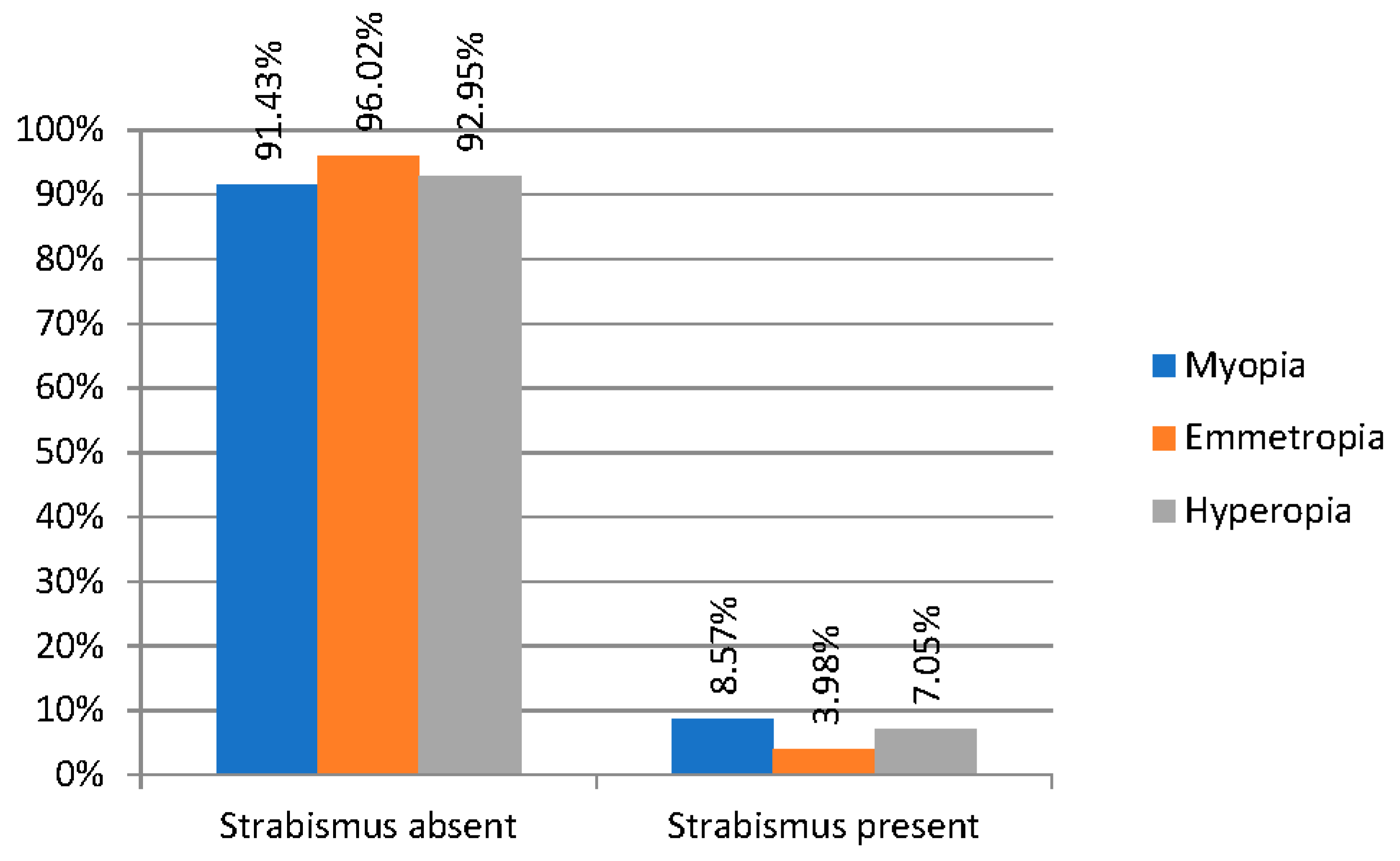
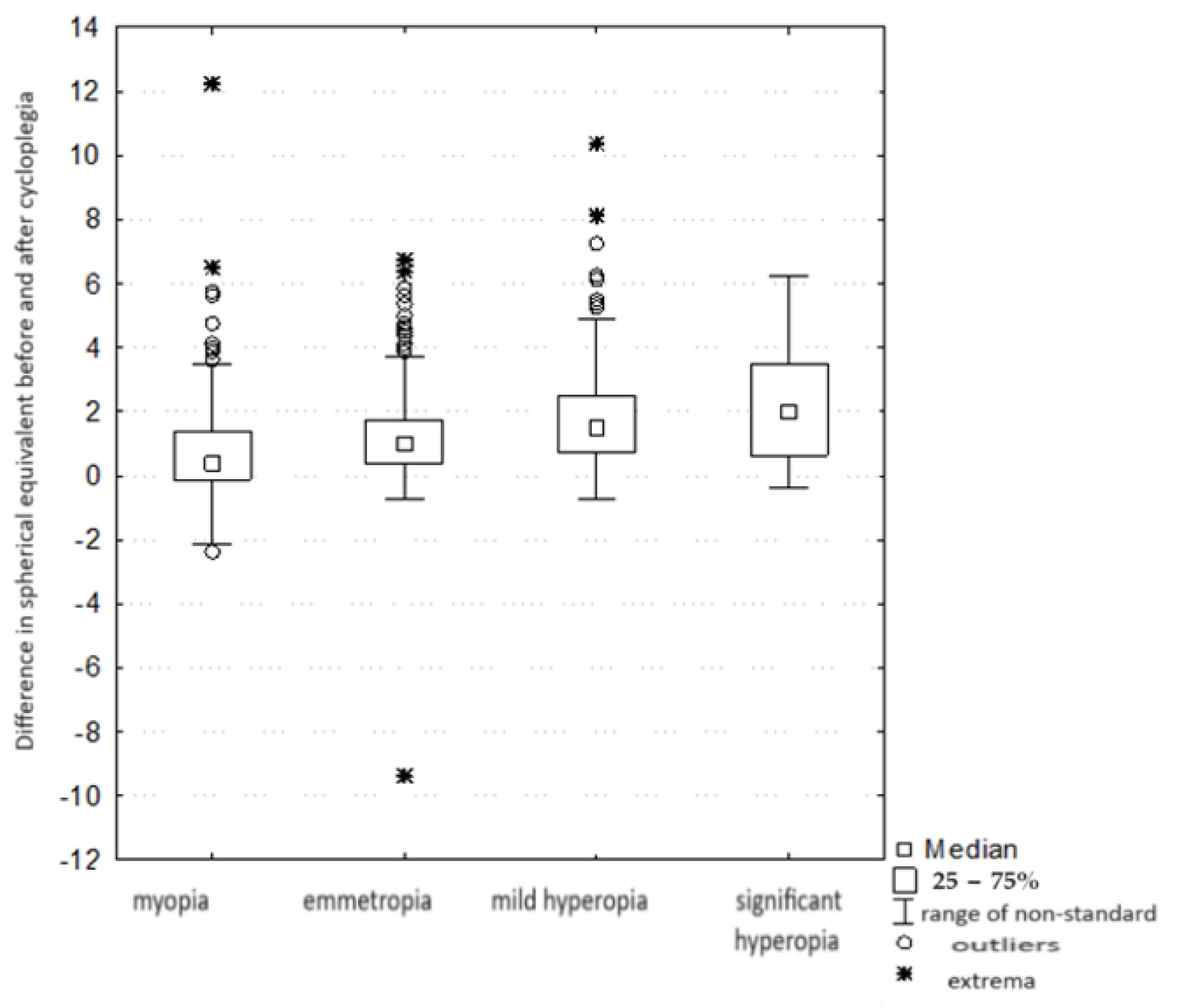
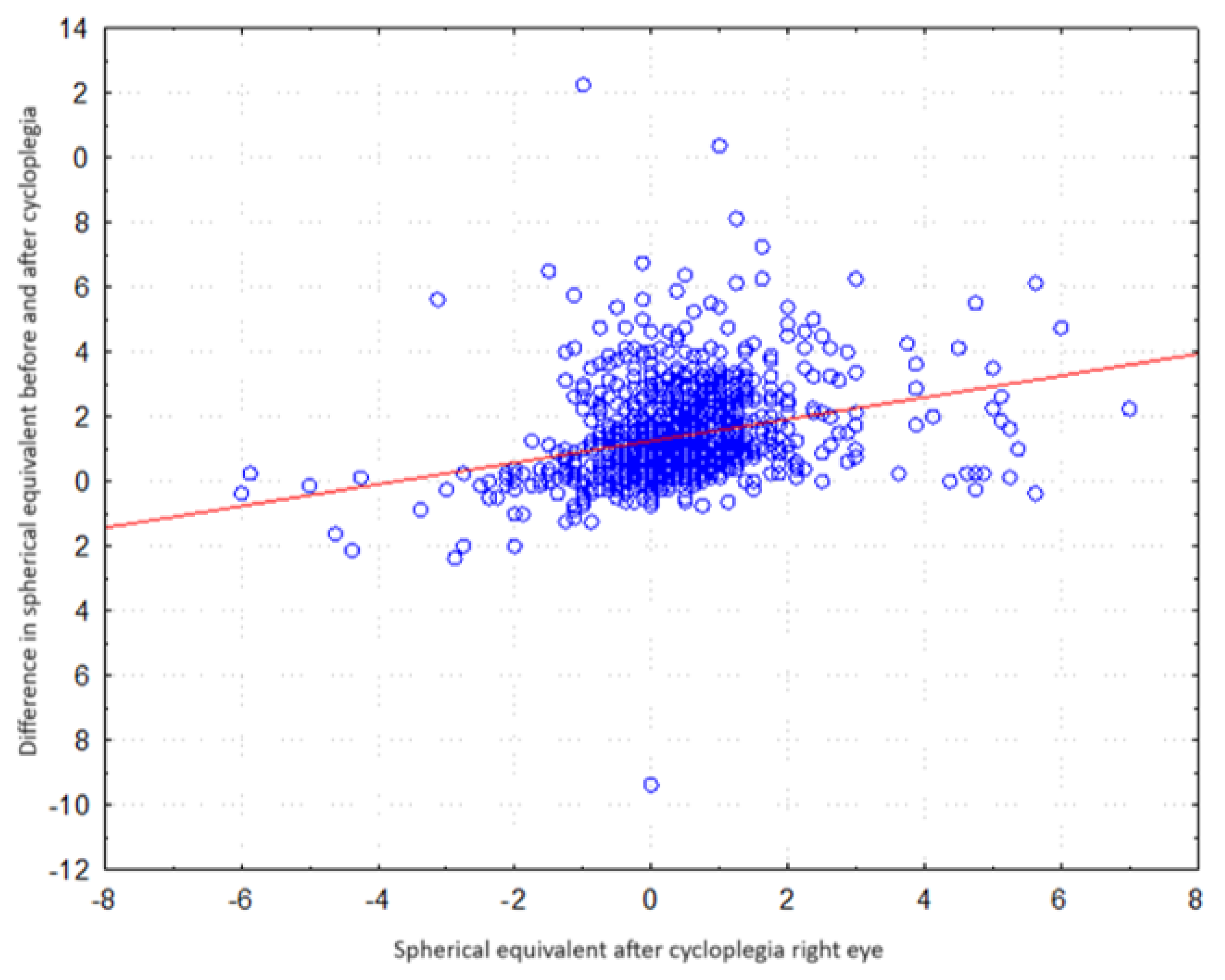
| Refraction Parameter | Spherical Equivalent (D) |
|---|---|
| Emmetropia | −0.5 to ≤+0.5° |
| Pre-myopia | −0.50 D–+0.75 |
| Myopia | ≥−0.50 |
| Mild hyperopia | ≥+0.50–≤+2.0 |
| Significant hyperopia | ≥+2.00 |
| Spherical Equivalent after Drops, Right Eye | Gender (M/F) F | Gender (M/F) M | Overall |
|---|---|---|---|
| below −0.50 | 61 | 79 | 140 |
| from −0.50 to +0.75 PRE-MYOPIA | 328 | 376 | 704 |
| above +0.75 | 161 | 143 | 304 |
| overall | 550 | 598 | 1148 |
| Chi2 Pearsona | 4.653970 | df = 2 | p = 0.09759 |
| Comparison of the Magnitude of the Change in Equivalents between Groups with Different Refractive Errors Kruskal–Wallis Test: H (3, n = 1144) = 93.40315 p = 0.000 | ||||
|---|---|---|---|---|
| Myopia R: 396.78 | Emmetropia R: 531.25 | Mild Hyperopia R: 670.19 | Significant Hyperopia R: 717.90 | |
| Myopia (group IV) | 0.000114 | 0.000000 | 0.000000 | |
| Emmetropia (group I) | 0.000114 | 0.000000 | 0.000337 | |
| mild hyperopia (group II) | 0.000000 | 0.000000 | 1.000000 | |
| significant hyperopia (group III) | 0.000000 | 0.000337 | 1.000000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modrzejewska, M.; Durajczyk, M. The Relationship between Selected Parameters and the Occurrence of Premyopia in a Group of 1155 Children Aged 8 in Northwestern Poland. J. Clin. Med. 2024, 13, 1977. https://doi.org/10.3390/jcm13071977
Modrzejewska M, Durajczyk M. The Relationship between Selected Parameters and the Occurrence of Premyopia in a Group of 1155 Children Aged 8 in Northwestern Poland. Journal of Clinical Medicine. 2024; 13(7):1977. https://doi.org/10.3390/jcm13071977
Chicago/Turabian StyleModrzejewska, Monika, and Magdalena Durajczyk. 2024. "The Relationship between Selected Parameters and the Occurrence of Premyopia in a Group of 1155 Children Aged 8 in Northwestern Poland" Journal of Clinical Medicine 13, no. 7: 1977. https://doi.org/10.3390/jcm13071977
APA StyleModrzejewska, M., & Durajczyk, M. (2024). The Relationship between Selected Parameters and the Occurrence of Premyopia in a Group of 1155 Children Aged 8 in Northwestern Poland. Journal of Clinical Medicine, 13(7), 1977. https://doi.org/10.3390/jcm13071977





